Introduction
Essential hypertension (EH) is a progressive
cardiovascular syndrome caused by multiple factors and its
pathogeneses is still being investigated. Epidemiological
investigation indicated that the prevalence of hypertensive Kazakh
patients in Xinjiang is significantly higher compared with those of
the other nationalities in the same region, while their awareness,
treatment and control rates were significantly lower (1). Recently, researchers have proposed that
EH is a chronic low-grade inflammatory disease (2–4). The
activation of T-lymphocytes, important participants in the human
immune system, is closely correlated with the occurrence and
development of EH (5,6). There are two potassium channels on the
T-lymphocyte membrane: The voltage-gated K+ channel 1.3
(Kv1.3) and the calcium-activated K+ channel.
The Kv1.3 channel serves a vital role in T-lymphocyte
activation (7–9). Our previous study has demonstrated that
the Kv1.3 channel of peripheral T-lymphocytes in
hypertensive Kazakh patients in Xinjiang serves a role in the onset
of hypertension (10); however, the
specific underlying mechanisms were unclear. There is evidence that
NLR family pyrin domain containing 3 (NLRP3) (also named cryopyrin,
CIAS1 or NALP3) participates in the formation of inflammasome,
which is involved in the onset of hypertension along with its main
downstream product, interleukin-1β (IL-1β) (11–13).
Declined intracellular K+ level is considered to be one
of the main mechanisms of NLRP3 inflammasome activation (14,15).
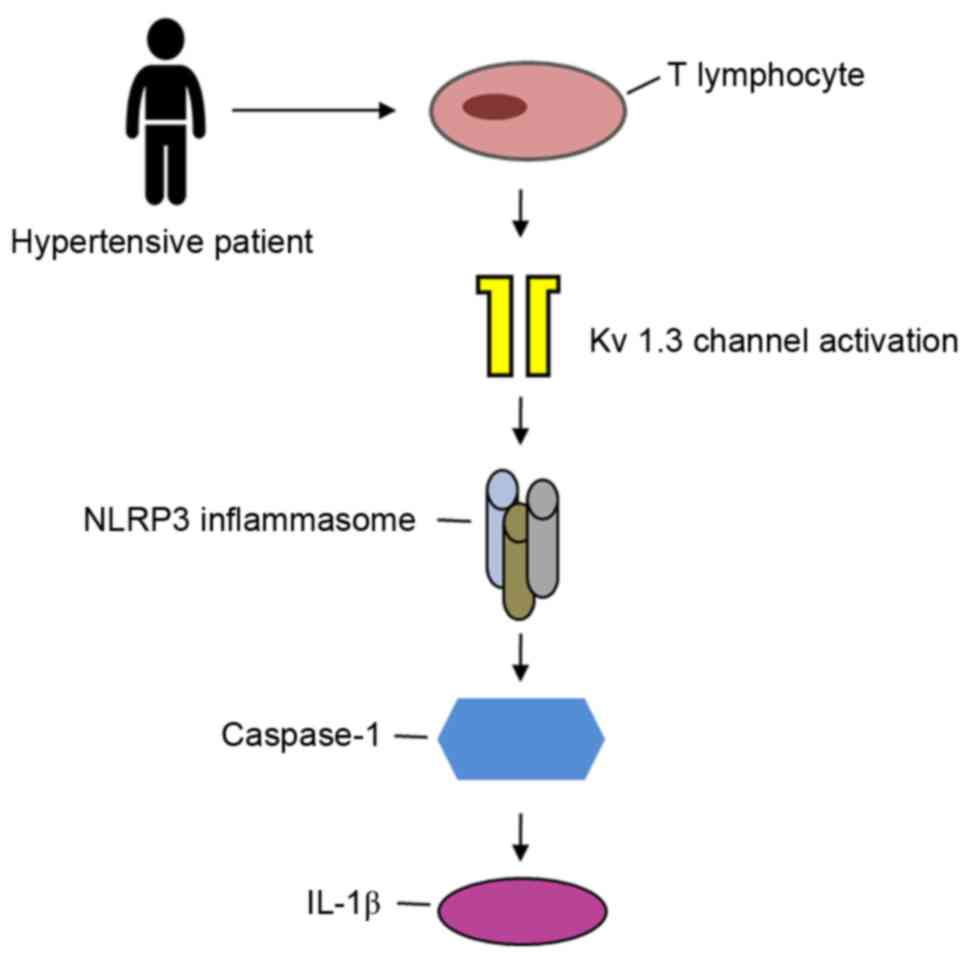
Therefore, it can be inferred that the T-lymphocyte
Kv1.3 channel may be involved in the regulation of
hypertension through activation of the NLRP3 inflammasome pathway
(Fig. 1).
The present study aimed to further verify the
expression levels of molecules associated with the NLRP3 pathway in
the peripheral blood T-lymphocytes of hypertensive Kazakh patients.
In addition, the correlation of these molecules with the
Kv1.3 channel was explored, attempting to provide novel
experimental evidence and theoretical basis for the inflammatory
mechanisms of hypertension.
Materials and methods
Subjects
A total of 30 hypertensive (defined as systolic
blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg)
Kazakh patients, who were not receiving antihypertensive drug
treatment and were first admitted to the Cardiology Center of the
First Affiliated Hospital of Xinjiang Medical University (Urumqi,
China) between June and December 2014, were randomly enrolled into
the present study as the hypertension group. The mean age of the
hypertensive patients was 57.4±5.6 years and their mean blood
pressure was 170.1±8.5/103.5±7.6 mmHg. In addition, 30 healthy
Kazakh subjects, with a mean age of 55.8±4.8 years and mean blood
pressure of 122.2±6.6/71.1±6.4 mmHg, were also enrolled during the
same period and served as the control group. The male to female
ratio was 1:1 in each group. The 1999 World Health Organization
International Society of Hypertension Guidelines for the Management
of Hypertension (16) were used as
the diagnostic criteria for hypertension in the present study.
Based on laboratory results and clinical examinations, any patients
with secondary hypertension, acute cardiovascular disease,
atherosclerosis, rheumatic heart disease, congenital heart disease,
acute and chronic infections, systemic autoimmune diseases,
diabetes and vital organ failure were excluded from the study. All
protocols were approved by the Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University and all patients
provided informed consent.
Reagents
Human lymphocyte separation medium, 4-aminopyridine
(4-AP), N-2-hydroxyethylpiperazine-N'-2′-ethanesulfonic acid
(HEPES), and K+ asparate were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The TRIzol reagent,
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium
(BCIP/NBT) solution and the reverse transcription kit (catalog no.
K1622) were purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) kit (catalog no. 204054) was obtained from
Qiagen (Hilden, Germany). In addition, the anti-NLRP3 (catalog no.
13158S; 1:1,000), anti-caspase-1 (catalog no. 3866S; 1:1,000),
anti-IL-1β (catalog no. 31202S; 1:1,000) and anti-GAPDH (catalog
no. 2118S; 1:1,000) rabbit monoclonal antibodies, as well as the
anti-rabbit IgG, alkaline phosphatase-linked secondary antibody
(catalog no. 7054S, 1:2,000), were from Cell Signaling Technology,
Inc. (Beverly, MA, USA). The IL-1β ELISA kit (catalog no. BMS224/2)
was obtained from eBioscience (San Diego, CA, USA), while the
FITC-labeled anti-CD3 antibody (catalog no. 340571) and recombinant
human IL-2 (rIL-2) were from BD Biosciences (San Jose, CA, USA).
The RPMI 1640 culture medium and fetal bovine serum were from GE
Healthcare (Chicago, IL, USA). The other patch-clamp associated
reagents were obtained from Amresco (Solon, OH, USA).
Sample collection
Approximately 12 ml peripheral venous blood was
collected from each subject. All samples were then treated with
heparin for anticoagulation and divided into two portions of 2 and
10 ml each. The 10 ml sample was separated by the lymphocyte
separation medium to isolate mononuclear cells, which were then
treated by negative magnetic-activated cell sorting to obtain the
T-lymphocyte suspension. The acquired cells were then labeled by
the FITC-CD3 monoclonal antibody and measured by flow cytometry
sorting, which proved that >95% of cells were T-lymphocytes.
Next, the mRNA and proteins of T-lymphocytes were extracted and
stored at −80°C in the refrigerator. T-lymphocytes from the
hypertension group were equally divided into two portions, with one
portion prepared for mRNA and protein extraction and the other for
the drug intervention experiment. The remaining 2 ml of the
peripheral venous blood samples was centrifuged at 1,000 x g for 5
min to collect the supernatant serum, which was then stored in the
refrigerator at −80°C.
In vitro proliferation of
T-lymphocytes from the hypertension group
After a 24-h proliferation period in the RPMI 1640
culture medium containing 10% fetal bovine serum and 50 U/ml rIL-2,
the T-lymphocytes were harvested and equally divided into two
portions. One portion was cultured in medium containing 4-AP (4-AP
group, with a final 4-AP concentration of 3 mmol/l) and the other
in medium without 4-AP (blank group). Subsequent to separate
culturing for 48 h, the T-lymphocytes were collected for mRNA,
protein extraction and patch clamp recording, while the cell
suspensions were harvested and stored in a −80°C refrigerator.
Whole-cell patch-clamp recording
Patch-clamp current recording for each sample (2–4
cells) was completed within 4 h under 20–24°C. The separated
T-lymphocytes were placed into the extracellular fluid, which
contained 150 mmol/l NaCl, 4.5 mmol/l KCl, 1.0 mmol/l
CaCl2, 1.0 mmol/l MgCl2 and 10 mmol/l HEPES,
whose pH was adjusted to 7.35 using NaOH. The microelectrodes were
fabricated by a two-step microelectrode puller; then, they were
filled with a suitable pipette solution, which contained 150 mmol/l
KCl, 1.0 mmol/l CaCl2, 1.0 mmol/l MgCl2, 10
mmol/l HEPES and 10 mmol/l EDTA, and was adjusted to a pH of 7.2
using NaOH. The resistance of the microelectrode ranged between 3
and 6 MΩ. The T-lymphocyte suspension was placed under the
microscope using a perfusion slot worktable. After 30 min of
standing, when the lymphocytes were fully adhered, the whole-cell
patch-clamp recording was initiated under room temperature. The
EPC10 amplifier (HEKA Elektronik, Lambrecht, Germany) was adopted
to record the potassium current of the individual lymphocytes under
the voltage clamp mode. The outer and inner diameters of the
recording electrode were 1.5 and 0.8 mm, respectively. The tip
diameter of the recording electrode was ~1 µm and the electrode
resistance was ~5 MΩ, while the electrodes were filled with a
pipette solution. Subsequent to contacting the cells with a
micromanipulator, negative pressure was applied to create a >1
GΩ condition for sealing, with a general sealing resistance of 1–20
MΩ. After compensating the electrode capacitance, transient high
negative pressure or strong electric stimulation was applied to
break the cell membrane and form a whole-cell patch clamp. The
membrane current was filtered by a 10 kHz (−3 dB) low frequency
filter, and the bioelectric signals were recorded using a PC. After
achieving the whole-cell recording mode, the clamping voltage was
−80 mV. Following the administration of depolarization-impulse
stimulation from −80 mV to +80 mV for 200 msec, the potassium
current was measured. Subsequently, T-lymphocytes were perfused by
4-AP with a final concentration of 3 mmol/l in order to observe the
current changes. The cutoff frequency of the signal was 10 kHz, and
the signals were sampled and stored.
RNA extraction and RT-qPCR
The total RNA of peripheral blood T-lymphocytes was
extracted with TRIzol reagent, and the A260/A280 absorbance was
found to be between 1.8 and 2.0. Next, 1 µg RNA was used for
reverse transcription with the following reaction conditions: 42°C
for 60 min and 70°C for 5 min. The acquired cDNA was used in the
sequential PCR reaction. The reaction system of quantitative
fluorescent PCR was as follows: 10 µl SYBR Green PCR Master Mix
(2X; Qiagen), 2 µl cDNA template, 0.5 µl (10 µmol/l)
forward/reverse primer and 7 µl ddH2O, at a total reaction volume
of 20 µl. The primers for NLRP3, caspase-1 and IL-1β were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), and
human β-actin was used as the internal control. Specific primer
fragments and annealing temperatures are listed in Table I. PCR was initiated at 95°C for 3 min
followed by 40 cycles of 95°C for 30 sec, annealing for 30 sec (see
Table I for annealing temperatures),
and 72°C for 30 sec, with the final cycle extended to 72°C for 5
min, followed by termination at 4°C. When the reactions ended, the
PCR products were placed on 2% agarose gels and analyzed by the
2−ΔΔCq method to obtain the mRNA expression.
 | Table I.Primer sequences for human NLRP3,
caspase-1, IL-1β and β-actin. |
Table I.
Primer sequences for human NLRP3,
caspase-1, IL-1β and β-actin.
| Gene | Sequence
(5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| NLRP3 | F:
AAGCACCTGTTGTGCAATCTGAAG | 60 | 103 |
|
| R:
GGGAATGGCTGGTGCTCAATAC |
|
|
| Caspase-1 | F:
GCCTGTTCCTGTGATGTGGA | 55 | 175 |
|
| R:
TTCACTTCCTGCCCACAGAC |
|
|
| IL-1β | F:
AGTGGCAATGAGGATGACTTGT | 55 | 127 |
|
| R:
TGTAGTGGTGGTCGGAGATTC |
|
|
| β-actin | F:
CTAAGTCATAGTCCGCCTAGAAGCA | 55 | 186 |
|
| R:
TGGCACCCAGCACAATGAA |
|
|
Western blot analysis
Total proteins of T-lymphocytes were extracted for
the measurement of the protein concentration of NLRP3, caspase-1
and IL-1β. Briefly, 20 µg protein and adequate loading buffer were
added per well, denatured in a 95°C waterbath and analyzed by 12%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Subsequent to electrophoresis, the proteins were electronically
transferred onto a polyvinylidene difluoride filter membrane,
blocked by skimmed milk for 1 h at room temperature and
co-incubated overnight with the appropriate rabbit anti-human
primary antibody (1:1,000) on a shaker at 4°C. After rinsing, the
membrane was co-incubated for 2 h with the AP-labeled goat
anti-rabbit secondary antibody (1:2,000) on a shaker at room
temperature. Following additional rinsing, the membrane was treated
with 5 ml BCIP/NBT solution according to the manufacturer's
instructions. The Quantity One 1-D analysis software version 4.6.3
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to analyze
the protein ladders. Protein expression levels were normalized to
those of internal controls (GAPDH).
Detection of IL-1β content in the
serum and cell culture medium supernatants
For ELISA detection, the manufacturer's instructions
were strictly followed using the original reagents from the IL-1β
ELISA kit. IL-1β levels were evaluated in samples from the
normotension vs. hypertension groups (serum), and the 4-AP treated
vs. untreated groups (supernatants). Triplicated wells were set for
the samples and standard reference. The final absorbance values of
proteins were read by a microplate reader at 450 nm, while the
means were used to calculate the IL-1β contents of the
corresponding samples based on the established standard curves.
Statistical analysis
The SPSS version 17.0 software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Numerical data are
expressed as the means ± standard deviation, while their pairwise
comparison was analyzed by Student's t test, with P<0.05
indicating statistically significant differences.
Results
Comparison of baseline data between
the hypertension and normotension groups
No significant differences were identified in the
age, hypertension history, smoking or drinking history, body mass
index, fasting glucose, C-reaction protein, triglycerides, high
density lipoprotein cholesterol or low-density lipoprotein
cholesterol levels between the two groups (P>0.05), indicating
comparability (Table II).
 | Table II.Baseline data of both the
normotension and hypertension groups. |
Table II.
Baseline data of both the
normotension and hypertension groups.
| Variables | Normotension
(n=30) | Hypertension
(n=30) | P-value |
|---|
| Age (years) | 55.8±4.8 | 57.4±5.6 | 0.220 |
| Hypertension
history (%) | 40.0 | 43.3 | >0.999 |
| Smoking history
(%) | 40.0 | 36.7 | >0.999 |
| Drinking history
(%) | 46.7 | 50.0 | >0.999 |
| BMI
(kg/m2) | 25.6±1.2 | 26.1±1.6 | 0.610 |
| Fasting glucose
(mmol/l) |
5.1±0.6 |
5.3±0.4 | 0.330 |
| CRP (mg/l) |
9.2±1.5 |
9.8±1.2 | 0.124 |
| TG (mmol/l) |
1.8±0.3 |
1.9±0.5 | 0.725 |
| HDL (mmol/l) |
1.4±0.3 |
1.3±0.2 | 0.455 |
| LDL (mmol/l) |
3.3±0.8 |
3.5±0.8 | 0.289 |
Current density of the T-lymphocyte
Kv1.3 channel
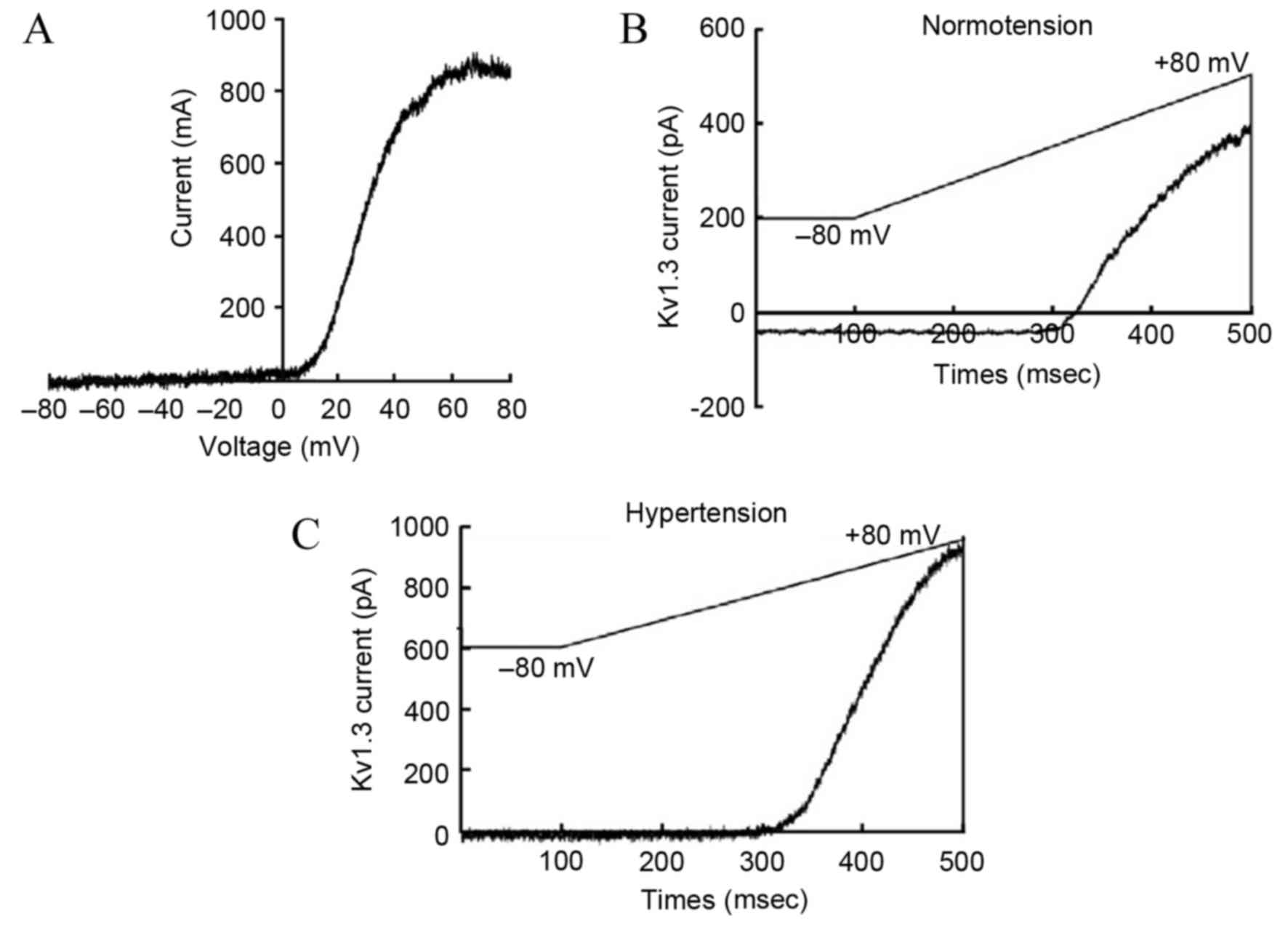
The acquired outward current of the T-lymphocyte
Kv1.3 channel after administering stimulation according to the
aforementioned plan was similar to that reported previously
(17). The current-voltage curve of
the Kv1.3 channel indicated that the current was voltage-dependent,
which was consistent with the current characteristics of the Kv1.3
channel (Fig. 2A). Compared with the
normotension group, the activation degree of the Kv1.3 channel in
the peripheral blood T-lymphocytes of patients from the
hypertension group was significantly higher (179±51 vs. 280±74
pA/pF, respectively; P<0.05; Fig. 2B
and C).
mRNA and protein expression levels of
NLRP3, caspase-1 and IL-1β in the T-lymphocytes
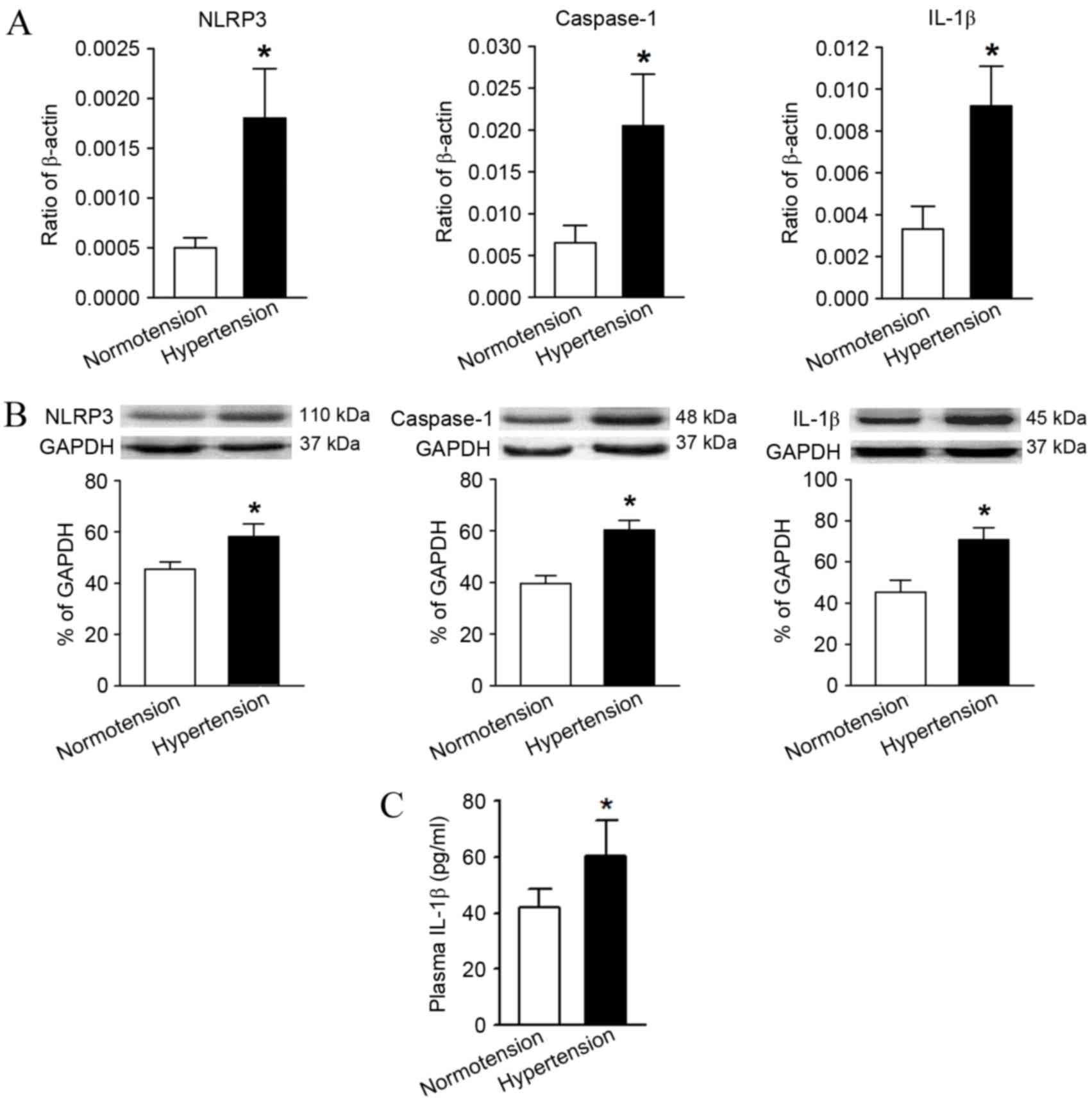
Compared with the normotension group, the mRNA and
protein relative expression levels of NLRP3, caspase-1 and IL-1β in
the hypertension group were significantly higher as determined by
RT-qPCR and western blot analysis (Fig.
3A and B). In addition, the serum IL-1β content in the
peripheral blood of hypertensive patients measured by ELISA was
also significantly higher in comparison with the levels in the
normotension group (Fig. 3C).
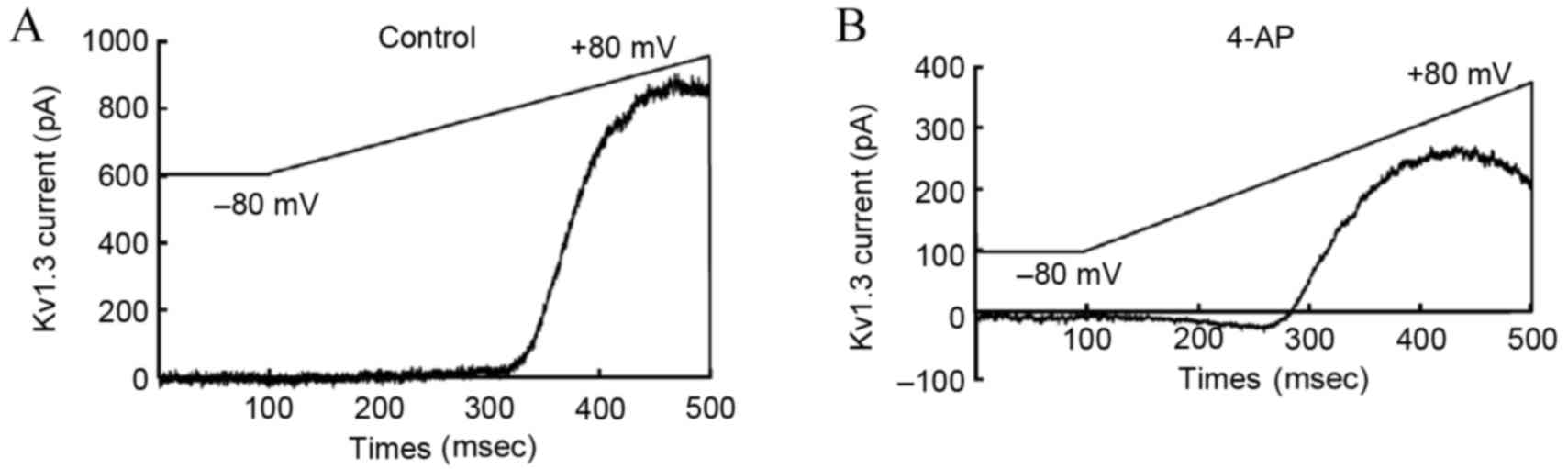
Impact of 4-AP upon the
Kv1.3 channel current
According to the previously described Kv1.3 channel
stimulation procedures, upon perfusion with 4-AP, the Kv1.3 channel
current of T-lymphocytes from the hypertensive subjects recorded by
the patch clamp method was significantly lower in the 4-AP treated
group in comparison with that in the blank group (Fig. 4). This observation confirmed that
exposure to 4-AP with a concentration of 3 mmol/l was able to
inhibit the current activity of T-lymphocyte Kv1.3.
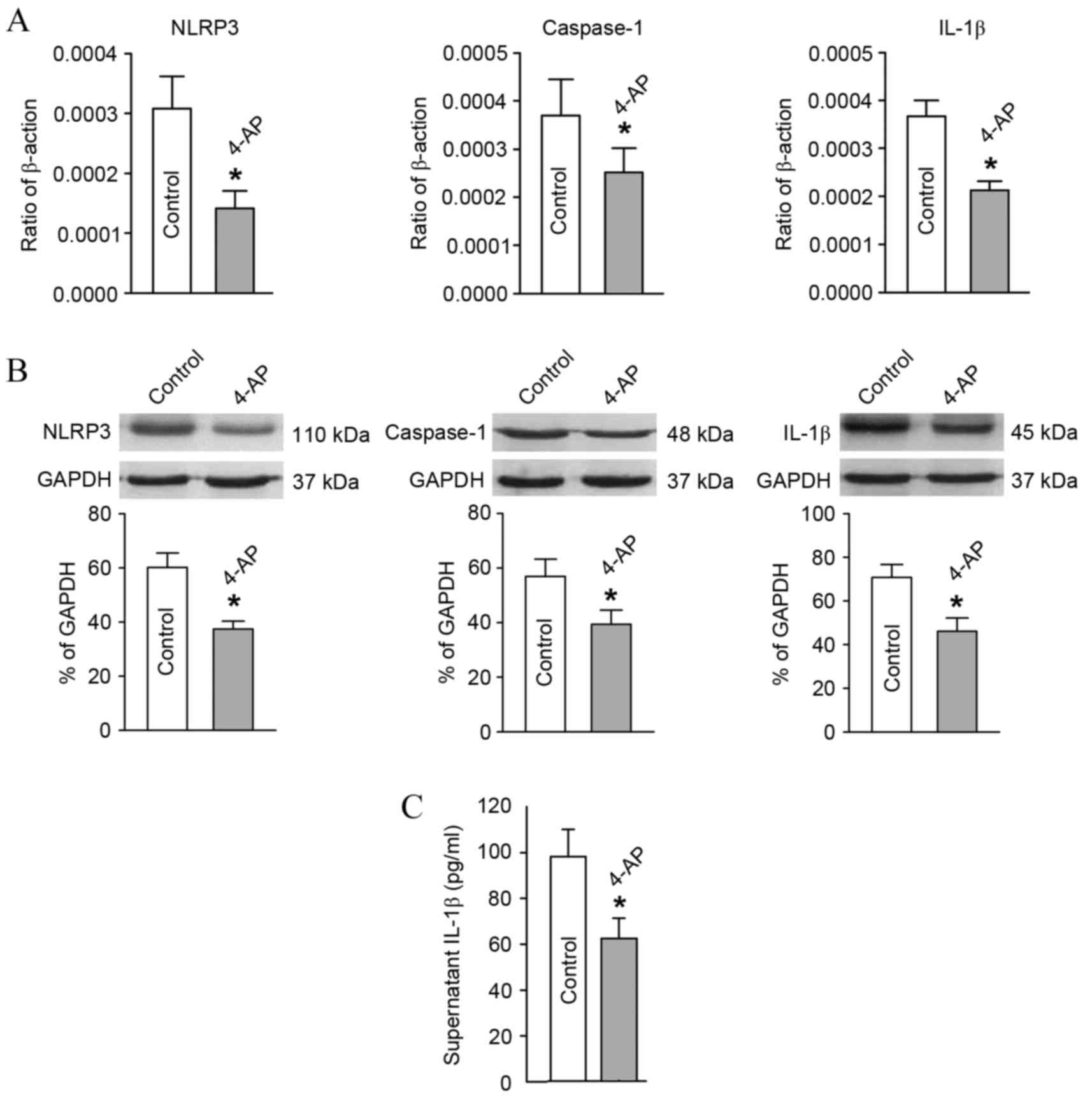
Expression changes in key molecules in
the NLRP3 signaling pathway of T-lymphocytes subsequent to
Kv1.3 channel blockage
In comparison with the blank group, after blocking
the T-lymphocyte Kv1.3 channel from the hypertensive subjects with
4-AP, the mRNA and protein expression levels of NLRP3, caspase-1
and IL-1β declined significantly as detected by RT-qPCR and western
blot analysis (Fig. 5A and B).
Furthermore, the IL-1β content in the culture supernatant of
T-lymphocytes from the hypertensive subjects also decreased
significantly as determined using ELISA (Fig. 5C).
Discussion
Hypertension is a chronic low-grade inflammatory
process characterized by activation of the lymphatic and
mononuclear systems (5–6,18). The
activation and function of lymphocytes is closely correlated with
the ionic channels distributed on their surface. The
Kv1.3 channel is mainly involved in the activation
process of T-lymphocytes. After activation and opening, the
Kv1.3 channel promotes the influx of extracellular
Ca2+, indirectly regulating the division, proliferation,
apoptosis and function of T-lymphocytes (19). Due to the dependence of this membrane
potential-calcium signal, it is predicated that Kv1.3
channel blockers may inhibit the activation and proliferation of
T-lymphocytes and result in immune suppression. This prediction has
been proven in various in vivo and in vitro
experiments (20,21). A previous study by the current
authors in a spontaneously hypertensive rat (SHR) model indicated
that the mRNA and protein levels of the Kv1.3 channel
structural protein (Gene ID: 29731) in high-grade SHRs (16-week
old) were significantly higher compared with those of the low-grade
SHRs (4-week old) (22). The
patch-clamp records also revealed that the current density of the
T-lymphocyte Kv1.3 channel was significantly higher in
the high-grade SHRs compared with low-grade SHRs (23). Furthermore, this channel could be
effectively blocked by 4-AP in a significantly
concentration-dependent manner (23,24).
Sequentially, it was further observed that the current density of
the T-lymphocyte Kv1.3 channel in hypertensive Kazakh
patients was significantly higher compared with that of the
normotension group (10). These
previous findings (10,22–24)
indicate that the activation of T-lymphocytes and the
Kv1.3 channel on its membrane are closely correlated
with hypertension; however, the specific mechanisms and downstream
signaling pathways remain unknown.
Inflammasome, a vital component of the innate immune
system, is an intracellular multiprotein complex consisting of the
NLRs family and caspase-1 (25).
NLRP3 is the crucial support of the protein complex, while
apoptosis-associated speck-like protein containing CARD is the
bridge between NLRP3 and pro-caspase-1 (26). The NLRP3 inflammasome is widely
expressed in dendritic cells and monocyte-macrophages. When
activated, NLRP3 shears pro-caspase-1 into its activated form,
caspase-1, which is capable of shearing the precursor form of the
IL-1 superfamily members, resulting in maturity and release of the
IL-1 superfamily members (26). In
this way, it actively participates in immune defense or injury, and
leads to vascular remodeling and blood pressure increase. Recent
research demonstrated that NLRP3 inflammasome may become a
potential biomarker and therapeutic target for early hypertension
(27). Therefore, it can be inferred
that the NLRP3 inflammasome pathway of peripheral blood
T-lymphocytes in hypertensive Kazakh patients in Xinjiang may also
be activated, while the NLRP3 inflammasome may be an effective
downstream molecule of the Kv1.3 channel, involved in
hypertension development.
In the present study, the expression levels of key
molecules (NLRP3, caspase-1 and IL-1β) in the NLRP3 inflammasome
pathway of peripheral blood T-lymphocytes were measured, while the
association between the T-lymphocyte Kv1.3 channel and
the NLRP3 inflammasome pathway was also analyzed. The results
indicated that peripheral blood T-lymphocyte Kv1.3
channel in hypertensive Kazakh patients was activated, which
confirmed our previous findings (23,24).
Since the Kv1.3 channel can influence the cell membrane
potential and activate the calcium signaling pathway, it serves an
important role during the activation of T-lymphocytes (17). Therefore, the current results
indicate that T-lymphocytes in the selected hypertensive population
were activated to a certain extent. Studies have demonstrated that
activated T-lymphocytes, induced by Angiotensin II (Ang II), served
an important role in the occurrence of hypertension and endothelial
dysfunction (5). Schiffrin (6) also documented the influential role of
T-lymphocytes during hypertension and vascular remodeling. The
study also pointed out that the transcription factor Foxp3
expressed by T-lymphocytes, including the T helper cells (Th1, Th2
and Th17) and suppressor T cells (including the regulatory T
cells), was closely correlated with the production of Ang II
(6). Therefore, T-lymphocytes served
a critical role during the onset and progression of EH. These
T-lymphocytes and functional states of Kv1.3 channels
influenced the activity of the NLRP3 inflammasome pathway. The
sequential results of the present study revealed that the mRNA and
protein expression levels of key molecules in the NLRP3
inflammasome signal pathway (namely NLRP3, caspase-1, and IL-1β)
were significantly higher in the hypertension group when compared
with the healthy normotension group. For the mechanisms underlying
NLRP3 inflammasome activation, multiple endogenous and exogenous
stimuli, such as K+ efflux, lysosomal instability and
increased levels of intracellular reactive oxygen, have been
demonstrated to activate the NLRP3 inflammasome pathway (28). Among these, the low intracellular
levels of K+ are considered the final common mechanism
through which the NLRP3 inflammasome activates caspase-1 (14,29). The
results of the current study support the aforementioned
observations. Therefore, it is suggested that the activation of
potassium channels is the trigger for activation of the NLRP3
inflammasome pathway in the present study, since additional in
vitro drug intervention experiments have verified that blockage
of the Kv1.3 channel with the aim to reduce the
K+ efflux may inhibit the activation of the inflammasome
pathway and the release of its downstream product IL-1β. It should
be stated here that the classic potassium channel blocker 4-AP was
found to effectively block the Kv1.3 channel at a
concentration of 3 mmol/l, which is consistent with previous
reports (30,31). The NLRP3 inflammasome pathway
inactivation caused by potassium channel blockage is of great
importance, since IL-1β, the key downstream molecule of the NLRP3
inflammasome pathway, is closely correlated with the occurrence of
hypertension. Studies have demonstrated that IL-1β can lead to
changes in the vascular endothelial cell (VEC) morphology and
intracellular skeletal structure, resulting in VEC functional
damage and a massive release of cytokines, such as endothelin 1,
which may have significant biological effects, including an
increase in blood pressure (32,33).
Furthermore, IL-1β can also upregulate the expression of type 1 Ang
receptor II (11), directly
impacting blood pressure regulation. IL-1β is also considered to be
one of the predictors of blood pressure problems in the elderly
(34).
In conclusion, the findings of the present study
revealed that the Kv1.3 channel was activated, while the
expression levels of molecules associated with the NLRP3
inflammasome pathway and its key downstream product were increased.
Blocking the Kv1.3 channel could result in inactivation
of this pathway, indicating that the NLRP3 inflammasome may be the
downstream effector molecule of the Kv1.3 channel.
However, due to the limited sample size, this is only a preliminary
exploration of the correlation between the Kv1.3 channel
on the membrane of peripheral blood T-lymphocytes and the NLRP3
inflammasome pathway in hypertensive Kazakh patients in the
Xinjiang region, China. In the future, studies with expanded
population size comparing the Han and Kazakh populations from the
same region, and animal experiments to verify the paradigm are
required for a deeper understanding of the association between the
T-lymphocyte NLRP3 inflammasome pathway activation and the
T-lymphocyte Kv1.3 channel. Such studies would provide
additional experimental evidence and theoretical basis for the
pathogenesis of EH.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81160039).
References
|
1
|
Liu F, Ma YT, Yang YN, Xie X, Li XM, Huang
Y, Ma X, Chen BD, Gao X and Du L: Current status of primary
hypertension in Xinjiang: An epidemiological study of Han, Uygur
and Hazakh populations. Zhonghua Yi Xue Za Zhi. 90:3259–3263.
2010.(In Chinese). PubMed/NCBI
|
|
2
|
Harrison DG, Guzik TJ, Lob HE, Madhur MS,
Marvar PJ, Thabet SR, Vinh A and Weyand CM: Inflammation, immunity,
and hypertension. Hypertension. 57:132–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montecucco F, Pende A, Quercioli A and
Mach F: Inflammation in the pathophysiology of essential
hypertension. J Nephrol. 24:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dinh QN, Drummond GR, Sobey CG and
Chrissobolis S: Roles of inflammation, oxidative stress, and
vascular dysfunction in hypertension. Biomed Res Int.
2014:4069602014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schiffrin EL: T lymphocytes: A role in
hypertension? Curr Opin Nephrol Hypertens. 19:181–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu L, Pennington M, Jiang Q, Whartenby KA
and Calabresi PA: Characterization of the functional properties of
the voltage-gated potassium channel Kv1.3 in human CD4+ T
lymphocytes. J Immunol. 179:4563–4570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wulff H, Calabresi PA, Allie R, Yun S,
Pennington M, Beeton C and Chandy KG: The voltage-gated Kv1.3 K(+)
channel in effector memory T cells as new target for MS. J Clin
Invest. 111:1703–1713. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitz A, Sankaranarayanan A, Azam P,
Schmidt-Lassen K, Homerick D, Hänsel W and Wulff H: Design of
PAP-1, a selective small molecule Kv1.3 blocker, for the
suppression of effector memory T cells in autoimmune diseases. Mol
Pharmacol. 68:1254–1270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang QB, Zhang YM, Cheng LF, Yuan QY,
Zhang GM, Liang P and Gou F: Voltage-dependent potassium channel
and calcium-activated potassium channel current changes of
peripheral blood T-lymphocytes from hypertensive patients in
Xinjiang Kazakh. Zhonghua Xin Xue Guan Bing Za Zhi. 41:1020–1024.
2013.(In Chinese). PubMed/NCBI
|
|
11
|
Sasamura H, Nakazato Y, Hayashida T,
Kitamura Y, Hayashi M and Saruta T: Regulation of vascular type 1
angiotensin receptors by cytokines. Hypertension. 30:35–41. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorffel Y, Lätsch C, Stuhlmüller B,
Schreiber S, Scholze S, Burmester GR and Scholze J: Preactivated
peripheral blood monocytes in patients with essential hypertension.
Hypertension. 34:113–117. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omi T, Kumada M, Kamesaki T, Okuda H,
Munkhtulga L, Yanagisawa Y, Utsumi N, Gotoh T, Hata A, Soma M, et
al: An intronic variable number of tandem repeat polymorphisms of
the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies
gene expression and is associated with essential hypertension. Eur
J Hum Genet. 14:1295–1305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munoz-Planillo R, Kuffa P, Martínez-Colon
G, Smith BL, Rajendiran TM and Núñez G: K+ efflux is the common
trigger of NLRP3 inflammasome activation by bacterial toxins and
particulate matter. Immunity. 38:1142–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marina-García N, Franchi L, Kim YG, Miller
D, McDonald C, Boons GJ and Núñez G: Pannexin-1-mediated
intracellular delivery of muramyl dipeptide induces caspase-1
activation via cryopyrin/NLRP3 independently of Nod2. J Immunol.
180:4050–4057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chalmers J: The 1999 WHO-ISH guidelines
for the management of hypertension. Med J Aust. 171:458–459.
1999.PubMed/NCBI
|
|
17
|
Panyi G, Possani LD, Rodríguez de laVega
RC, Gáspár R and Varga Z: K+ channel blockers: Novel tools to
inhibit T cell activation leading to specific immunosuppression.
Curr Pharm Des. 12:2199–2220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harrison DG, Marvar PJ and Titze JM:
Vascular inflammatory cells in hypertension. Front Physiol.
3:1282012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis RS: Calcium signaling mechanisms in
T lymphocytes. Annu Rev Immunol. 19:497–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damjanovich S, Gáspár R and Panyi G: An
alternative to conventional immunosuppression: Small-molecule
inhibitors of Kv1.3 channels. Mol Interv. 4:250–254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leonard RJ, Garcia ML, Slaughter RS and
Reuben JP: Selective blockers of voltage-gated K+ channels
depolarize human T lymphocytes: Mechanism of the antiproliferative
effect of charybdotoxin. Proc Natl Acad Sci USA. 89:pp.
10094–10098. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Zhang YM, Ma KT, Si JQ and Liang P:
Difference in the expression of Kv channel in lymphocytes between
spontaneously hypertensive rats and Wistar rats. Sheng Li Xue Bao.
62:382–386. 2010.(In Chinese). PubMed/NCBI
|
|
23
|
Luo J, Ma KT, Zhang YM, Si JQ, Liang P and
Li J: Effects of telmisartan on 4-Aminopyridine-sensitive voltage
dependant potassium channel of lymphocyte derived from
spontaneously hypertensive rat. Zhonghua Xin Xue Guan Bing Za Zhi.
38:751–754. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Zhang Q, Zhang Y, Cheng L, Yuan Q, Zhang G
and Gou F: Effects of telmisartan on IKCa1 potassium channel after
T-lymphocyte activation and proliferation in peripheral blood of
hypertensive patients in Xinjiang Kazakh. Zhonghua Yi Xue Za Zhi.
94:182–186. 2014.(In Chinese). PubMed/NCBI
|
|
25
|
Koizumi Y, Toma C, Higa N, Nohara T,
Nakasone N and Suzuki T: Inflammasome activation via intracellular
NLRs triggered by bacterial infection. Cell Microbiol. 14:149–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Miguel C, Rudemiller NP, Abais JM and
Mattson DL: Inflammation and hypertension: New understandings and
potential therapeutic targets. Curr Hypertens Rep. 17:5072015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pétrilli V, Papin S, Dostert C, Mayor A,
Martinon F and Tschopp J: Activation of the NALP3 inflammasome is
triggered by low intracellular potassium concentration. Cell Death
Differ. 14:1583–1589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung G, Sun W, Brookes S, Smith D and Shi
R: Potassium channel blocker, 4-aminopyridine-3-methanol, restores
axonal conduction in spinal cord of an animal model of multiple
sclerosis. Exp Neurol. 227:232–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Panyi G, Varga Z and Gáspár R: Ion
channels and lymphocyte activation. Immunol Lett. 92:55–66. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ziesche R, Petkov V, Williams J, Zakeri
SM, Mosgöller W, Knöfler M and Block LH: Lipopolysaccharide and
interleukin 1 augment the effects of hypoxia and inflammation in
human pulmonary arterial tissue. Proc Natl Acad Sci USA. 93:pp.
12478–12483. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nemati F, Rahbar-Roshandel N, Hosseini F,
Mahmoudian M and Shafiei M: Anti-inflammatory effects of
anti-hypertensive agents: Influence on interleukin-1β secretion by
peripheral blood polymorphonuclear leukocytes from patients with
essential hypertension. Clin Exp Hypertens. 33:66–76. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barbieri M, Ferrucci L, Corsi AM, Macchi
C, Lauretani F, Bonafè M, Olivieri F, Giovagnetti S, Franceschi C
and Paolisso G: Is chronic inflammation a determinant of blood
pressure in the elderly? Am J Hypertens. 16:537–543. 2003.
View Article : Google Scholar : PubMed/NCBI
|