Introduction
Lung cancer is a disease with high morbidity and
mortality rates (1). The prevalence
of lung cancer is increasing in China, particularly in large cities
(2). Data released by the World
Health Organization in 2003 indicated that lung cancer is one of
the most malignant cancers, and seriously affects the health and
mortality of patients (3). Tumor
necrosis factor (TNF)-α is indicated to be a key cytokine for use
in the treatment of cancer (4).
TNF-α has been found to have direct antitumor effects and strong
biological activity (5).
Furthermore, elevated levels of TNF-α have been found to be
associated with the development and treatment of lung cancer
(6). Glycogen synthase kinase
(GSK)-3β has been suggested as a therapeutic target for numerous
diseases, including cancer, because of its diverse cellular
functions (7). GSK-3β phosphorylates
a variety of proteins associated with cell cycle regulation,
apoptosis and cell survival (8);
therefore, the regulation of GSK-3β expression may have an
important role in the prevention and treatment of lung cancer.
Rutin is a pharmacological agent that has been used
clinically to regulate cardiovascular disease for many years and
may be effective in the treatment of tumors (9,10). In
the present study, the effect of rutin treatment on GSK-3β and
TNF-α expression in lung cancer was investigated.
Materials and methods
Materials
4′,6-Diamino-2-phenylindole (DAPI) and antibodies
against TNF-α (MK1169) and GSK-3β (27C10) were purchased from Wuhan
Boster Biological Technology, Ltd. (Wuhan, China). Other reagents
were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
The MCO-5AC CO2 thermostat incubator was obtained from
Sanyo Electrical Biomedical Co., Ltd. (Osaka, Japan).
Cell culture
A549 lung carcinoma cells were provided by the
School of Pharmaceutical Sciences of Jilin University (Changchun,
China). The A549 cells were maintained in plastic dishes (150 mm)
with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Shanghai BaiJin
Chemical Group Co., Ltd., Shanghai, China), 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. The cells were divided into five
groups: Control, cisplatin and rutin (low, medium and high) groups.
Cells were seeded into 96-well plates (5×10−5
cells/well) in Dulbecco's modified Eagle's medium (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) supplemented with 10% fetal
bovine serum and incubated with 1×10−8 (low),
2×10−8 (medium) or 4×10−8 mol/l (high) rutin
or 1×10−9 mol/l cisplatin (both from Sigma-Aldrich;
Merck KGaA) for 24 h. Control cells were treated with PBS under the
same culture conditions.
ELISA
Cells were lysed using tissue Total Protein Lysis
Buffer (Yi Li Biotechnology Co., Ltd., Shanghai, China) and
centrifuged at 10,000 to 14,000 × g for 15 sec at 4°C. Lysates were
subsequently analyzed according to the instructions of a TNF-α
ELISA kit (EH3TNFA; Nanjing Zhi Bai Cui Biology Technology Co.
Ltd., Nanjing, China), with measurement of the absorbance value at
450 nm.
Western blot analysis
A549 cells were homogenized in
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA).
Cell suspensions were centrifuged at 10,000 to 14,000 × g for 4°C
at 15 sec. The total protein concentration of the homogenates was
measured using a bicinchoninic acid assay reagent. Equal amounts of
protein extract (20 µl) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes via
electroblotting. Separated proteins were transferred to
polyvinylidene difluoride membranes (Sigma-Aldrich; Merck KGaA).
After blocking with 5% non-fat milk (4°C, 2 h), the membranes were
probed with primary antibodies against TNF-α (1:500; MK1169; Wuhan
Boster Biological Technology, Ltd.) and GAPDH (1:500; SAB2100894;
Sigma-Aldrich; Merck KGaA) at 20–27°C for 2 h, followed by
incubation with anti-mouse immunoglobulin G secondary antibodies
(1:400; AP130P; Sigma-Aldrich; Merck KGaA) at 37°C for 20 min.
Immunocomplexes were visualized using an enhanced chemiluminescence
detection system (EMD Millipore, Billerica, MA, USA).
Immunoreactive bands were analyzed using Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Immunofluorescence and DAPI
staining
Cells were fixed with 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature, then
washed three times with PBS for a total of 10 min. Cells were
incubated with GSK-3β antibody (1:200) at 4°C overnight. Following
this, the cells were washed three times for 5 min and subsequently
incubated with anti-mouse immunoglobulin G secondary antibodies
(1:500; AP130P; Sigma-Aldrich; Merck KGaA) at 4°C for 2 h. DAPI was
then used for nuclear staining. Cells were mounted and images were
captured using a Nikon Eclipse 80i fluorescence microscope (Nikon
Corporation, Tokyo, Japan). The protein expression of GSK-3β and
the number of apoptotic cells (DAPI) were analyzed using Image Pro
Plus 6.0 software.
Statistical analysis
All data were obtained from at least three separate
experiments and are presented as the mean ± standard error of the
mean. Statistical comparisons were made using the Student's t-test.
Statistical analysis of the data was performed using SPSS version
11 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
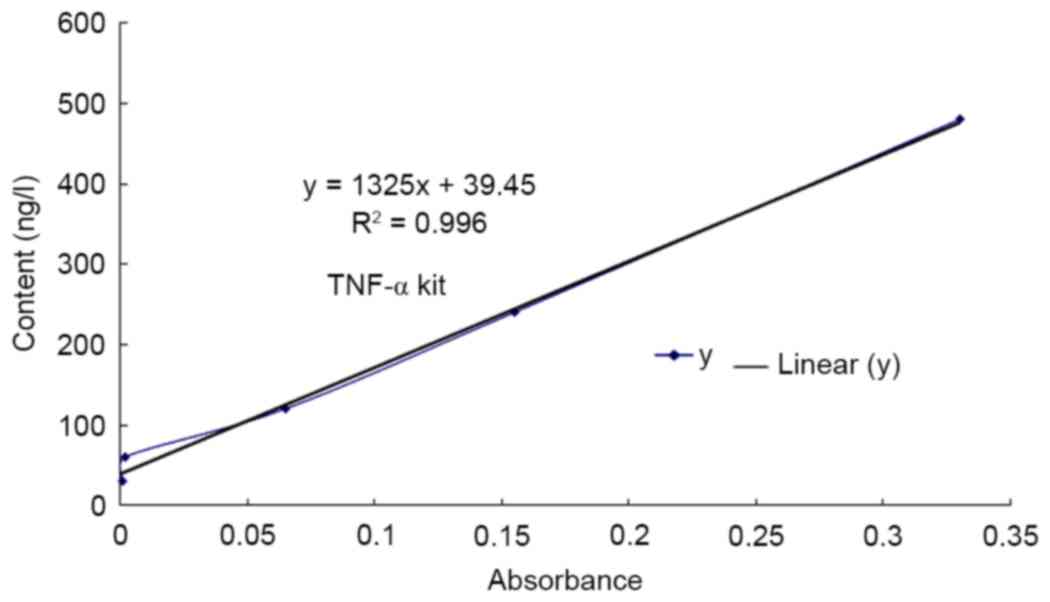
TNF-α content determined using
ELISA
An ELISA kit was used to assess the expression of
TNF-α in different groups. The results of the ELISA demonstrate
that the expression of TNF-α protein in the cisplatin group was
significantly increased compared with that in the control group
(P<0.01; Fig. 1, Table I). Furthermore, the expression of
TNF-α in the low, medium and high rutin groups was also increased
significantly compared with that in the control group (P<0.01;
Table I).
 | Table I.TNF-α content in the five groups. |
Table I.
TNF-α content in the five groups.
| Groups | TNF-α (ng/l) |
|---|
| Control | 50.49±2.02 |
| Cisplatin |
229.37±27.58a |
| Rutin (low) |
114.09±9.40a |
| Rutin (medium) |
160.03±18.55a |
| Rutin (high) |
154.73±10.00a |
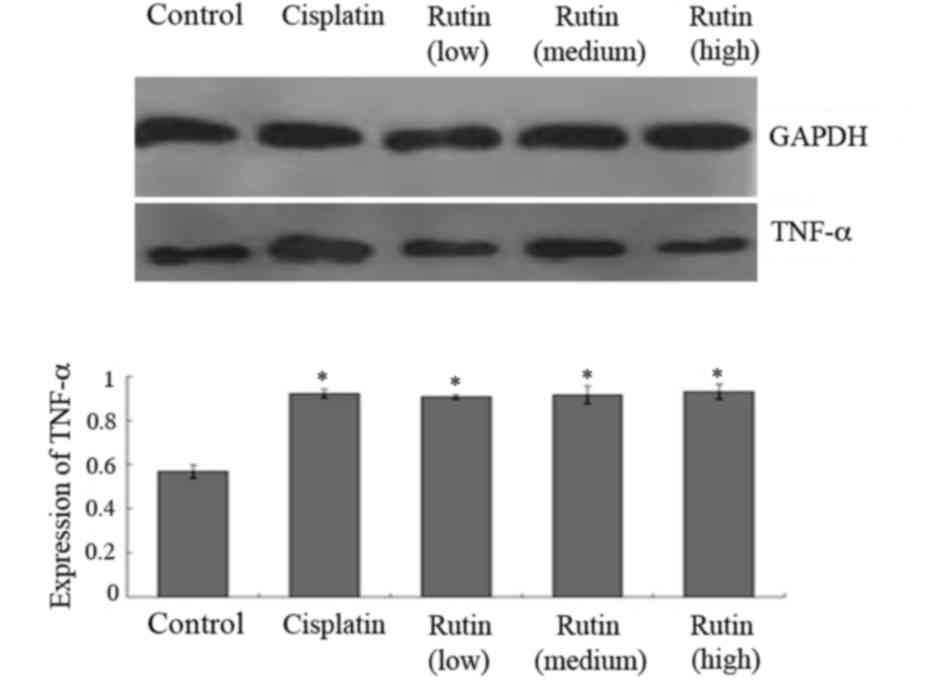
Western blot analysis
Western blotting was conducted to further
investigate the expression of TNF-α. The results were analyzed
semi-quantitatively according to the grayscale value. TNF-α levels
were significantly increased in the cisplatin group compared with
the control group (Fig. 2;
P<0.05). Additionally, the expression of TNF-α in the low,
medium and high rutin groups was also significantly higher compared
with that in the control group (Fig.
2; P<0.05).
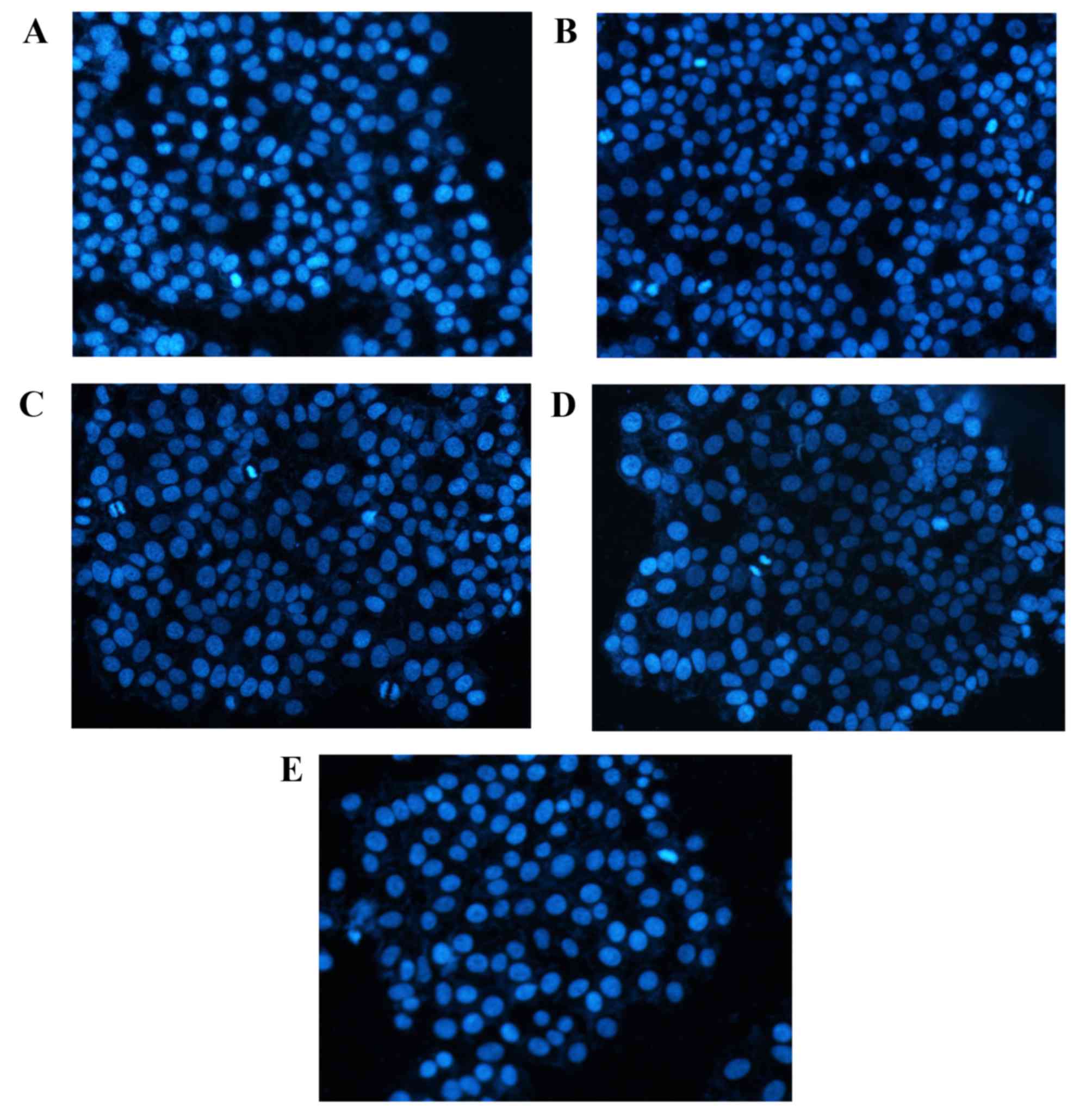
DAPI staining
DAPI staining results (Fig. 3) demonstrated that cells in the
control group had uniform chromatin, a large nucleus and integrity
of the nuclear envelope. By contrast, apoptotic morphological
changes of A549 cells after 48 h rutin or cisplatin treatment were
observed. Relative to the control group, cells groups treated with
rutin and cisplatin exhibited higher numbers of detached cells with
round and shrunken morphologies and condensed nuclei.
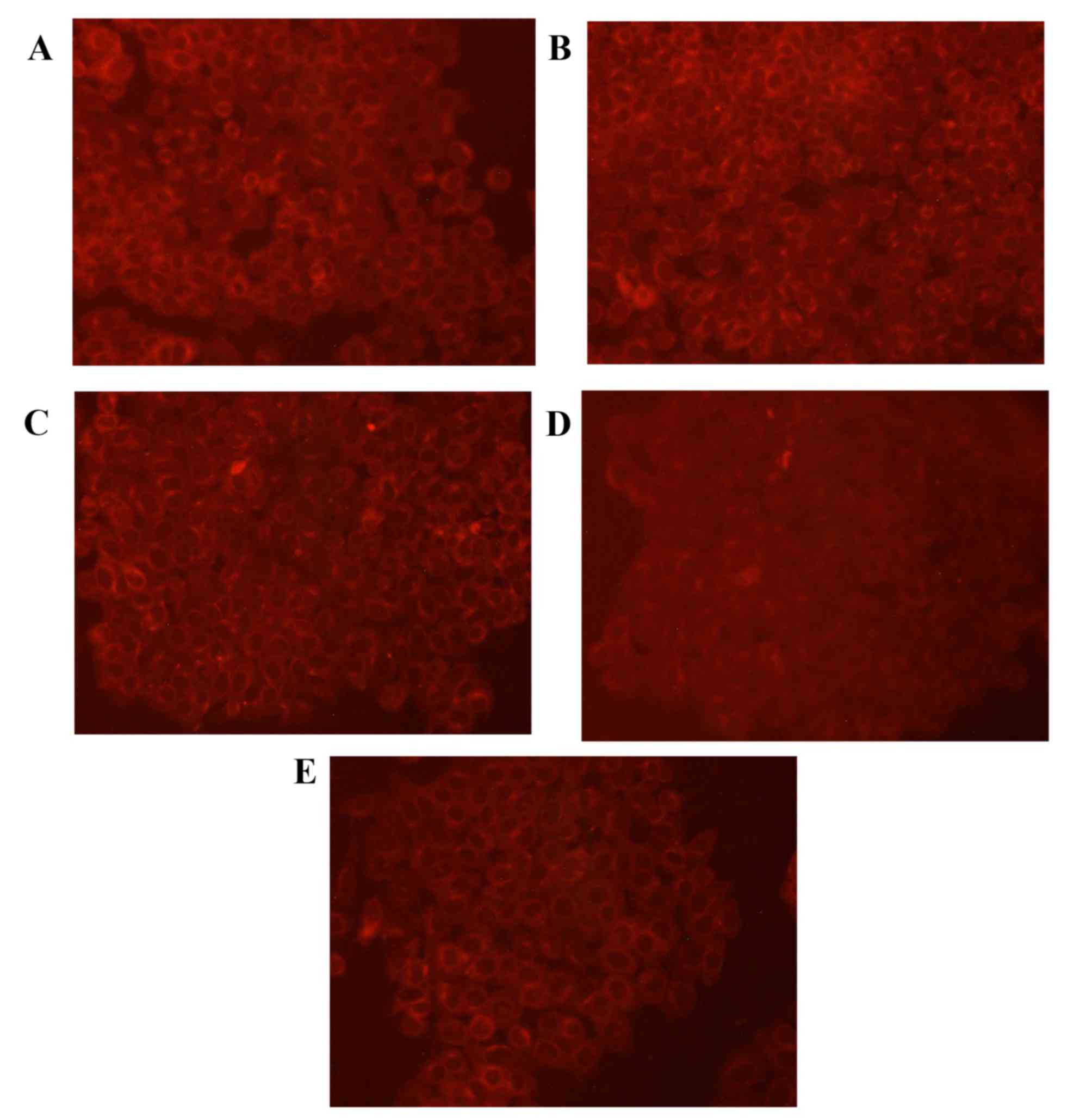
Expression of GSK-3β protein
To validate the expression pattern of GSK-3β in lung
cancer cells, GSK-3β specific fluorescent staining was performed on
A549 cells (Fig. 4). The results of
the staining assay revealed that the cells in the control group had
uniform chromatin and a large nucleus. Relative to the control
group, levels of GSK-3β protein were markedly increased in the
cisplatin group. In addition, GSK-3β protein expression was
increased in the low, medium and high rutin groups compared with
the control group.
Discussion
Human TNF-α is composed of 233 amino acids
(molecular weight, 26 kDa) and contains a signal peptide composed
of 76 amino acid residues (11). It
has previously been suggested to be an important tumor-related
factor (12). One of the aims of the
present study was to determine whether rutin promotes TNF-α
expression. The results of the present study demonstrated that
TNF-α expression was significantly increased in the low, medium and
high rutin groups compared with the control group. These results
suggest that rutin may stimulate the expression of TNF-α in A549
human lung carcinoma cells, and serve a role in killing tumor
cells.
GSK-3 is a serine/threonine kinase that is highly
evolutionarily conserved and is present in many mammalian
eukaryotic cells, functioning to remove active glycogen synthase,
which regulates cell differentiation, proliferation, survival and
apoptosis (12). GSK-3 was first
isolated from rabbit skeletal muscle tissues and there are two
subtypes; GSK-3α and GSK-3β (13).
GSK-3β is a key enzyme associated with glycogen metabolism
(14). This in turn affects
mitochondrial permeability and the release of cytochrome c, which
is associated with apoptosis regulation (13). In the present study, the expression
of TNF-α was significantly increased in the rutin groups compared
with the control group and the expression of GSK-3β was increased
concurrently, which indicates that TNF-α may have promoted GSK-3β
protein expression in the rutin group. These results suggest that
rutin stimulated the expression of TNF-α.
In conclusion, the results of the present study
suggest that rutin may serve as an effective antitumor treatment.
Rutin treatment may increase the expression of TNF-α, and promote
the expression of GSK-3β.
Acknowledgements
The present study was supported by the Science and
Technology Department of Jilin Province (grant nos. 20140312002ZG
and YYZX201259) and the National Natural Science Foundation of
China (grant no. 81272875).
References
|
1
|
Varona P, Herrera D, García RG, Bonet M,
Romero T and Venero SJ: Smoking-attributable mortality in Cuba.
Medicc Rev. 11:43–47. 2009.PubMed/NCBI
|
|
2
|
Sun X, Liu W, Wu S, Han H, Lin Y and Dai
X: The morbidity and mortality trend and prediction of lung cancer
in residents of Nangang District of Harbin in China during the past
10 years. Zhongguo Fei Ai Za Zhi. 8:514–517. 2005.(In Chinese).
PubMed/NCBI
|
|
3
|
Rivera MP, Detterbeck F and Mehta AC:
American College of Chest Physicians: Diagnosis of lung cancer: The
guidelines. Chest. 123 1 Suppl:S129–S136. 2003. View Article : Google Scholar
|
|
4
|
Wang X, Yang L, Huang F, Zhang Q, Liu S,
Ma L and You Z: Inflammatory cytokines IL-17 and TNF-α up-regulate
PD-L1 expression in human prostate and colon cancer cells. Immunol
Lett. 184:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohammadpour H, Pourfathollah AA, Zarif M
Nikougoftar and Shahbazfar AA: Irradiation enhances susceptibility
of tumor cells to the antitumor effects of TNF-α activated adipose
derived mesenchymal stem cells in breast cancer model. Sci Rep.
6:284332016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kouklakis G, Efremidou EI, Pitiakoudis M,
Liratzopoulos N and Polychronidis ACh: Development of primary
malignant melanoma during treatment with a TNF-α antagonist for
severe Crohn's disease: A case report and review of the
hypothetical association between TNF-α blockers and cancer. Drug
Des Devel Ther. 7:195–199. 2013.PubMed/NCBI
|
|
7
|
Park SA, Lee JW, Herbst RS and Koo JS:
GSK-3α is a novel target of CREB and CREB-GSK-3α signaling
participates in cell viability in lung cancer. PLoS One.
11:e01530752016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phukan S, Babu VS, Kannoji A, Hariharan R
and Balaji VN: GSK3beta: Role in therapeutic landscape and
development of modulators. Br J Pharmacol. 160:1–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panchal SK, Poudyal H, Arumugam TV and
Brown L: Rutin attenuates metabolic changes, nonalcoholic
steatohepatitis, and cardiovascular remodeling in high-
carbohydrate, high-fat diet-fed rats. J Nutr. 141:1062–1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heck CI and de Mejia EG: Yerba mate tea
(Ilex paraguariensis): A comprehensive review on chemistry, health
implications, and technological considerations. J Food Sci.
72:R138–R151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tchorzewski H, Zeman K, Kantorski J,
Paleolog E, Kahan M, Feldmann M, Kwinkowski M, Guga P, Szymanska B,
Parniewski P, et al: The effect of tumour necrosis factor-alpha
(TNF-alpha) muteins on human neutrophils in vitro. Mediators
Inflamm. 2:41–48. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaidanovich-Beilin O, Lipina TV, Takao K,
van Eede M, Hattori S, Laliberté C, Khan M, Okamoto K, Chambers JW,
Fletcher PJ, et al: Abnormalities in brain structure and behavior
in GSK-3alpha mutant mice. Mol Brain. 2:352009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sequea DA, Sharma N, Arias EB and Cartee
GD: Greater filamin C, GSK3α, and GSK3β serine phosphorylation in
insulin-stimulated isolated skeletal muscles of calorie restricted
24 month-old rats. Mech Ageing Dev. 134:60–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow HM, Guo D, Zhou JC, Zhang GY, Li HF,
Herrup K and Zhang J: CDK5 activator protein p25 preferentially
binds and activates GSK3β. Proc Natl Acad Sci USA. 111:pp.
E4887–E4895. 2014; View Article : Google Scholar : PubMed/NCBI
|