Introduction
Cerebral stroke is associated with the highest rate
of disability in the world, seriously threatening human health
(1). Increasing evidence has shown
that the incidence rate of ischemic stroke secondary to decreased
distal blood flow induced by carotid artery stenosis is not high
(2). Similarly, previous findings
have shown that the composition of carotid atherosclerosis plaques
is closely related to the occurrence of cerebrovascular diseases.
Therefore, its pathogenic mechanism is thromboembolism caused by
the rupture of vulnerable plaques, rather than vascular lumen
stenosis (1,2). The detection and evaluation of
morphological changes in plaques may be more clinically valuable
than the evaluation of the degree of lumen stenosis. Previous
findings showed that vulnerable plaques have larger amounts of
eccentric lipids, thinner fibrous caps, substantial inflammatory
cell infiltration, intra-plaque hemorrhage, neovascularization, and
superficial ulcers (3–5).
Neovascularization in plaques is an independent
predictive factor of the rupture of vulnerable plaques, and is
currently considered the most potent independent predictive factor
of plaque rupture. Previous evidence demonstrated that the
development of atherosclerotic plaques may be related to
neovascularization (6). In 2005,
Virmani et al found that neovascularization in plaques can
promote the development of atherosclerotic lesions, and even induce
intra-plaque hemorrhage, plaque rupture, and complications, which
are important factors related to plaque instability (7). In addition, it was found that the
amount of neovascularization in plaques is closely related to
clinical manifestations. A higher density of neovascularization is
associated with an increased risk of typical clinical
manifestations (2–4). Therefore, the detection of
neovascularization is important for predicting plaque stability,
assessing the risk of stroke, and for guiding clinical
treatment.
Based on the above observations, new techniques for
assessing neovascularization in plaques are necessary. Ultrasound
micro-flow imaging (SMI) is a new blood flow imaging technique with
high sensitivity, high resolution, and low artifact (8,9). The
technique can effectively separate blood flow signals and
overlapping tissue motion artifacts by the adaptive calculation
method, and accurately detect low-speed blood flow signals
(10). However, to the best of our
knowlegde, there are no reports on SMI-mediated detection of
neovascularization in plaques. Therefore, the aim of the present
study was to investigate whether SMI and contrast-enhanced
ultrasound (CEUS) are consistent for the detection of
neovascularization in plaques that were verified by histology. We
further investigated whether clinical symptoms and severities were
associated with differences in SMI grading, to provide a basis for
early clinical diagnosis and treatment, and reduce the risk of
cerebral stroke caused by plaque rupture.
Patients and methods
Patients
A total of 39 patients (64 carotid atherosclerotic
plaques) who underwent cervical vascular examination between
February 2015 and February 2016 in the Affiliated Hospital of
Chengde Medical College were selected. There were 31 males and 8
females, with an average age of 66.8±7.4 years. Informed consent
was obtained from all the enrolled patients. There were 11 patients
with single carotid atherosclerotic plaques, and 28 patients with
multiple carotid atherosclerotic plaques. Inclusion criteria were:
patients with carotid atherosclerotic plaques in the common carotid
artery and internal carotid artery identified by ultrasound
examination (plaque thickness ≥2.0 mm, length ≥10 mm). Exclusion
criteria were: patients who were unconscious and could not
cooperate in the examination; patients with cardiac insufficiency
and severe coronary atherosclerotic cardiopathy, arrhythmia, or
allergic constitution; patients with carotid plaques associated
with strong echoes with sound shadows, and patients with
contraindications for SonoVue (Bracco spA, Milan, Italy).
Instruments and reagents
Aplio 500 ultrasonic diagnostic apparatus (Toshiba,
Tokyo, Japan) (11-L4 probe; frequency, 4–11 MHz), and contrast
agent, SonoVue were used in the present study.
Ultrasonic examination
Routine ultrasound examination was performed by two
experienced physicians in the ultrasound department. Patients were
placed in a horizontal position with their head towards the
opposite side of examination, with the neck exposed. The carotid
artery was scanned by the probe from top to bottom in transverse
and longitudinal directions. The largest thickness of plaques was
measured in the longitudinal section, and severe carotid stenosis
(70–99%) was determined according to the diagnostic criteria of the
maximum systolic velocity (PSV) >230 mm/sec, maximum diastolic
velocity (EDV) >100 mm/sec, PSV in the stenosed segment, distal
maximum systolic velocity >4.0, and low-speed low-beat change in
the distal stenosis spectrum. The probe was kept still, and the SMI
mode was turned on to analyze neovascularization in plaques and
store images. Examination of neovascularization was conducted again
using CEUS, and the mechanical index was reduced to 0.12–0.20 to
avoid the rupture of micro-bubbles at the early stage of
angiography. Contrast agent (5 ml) was dissolved in 5 ml of 0.9%
normal saline, followed by intravenous injection of 2.4 ml of
contrast agent into the elbow vein. The dose was adjusted according
to the specific conditions of the patient (not exceeding 4.8 ml).
The images were stored after continuous observation for 5 min, and
5 ml of 0.9% normal saline was injected following the contrast
agent.
CEUS
Before examination, the patients were asked to
minimize body movement and swallowing, and avoid excessive
breathing movement. The operator placed the probe on the surface of
the body, and chose a clear region of the plaque. The ultrasound
contrast mode and double-width real-time display were turned on,
simultaneously showing the two-dimensional gray-scale interface and
ultrasound contrast interface. The assistant was asked to inject
2.4 ml of contrast agent into the superficial vein in the elbow via
bolus injection, immediately followed by 5 ml of 0.9% normal saline
solution. At the same time, the built-in timer and image
acquisition were started. During the examination, the probe was
maintained to clearly display the sections of interest. After
continuous observation for 5 min, and automatic sequence
acquisition, the dynamic images were stored in the hard disk. In
patients with ≥2 plaques, the interval between examinations was at
least ≥15 min. If a patient received ≥2 examinations, the built-in
timer was restarted after entering the dynamic acquisition
interface. After examination, the images were stored in raw data
format, followed by in-machine and off-machine quantitative
analysis. Intensity-over-time curve (ITC) Qanalysis quantitative
software (Nanjing Jiancheng Biotech Co., Nanjing, China) was used
for the in-machine analysis: after entering the ITC Qanalysis
interface, the continuous dynamic images were opened, and the
region of interest (ROI) was drawn manually according to the size
and shape of different plaques. Another ROI was drawn in the
carotid artery lumen in the proximal part of the plaque as the
control, and the ROI was manually adjusted frame-by-frame in the
in-machine analysis. The software could automatically generate the
ITC in the plaque and arterial lumen.
Carotid plaque grouping
Under conventional two-dimensional gray-scale
ultrasound, carotid plaques were divided according to the plaque
classification method proposed by Gray-Weale et al (1) combined with the echo of the
sternocleidomastoid into homogeneous hypoechoic plaques (Type I):
hypoecho in the plaque but with a fibrous cap that could reflect
the echo; heterogeneous hypoechoic plaques (Type II): hypoechoic
area prevailed in the plaque, and the hyperechoic area was <25%
of the total plaque area; heterogeneous hyperechoic plaques (Type
III): hyperechoic area prevailed in the plaque, and the hypoechoic
area was <25% of the total plaque area; homogeneous hyperechoic
plaques (Type IV): homogeneous hyperechoic plaques; unclassified
plaques (Type V): calcified plaques formed with a rear acoustic
shadow. Type V affected the results of analysis because of the rear
acoustic shadow. Therefore, it was not included in the scope of
analysis. Definitions: hypoecho (echo slightly higher than the
blood), isoecho (similar to the sternocleidomastoid echo), and
hyperecho (higher than the sternocleidomastoid echo).
Gray-scale median (GSM) analysis
Three sections in conventional two-dimensional
ultrasound in the longitudinal and transverse directions clearly
showed the stored images after plaque echo, and the images were
exported using a mobile hard disk. Power Showcase software
(Autodesk, Inc., San Rafael, CA, USA) was used to transform the raw
data format into JPG format. Adobe Photoshop Elements 11 (Adobe
Systems Incorporated, San Jose, CA, USA) was used for image editing
to normalize the image processing. The gray-scale range was from 0
(black) to 255 (white), and 2 reference points were selected, blood
(0) and adventitia (255). The image was imported to the software,
the whole contours of each plaque in the 4 kinds of echoes were
obtained using the ‘Automatic Selection Tool’, and the gray-scale
pixel histograms of plaques were obtained. The GSM values of
plaques were recorded, and the automatic selection tool was used to
draw the local area of the sternocleidomastoid in the same image to
obtain the gray-scale pixel histogram of the sternocleidomastoid,
and the GSM values were recorded. The GSMs of plaques and the
sternocleidomastoid in conventional two-dimensional gray-scale
ultrasound longitudinal and transverse sections were obtained, and
the means were compared. Among all plaques, mean GSM values lower
than those of the sternocleidomastoid were classified as hypoechoic
plaques, while mean GSM values higher than those of the
sternocleidomastoid were classified as hyperechoic plaques, and the
echo type of the plaque was determined by naked eye
examination.
Visual score of CEUS
According to the scoring criterion of Shah et
al (6), the degree of
development was divided into 0–3 points: 0 points, no enhancement
in plaques; 1 point, point-like enhancement in plaques; 2 points,
point-like and 1–2 short line-like enhancements; 3 points,
line-like enhancement in plaques throughout the plaque, or with
blood flow sign. Double-blind scoring was performed by two
physicians; when there was disagreement, the scoring results of the
more experienced one prevailed.
Examination method of SMI
The SMI mode was turned on to detect
neovascularization in plaques. The sites of neovascularization were
divided according to the plaque site into the proximal part, distal
part, top part, and basilar part. Neovascularization was the
short-line or strip-like hyper echo, although multi-section
conventional ultrasound images were required to exclude the
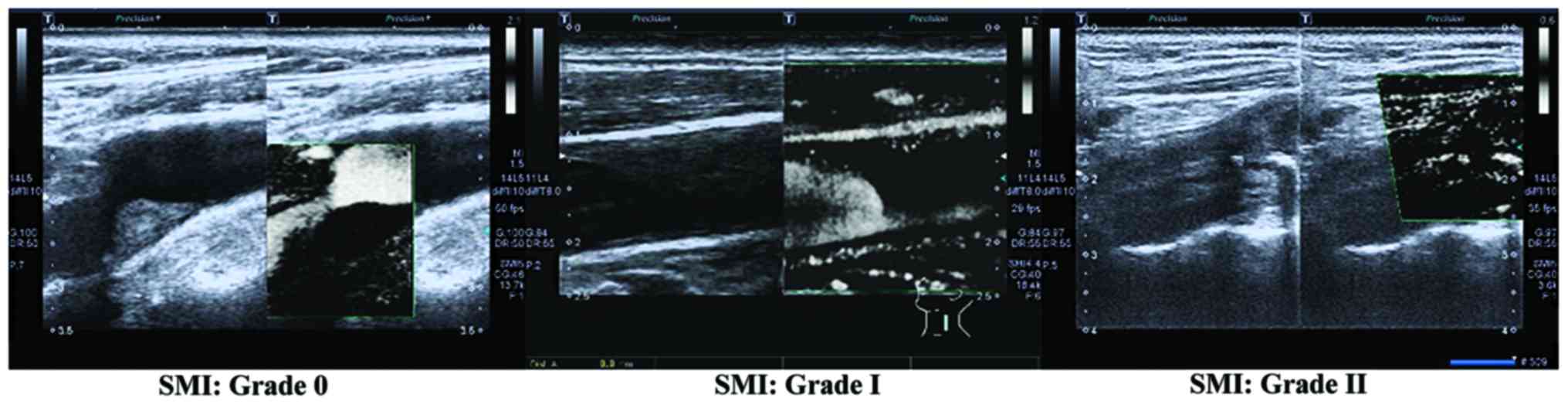
calcified echo in plaques. Grading criteria of neovascularization
in plaques detected by SMI (5):
Grade 0, no blood flow signal in plaques; Grade I, visible blood
flow signal in the shoulder or basilar part of plaques; Grade II,
visible blood flow signals in the shoulder and basilar part of
plaques.
Observational indexes of SMI
The results of the diagnostic methods were recorded,
and neovascularization in carotid plaques was divided according to
the grading criteria of Nakamura et al (11): Grade 0, no obvious micro-bubbles in
plaques; Grade I, micro-bubbles were limited to one side of the
shoulder or outer membrane of plaques; Grade II, visible diffuse
micro-bubbles in plaques.
Diagnostic criteria of SMI
According to the unified test standard of the North
American Symptomatic Carotid Endarterectomy Trial approved by the
ultrasound conference of the Radiological Society of North America
(1), the degree of carotid artery
stenosis was evaluated. Carotid diameter stenosis was divided into
4 grades: i) mild stenosis, stenosis rate <50%; ii) moderate
stenosis with a stenosis rate of 50–69%; iii) severe stenosis with
a stenosis rate of 70–99%; and iv) total occlusion with a stenosis
rate of 100%.
Statistical analysis
SPSS 17.0 statistical software (IBM Corp., Armonk,
NY, USA) was used. The κ test was used to test the consistency of
grading results of SMI and CEUS. κ >0 indicated the
significance, and larger κ value indicated better consistency.
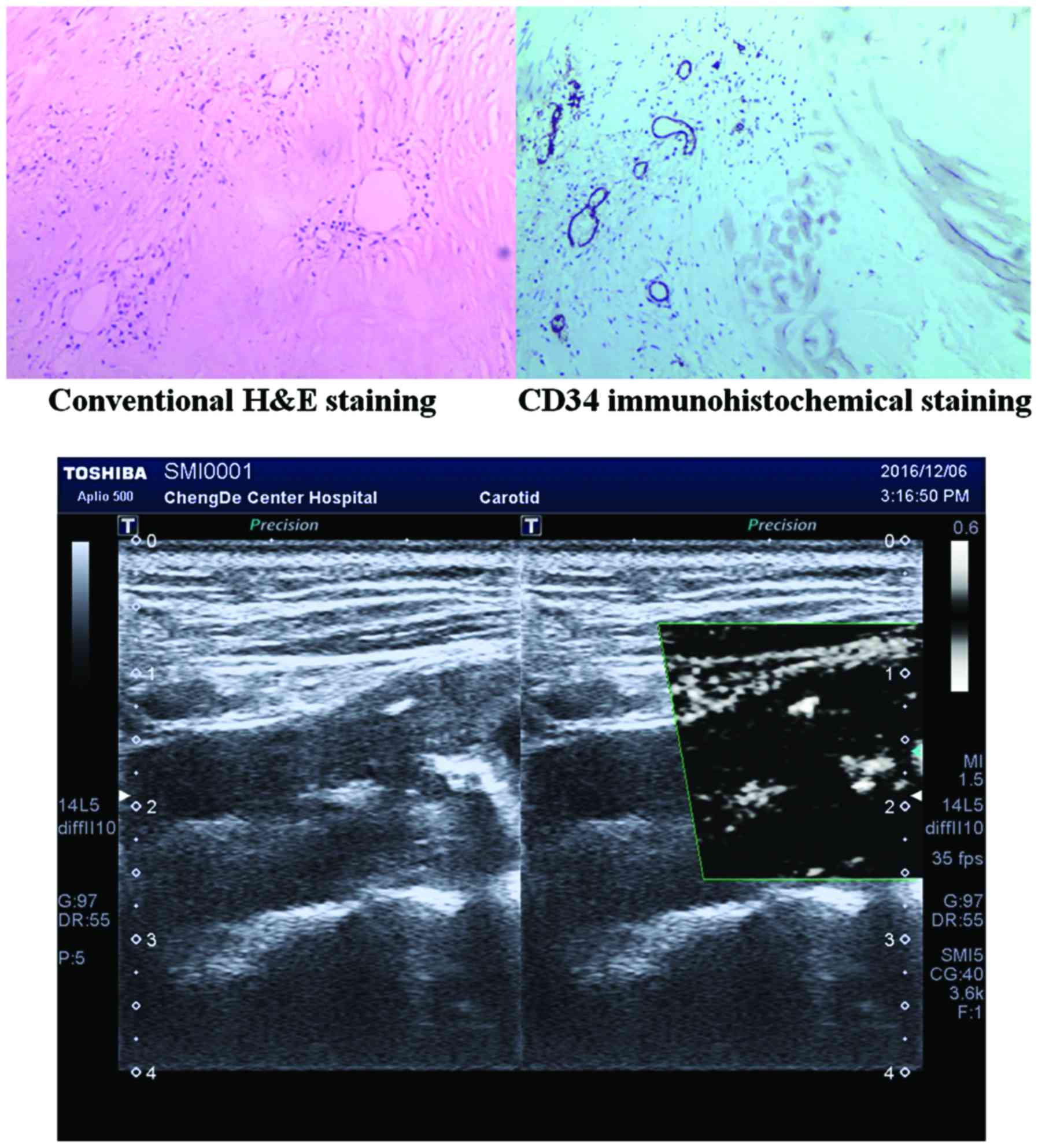
One-way ANOVA was used to investigate the differences of
neovascularization density in plaques with different SMI grading,
demonstrated by CD34 immunohistochemical staining. P<0.05 was
considered statistically significant. Spearman's rank correlation
test was used to investigate the correlation between SMI and
clinical symptoms and severity. The correlation coefficient r>0
indicated a positive correlation, r<0 indicated a negative
correlation, and r=0 indicated no correlation.
Results
Ultrasonic examination results
A total of 39 patients underwent carotid
endarterectomy, and 39 carotid plaques were removed. κ test result
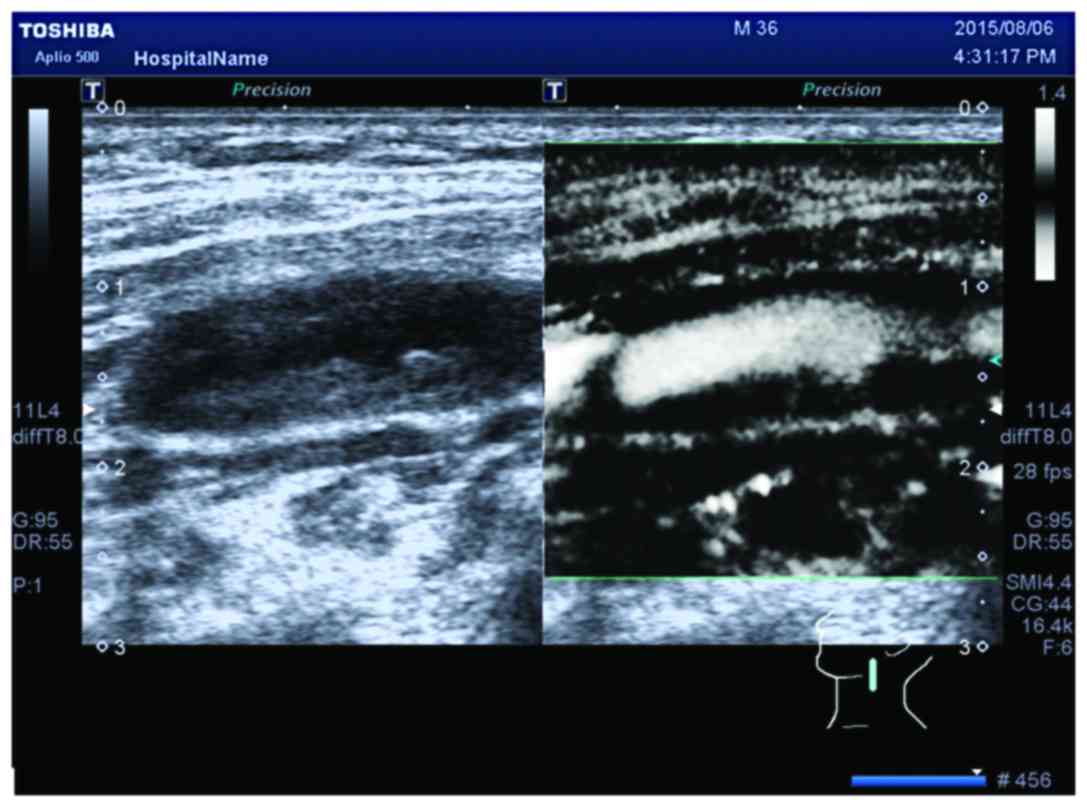
of the consistency of SMI and CEUS: κ=0.860 >0 (Fig. 1; Table
I).
 | Table I.Grading results of CEUS. |
Table I.
Grading results of CEUS.
|
| SMI grading |
|---|
|
|
|
|---|
| CEUS grading | 0 | I | II | Total |
|---|
| 0 | 7 | 0 | 0 | 7 |
| I | 1 | 6 | 2 | 9 |
| II | 0 | 0 | 23 | 23 |
| Total | 8 | 6 | 25 | 39 |
Immunohistochemical examination
results
A total of 39 patients underwent carotid
endarterectomy, and 39 carotid plaques were removed. One-way ANOVA
was performed for neovascularization density of immunohistochemical
staining in different SMI gradings (P<0.001). There was no
significant difference between Grade 0 and Grade I SMI
(P0-I=0.836), while there were significant differences between the
other 2 groups (P0-II=0.001, PI-II=0.041) (Table II).
 | Table II.Neovascularization density of CD34
immunohistochemical staining. |
Table II.
Neovascularization density of CD34
immunohistochemical staining.
| SMI grading | Neovascularization
density (mm2) |
|---|
| Grade 0 | 1.79±0.41 |
| Grade I | 2.26±0.73 |
| Grade II | 3.11±0.48 |
Dynamic imaging performances and
visual scores of the 4 groups of plaques
A total of 64 carotid plaques from 39 patients were
divided into 4 groups according to different echo types, and into 4
grades according to CEUS visual score: 0, 1, 2, and 3 points; with
5, 23, 23, and 13, respectively, in each grade (Table III). The consistency rate of the
objects was 91% (κ=0.910, P<0.001). There were statistically
significant differences in the enhancement grading and intensity of
CEUS visual score among the carotid atherosclerotic plaques with
different echo types (χ2=17.951, P<0.001). Lower echo
of carotid atherosclerotic plaques was associated with more obvious
enhancement of CEUS visual score (Table III). The enhancement scores of the
homogeneous hypoechoic plaque group and heterogeneous hypoechoic
plaque group were 2 and 3 points, accounting for approximately
66.7% (14/21) and 92.3% (12/13), respectively. However, one
homogeneous hypoechoic plaque showed no enhancement, and the
enhancement scores of the homogeneous hyperechoic plaque group and
heterogeneous hyperechoic plaque group were 1 point, accounting for
approximately 58.8% (10/17) and 46.2% (6/13), respectively.
 | Table III.Grading of CEUS visual scores of
carotid atherosclerotic plaques. |
Table III.
Grading of CEUS visual scores of
carotid atherosclerotic plaques.
| CEUS grading | No. of plaques | Percentage |
|---|
| 0 | 5 | 7.9 |
| 1 | 23 | 35.9 |
| 2 | 23 | 35.9 |
| 3 | 13 | 20.3 |
| Total | 64 | 100 |
CEUS parameters of the 4 groups of
plaques
Grading results of 64 plaques of the 39 patients are
shown in Table IV. There were 5
plaques with no enhancement in CEUS. Therefore, 59 plaques were
included in the CEUS quantitative analysis for the intensities and
densities of the 4 groups of plaques according to different echo
types, the results showed that the enhancement intensity was
increased in the order of homogeneous hyperechoic plaques,
heterogeneous hyperechoic plaques, homogeneous hypoechoic plaques,
and heterogeneous hypoechoic plaques. The enhancement density was
increased in the order of heterogeneous hyperechoic plaques,
homogeneous hyperechoic plaques, homogeneous hypoechoic plaques,
and heterogeneous hypoechoic plaques. The H test showed that there
were statistically significant differences in the enhancement
intensity among the 4 groups (χ2=29.025, P<0.001).
Pairwise comparisons showed that there were statistically
significant differences between the homogeneous hypoechoic plaque
group and homogeneous hyperechoic plaque group and heterogeneous
hyperechoic plaque group (P<0.05). There were statistically
significant differences between the heterogeneous hypoechoic plaque
group and homogeneous hyperechoic plaque group and heterogeneous
hyperechoic plaque group (P<0.05). There were no statistically
significant differences between the other groups (Tables IV and V).
 | Table IV.Comparisons of enhanced intensity
values of carotid plaques with different echo types: median
(interquartile range). |
Table IV.
Comparisons of enhanced intensity
values of carotid plaques with different echo types: median
(interquartile range).
| Echo type | Median
(interquartile range) (×10-5AU) |
|---|
| Homogeneous
hypoechoic plaque (Group 1) | 0.0510
(0.0269–0.1877) |
| Heterogeneous
hypoechoic plaque (Group 2) | 0.1021
(0.0679–0.2013) |
| Homogeneous
hyperechoic plaque (Group 3) | 0.0125
(0.0036–0.0352)a,b |
| Heterogeneous
hyperechoic plaque (Group 4) | 0.0137
(0.0032–0.0300)a,b |
 | Table V.CEUS visual scores of carotid
atherosclerotic plaques with different echo types (n). |
Table V.
CEUS visual scores of carotid
atherosclerotic plaques with different echo types (n).
| Echo type | No. of plaques | 0 | 1 | 2 | 3 |
|---|
| Homogeneous
hypoechoic plaque | 21 | 1 | 6 | 7 | 7 |
| Heterogeneous
hypoechoic plaque | 13 | 0 | 1 | 7 | 5 |
| Homogeneous
hyperechoic plaque | 17 | 2 | 10 | 5 | 0 |
| Heterogeneous
hyperechoic plaque | 13 | 2 | 6 | 4 | 1 |
Grading results of SMI and CEUS
The κ test result was κ=0.860 >0, indicating that
SMI and CEUS have good consistency for the detection of
neovascularization in plaques (Table
V).
Quantitative parameters of CEUS visual
score enhancement
There were 5 plaques with no enhancement in CEUS
with 0 points. Therefore, 59 plaques with >0 points were
included in the CEUS quantitative analysis. The enhanced intensity
of CEUS visual scores is shown in Table
VI. The visual score of intensity was increased from 1 point, 2
points, and 3 points in turn. There were statistically significant
differences in the enhanced intensity values with different scores
(χ2=23.709, P<0.001). Pairwise comparison showed that
there were statistically significant differences in the enhanced
intensity values between the visual score of 1 point and the visual
score of 2 and 3 points (P<0.05), and there was no statistically
significant difference between the other groups.
 | Table VI.Comparisons of enhanced intensity of
carotid atherosclerotic plaques with different scores [median
(interquartile range)]. |
Table VI.
Comparisons of enhanced intensity of
carotid atherosclerotic plaques with different scores [median
(interquartile range)].
| Score | No. of plaques | Median
(interquartile range) (×10-5AU) |
|---|
| 1 | 23 | 0.0086
(0.0032–0.0324) |
| 2 | 23 | 0.0546
(0.0300–0.1019) |
| 3 | 13 | 0.1021
(0.0478–0.3468) |
Comparison of the consistency of SMI
and CEUS for the diagnosis of neovascularization in plaques
The sensitivity, specificity, and accuracy of SMI
for the detection of neovascularization in carotid plaques were
100, 65.0 and 68.8%, respectively. The comparison of the
consistency of SMI and CEUS for the diagnosis of neovascularization
in plaques showed κ=0.754 and P<0.05 (Table VII).
 | Table VII.Comparison of the consistency of SMI
and CEUS for the detection of neovascularization in plaques. |
Table VII.
Comparison of the consistency of SMI
and CEUS for the detection of neovascularization in plaques.
|
| Detection of
neovascularization in plaques via SMI |
|
|---|
|
|
|
|
|---|
| Item | Detected | Not detected | Total |
|---|
| Detection of
neovascularization in plaques via CEUS |
|
|
|
|
Detected | 44 | 7 | 51 |
| Not
detected | 0 | 13 | 13 |
| κ-value | 0.754 |
| 64 |
| P-value | <0.05 |
|
|
Discussion
Carotid atherosclerotic disease is one of the main
causes of cerebral stroke. Histological and morphological studies
suggest that carotid plaque-induced stroke depends on whether
severe arterial stenosis leads to intracranial hypoperfusion or
plaque rupture and ulceration, causing arterial thrombosis, which
is referred to as the stability of plaques (12). Therefore, the search for accurate
diagnostic and preventive methods, and a risk stratification and
treatment plan to reduce the incidence and severity of acute
cerebrovascular diseases, should no longer be limited to assessing
the degree of arterial stenosis (13). In recent years, it has been shown
that hypoechoic plaques share the pathological features of unstable
plaques, including large lipid nuclei, thin fibrous caps, a large
number of inflammatory cells, and the presence of
neovascularization. Hemorrhage and rupture of these plaques occur
more easily, resulting in a series of clinical symptoms.
Hyperechoic plaques are less prone to rupture because of increased
fiber composition and thicker fibrous caps (14–18).
According to De Blois et al (19), intima-media thickness and plaque
composition are evaluation indicators that can predict the
occurrence of cardiovascular and cerebrovascular diseases.
Therefore, the accurate evaluation of plaque stability is of great
significance for predicting the risk of plaque rupture, and
reducing the incidence of acute cerebrovascular disease.
Atherosclerotic plaques are primarily composed of a
fibrous cap, lipid necrotic core, inflammatory cells, adventitia,
and neovascularization (20–24). Sluimer et al (25) found that hypoxia in plaques is
related to thrombosis, atherosclerosis, vascular endothelial growth
factor, CD68 expression, and hypoxia-inducible factor. The mRNA and
protein expression of hypoxia-inducible factor, target genes, and
microvessel density are gradually increased from the early to the
stable stage of the lesion, indicating the correlation between the
development of hypoxia and atherosclerosis and neovascularization
in plaques. New capillaries in plaques, and proliferation of
adventitial vasa vasorum or ‘vessels of the vessels’ are important
features of vulnerable plaques, and are closely related to plaque
stability. Increased neovascularization is associated with poorer
plaque stability, and can more easily result in rupture, causing
embolism (26). Therefore, it is
highly important to detect and screen for dangerous lesions,
perform qualitative and quantitative analysis of the proliferative
degree of neovascularization in plaques with different echo types,
conduct risk stratification for plaques, predict plaque rupture
risk, and provide a valuable clinical basis for the prevention and
treatment of atherosclerosis-related cardiovascular and
cerebrovascular diseases. At present, various imaging methods, such
as three-dimensional computer tomography, high-resolution magnetic
resonance imaging, and CEUS, have been used to assess the
properties of plaques, and the application of SMI in evaluating
neovascularization in atherosclerotic plaques has great clinical
value (27). Our study showed that
the sensitivity, specificity, and accuracy of SMI for the detection
of neovascularization in carotid plaques were 100, 65.0, and 68.8%,
respectively. The comparison of consistency of SMI and CEUS for the
diagnosis of neovascularization in plaques showed κ=0.754 and
P<0.05, suggesting that SMI has a better power for the diagnosis
of neovascularization in plaques, and has good consistency compared
with CEUS.
To the best of our knowledge, there are no relevant
studies on the detection of neovascularization in carotid plaques
via SMI, although there are many studies on other diseases that
indicate that SMI can effectively detect blood vessels (27,28). In
this study, we found that 1 plaque had Grade 0 SMI and Grade I
CEUS, and 2 plaques had Grade II SMI and Grade I CEUS. The optimal
debugging condition of SMI therefore requires further study, as it
is a new technique for observing neovascularization. When using the
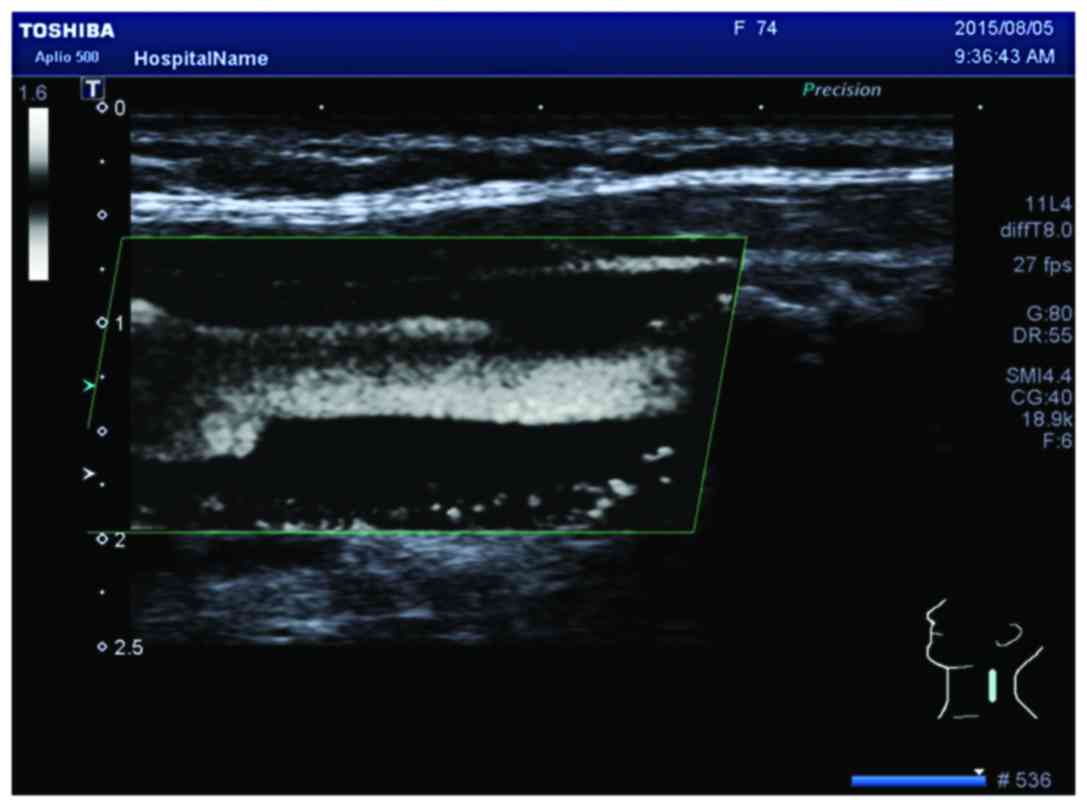
SMI mode to observe neovascularization in plaques, calcification in
plaques shows a similar performance to neovascularization in the
SMI mode. However, it can distinguish between them. New vessels
will flow with time, but the development of calcification is
static, brighter than new blood vessels, and can coincide with
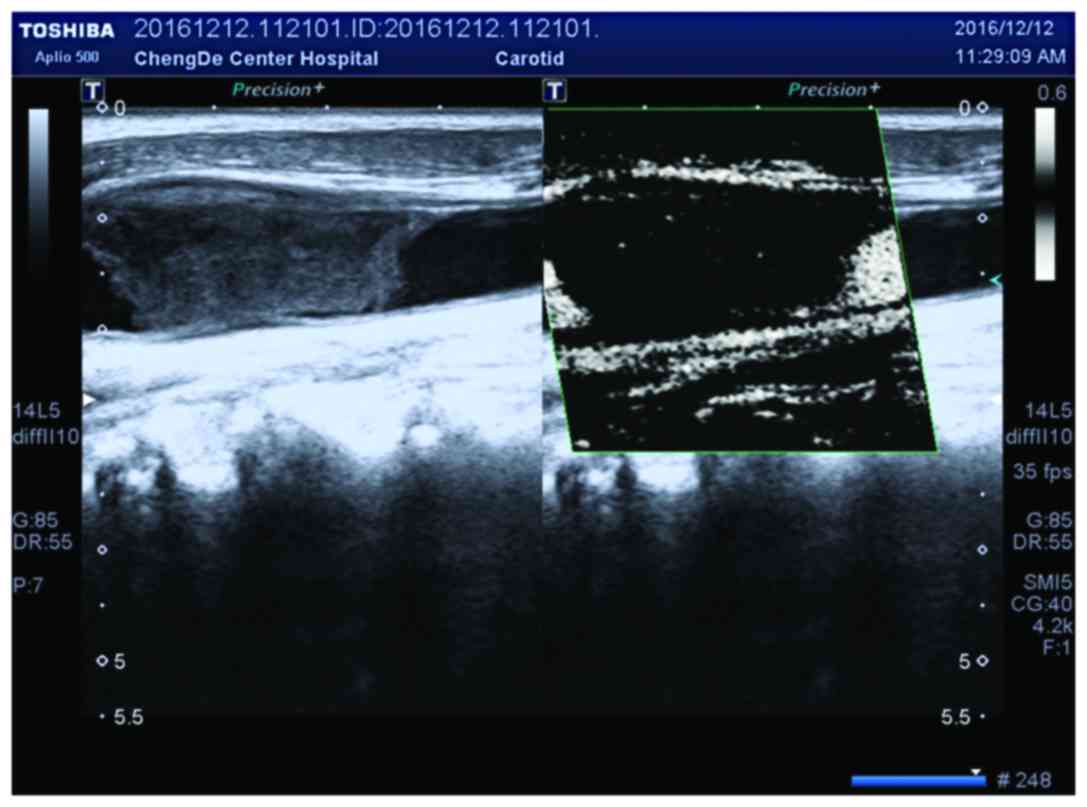
two-dimensional images (Fig. 2).
Therefore, it can eliminate the impact of calcification on the
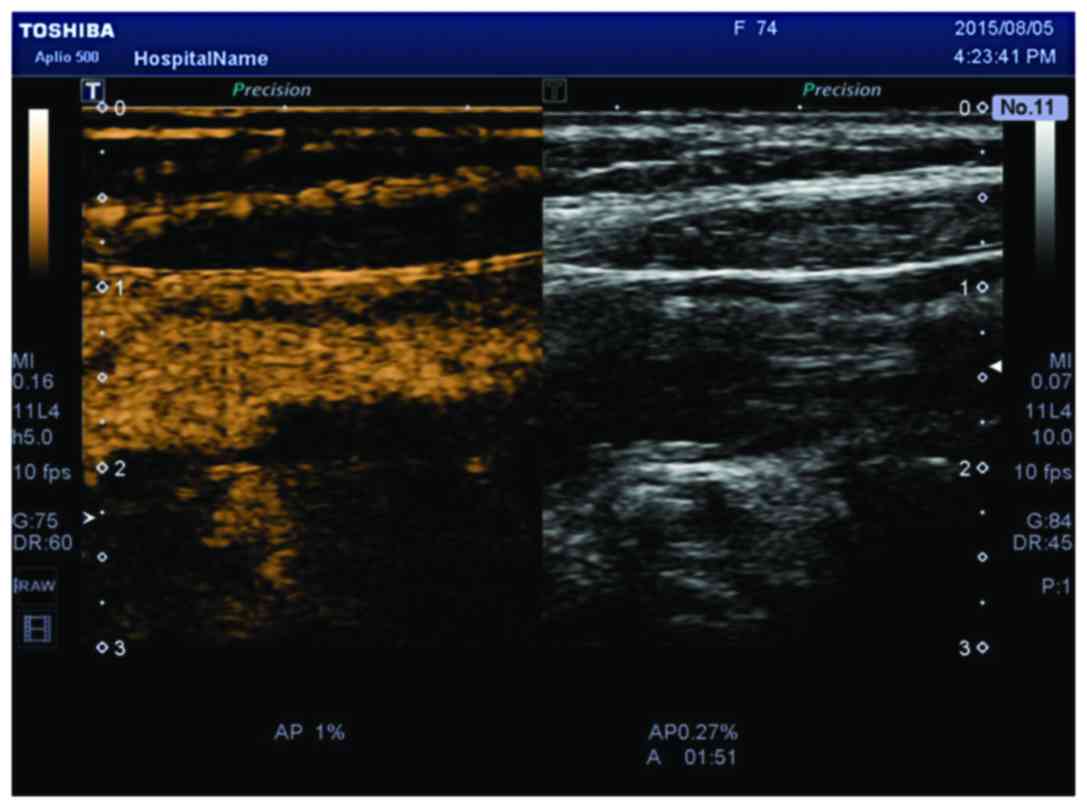
observation of neovascularization. Compared to CEUS, the SMI
technique uses a new self-adaptive algorithm to identify and
eliminate tissue motion artifacts, retain the most subtle
low-velocity blood flow signals, and can show unparalleled details
with high definition and clear neovascularization in plaques
(Figs. 3 and 4).
Before SMI, CEUS was commonly used to observe
neovascularization in carotid plaques, but the technique is
invasive and limited by the physical condition of the patient, with
relatively high costs. During routine ultrasound, we used the SMI
technique to evaluate neovascularization in plaque development. The
SMI procedure is basically the same as color Doppler flow imaging
without the waiting time for contrast agent to distribute to
vessels. Therefore, the time necessary for observing
neovascularization is much shorter than that of CEUS, reducing the
psychological and financial burden of patients, and reducing the
workload of ultrasound physicians. Patients with ulcerated plaques
received SMI to observe neovascularization in carotid plaques,
followed by risk stratification for ulcerated plaques, and
prediction of the risk of stroke. As shown in Figs. 5 and 6, the plaques in patients with ultra-low
echo plaques can be deformed with the heart, which may be because
of plaque rupture, causing thrombosis. SMI examination identified
the visible blood flow signals in ultra-low echo, which may have
occurred because most of the components of the plaques were liquid.
Regarding the detection of carotid plaques, when the common carotid
artery bifurcation site or plaque site is relatively high, the
vascular probe cannot meet the needs of clinical examination.
Therefore, a conventional abdominal probe should be used. The SMI
technique is not limited by the probe. Given that the SMI technique
is non-invasive with easy operation and no additional injury in
routine ultrasound examination, it is suitable for the evaluation
of carotid atherosclerotic plaques.
The results of our study showed that the GSM values
were increased in the order of homogeneous hypoechoic plaques
(34.78±11.16), heterogeneous hypoechoic plaques (44.55±11.97),
homogeneous hyperechoic plaques (50.20±13.61), and heterogeneous
hyperechoic plaques (77.50±16.04). Moreover, the differences in GSM
values between the 4 groups of plaques were statistically
significant (F=29.365, P<0.001), indicating that lower plaque
echo is associated with lower GSM value, which is consistent with
the previous histological study and GSM correspondence analysis
(29). The GSM values of the
heterogeneous hypoechoic plaque group were higher than those of the
homogeneous hypoechoic plaque group. The GSM values of the
heterogeneous hyperechoic plaque group were higher than those of
the homogeneous hyperechoic plaque group, and the differences in
the comparisons between the two groups were statistically
significant (P<0.05). These observations were related to the
histological composition and classification of plaques: hypoecho
prevailed in heterogeneous hypoechoic plaques, and the calcium and
fiber composition in the hyperechoic area (<25% of the total
plaque area) made the GSM values higher than those of the
homogeneous hypoechoic plaque group; hyperecho prevailed in
heterogeneous hyperechoic plaques, and the hypoechoic and echoless
areas (<25% of the total plaque area) could decrease the GSM
value of plaques; however, the hyperechoic area in heterogeneous
hyperechoic plaques accounted for the majority, and two-dimensional
ultrasonography showed that its gray-scale intensity was higher
than that of the homogeneous hypoechoic plaque group. According to
ultrasound imaging and the acoustic impedance principle, combined
with pathological tissue components, it may be related to a higher
calcareous component and higher GSM value. Therefore, the GSM
values of the heterogeneous hyperechoic plaque group were higher
than those of the homogeneous hyperechoic plaque group. In
addition, the differences between the heterogeneous hypoechoic and
homogeneous hyperechoic plaque groups were not statistically
significant (P=0.245), possibly because the calcareous component in
heterogeneous hypoechoic plaques increases the GSM value, thus it
is close to the GSM value of homogeneous hyperechoic plaques
containing fibrous tissue components. The GSM technique provides a
quantitative and objective evaluation method for the classification
of plaques with different echo types, avoiding the subjectivity of
judging the plaque echo types by naked eye evaluation. Therefore,
the repeatability is high (30–33).
According to Mathiesen et al (34), hypoechoic plaques can increase the
risk of ischemic cerebrovascular events, which is significantly
correlated with the plaque echo type (P=0.015), and detecting the
plaque echo type via two-dimensional ultrasound can predict the
risk of ischemic cerebrovascular events.
In this study, κ test results showed that SMI and
CEUS have good consistency for the detection of neovascularization
in plaques. One-way ANOVA showed that SMI can effectively
distinguish neovascularization density. However, there was no
statistically significant difference between Grade 0 and Grade 1
SMI. The rank-sum test and Spearmans rank correlation test showed
that SMI grading can predict the occurrence of stroke to some
extent. Higher SMI grading is associated with higher risk of
stroke.
In conclusion, our results suggest that the GSM
technique can evaluate plaque stability through the quantitative
classification of plaque echoes, for use as an important reference
index for predicting the risk of stroke. This is consistent with
the study by Ariyoshi et al (35), who measured the plaque echoes of
carotid atherosclerotic plaques in 84 patients with type 2 diabetes
mellitus using GSM, and investigated the correlations between
different echoes and different GSM values, and the incidence rate
of cardiovascular and cerebrovascular events. The GSM technique can
be used in combination with CEUS to directly observe the
proliferation of neovascularization in plaques, and obtain
significant quantitative parameters, to evaluate the stability of
plaque more objectively.
References
|
1
|
Gray-Weale AC, Graham JC, Burnett JR,
Byrne K and Lusby RJ: Carotid artery atheroma: comparison of
preoperative B-mode ultrasound appearance with carotid
endarterectomy specimen pathology. J Cardiovasc Surg (Torino).
29:676–681. 1988.PubMed/NCBI
|
|
2
|
Naghavi M, Libby P, Falk E, Casscells SW,
Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P,
Pasterkamp G, et al: From vulnerable plaque to vulnerable patient:
a call for new definitions and risk assessment strategies: part I.
Circulation. 108:1664–1672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellings WE, Peeters W, Moll FL, Piers SR,
van Setten J, van der Spek PJ, De Vries JP, Seldenrijk KA, De Bruin
PC, Vink A, et al: Composition of carotid atherosclerotic plaque is
associated with cardiovascular outcome: a prognostic study.
Circulation. 121:1941–1950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hingwala D, Kesavadas C, Sylaja PN, Thomas
B and Kapilamoorthy TR: Multimodality imaging of carotid
atherosclerotic plaque: going beyond stenosis. Indian J Radiol
Imaging. 23:26–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fleiner M, Kummer M, Mirlacher M, Sauter
G, Cathomas G, Krapf R and Biedermann BC: Arterial
neovascularization and inflammation in vulnerable patients: early
and late signs of symptomatic atherosclerosis. Circulation.
110:2843–2850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah F, Balan P, Weinberg M, Reddy V,
Neems R, Feinstein M, Dainauskas J, Meyer P, Goldin M and Feinstein
SB: Contrast-enhanced ultrasound imaging of atherosclerotic carotid
plaque neovascularization: a new surrogate marker of
atherosclerosis? Vasc Med. 12:291–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Virmani R, Kolodgie FD, Burke AP, Finn AV,
Gold HK, Tulenko TN, Wrenn SP and Narula J: Atherosclerotic plaque
progression and vulnerability to rupture: angiogenesis as a source
of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol.
25:2054–2061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Staub D, Partovi S, Schinkel AF, Coll B,
Uthoff H, Aschwanden M, Jaeger KA and Feinstein SB: Correlation of
carotid artery atherosclerotic lesion echogenicity and severity at
standard US with intraplaque neovascularization detected at
contrast-enhanced US. Radiology. 258:618–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russell DA, Wijeyaratne SM and Gough MJ:
Changes in carotid plaque echomorphology with time since a
neurologic event. J Vasc Surg. 45:367–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sztajzel R, Momjian S, Momjian-Mayor I,
Murith N, Djebaili K, Boissard G, Comelli M and Pizolatto G:
Stratified gray-scale median analysis and color mapping of the
carotid plaque: correlation with endarterectomy specimen histology
of 28 patients. Stroke. 36:741–745. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura J, Nakamura T, Deyama J, Fujioka
D, Kawabata K, Obata JE, Watanabe K, Watanabe Y and Kugiyama K:
Assessment of carotid plaque neovascularization using quantitative
analysis of contrast-enhanced ultrasound imaging is useful for risk
stratification in patients with coronary artery disease. Int J
Cardiol. 195:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher M, Paganini-Hill A, Martin A,
Cosgrove M, Toole JF, Barnett HJ and Norris J: Carotid plaque
pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke.
36:253–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ten Kate GL, van Dijk AC, van den Oord SC,
Hussain B, Verhagen HJ, Sijbrands EJ, van der Steen AF, van der
Lugt A and Schinkel AF: Usefulness of contrast-enhanced ultrasound
for detection of carotid plaque ulceration in patients with
symptomatic carotid atherosclerosis. Am J Cardiol. 112:292–298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vicenzini E, Giannoni MF, Sirimarco G,
Ricciardi MC, Toscano M, Lenzi GL and Di Piero V: Imaging of plaque
perfusion using contrast-enhanced ultrasound - clinical
significance. Perspect Med. 1:44–50. 2012. View Article : Google Scholar
|
|
15
|
Hoogi A, Adam D, Hoffman A, Kerner H,
Reisner S and Gaitini D: Carotid plaque vulnerability:
quantification of neovascularization on contrast-enhanced
ultrasound with histopathologic correlation. AJR Am J Roentgenol.
196:431–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nandalur KR, Baskurt E, Hagspiel KD,
Phillips CD and Kramer CM: Calcified carotid atherosclerotic plaque
is associated less with ischemic symptoms than is noncalcified
plaque on MDCT. AJR Am J Roentgenol. 184:295–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo Y, Watanabe S, Ishizu T, Moriyama N,
Takeyasu N, Maeda H, Ishimitsu T, Aonuma K and Yamaguchi I:
Echolucent carotid plaques as a feature in patients with acute
coronary syndrome. Circ J. 70:1629–1634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grønholdt ML, Nordestgaard BG, Schroeder
TV, Vorstrup S and Sillesen H: Ultrasonic echolucent carotid
plaques predict future strokes. Circulation. 104:68–73. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Blois J, Stranden E, Jogestrand T,
Henareh L and Agewall S: Echogenicity of the carotid intima-media
complex and cardiovascular risk factors. Clin Physiol Funct
Imaging. 32:400–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shalhoub J, Owen DR, Gauthier T, Monaco C,
Leen EL and Davies AH: The use of contrast enhanced ultrasound in
carotid arterial disease. Eur J Vasc Endovasc Surg. 39:381–387.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogata T, Yasaka M, Wakugawa Y, Kitazono T
and Okada Y: Morphological classification of mobile plaques and
their association with early recurrence of stroke. Cerebrovasc Dis.
30:606–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higashida T, Kanno H, Nakano M, Funakoshi
K and Yamamoto I: Expression of hypoxia-inducible angiogenic
proteins (hypoxia- inducible factor-1alpha, vascular endothelial
growth factor, and E26 transformation-specific-1) and plaque
hemorrhage in human carotid atherosclerosis. J Neurosurg.
109:83–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SH, Lee JH, Yoo SY, Hur J, Kim HS and
Kwon SM: Hypoxia inhibits cellular senescence to restore the
therapeutic potential of old human endothelial progenitor cells via
the hypoxia-inducible factor-1α-TWIST-p21 axis. Arterioscler Thromb
Vasc Biol. 33:2407–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Woo JS, Kim BY, Jang HH, Hwang SJ,
Kwon SJ, Choi EY, Kim JB, Cheng X, Jin E, et al: Biochemical and
clinical correlation of intraplaque neovascularization using
contrast-enhanced ultrasound of the carotid artery.
Atherosclerosis. 233:579–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sluimer JC, Gasc JM, van Wanroij JL,
Kisters N, Groeneweg M, Gelpke MD Sollewijn, Cleutjens JP, van den
Akker LH, Corvol P, Wouters BG, et al: Hypoxia, hypoxia-inducible
transcription factor, and macrophages in human atherosclerotic
plaques are correlated with intraplaque angiogenesis. J Am Coll
Cardiol. 51:1258–1265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao X, Kong J, Zhao Y, Wang X, Bu P,
Zhang C and Zhang Y: Gene silencing of TACE enhances plaque
stability and improves vascular remodeling in a rabbit model of
atherosclerosis. Sci Rep. 5:179392015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YH, Zheng QC, Yuan GX and Ren JH: The
application of ultrasound B-flow imaging in detection of carotid
atherosclerotic micro-vessel with ischemic cerebrovascular disease.
Saudi Med J. 33:1080–1086. 2012.PubMed/NCBI
|
|
28
|
Lind L, Andersson J, Rönn M, Gustavsson T,
Holdfelt P, Hulthe J, Elmgren A, Zilmer K and Zilmer M: Brachial
artery intima-media thickness and echogenicity in relation to
lipids and markers of oxidative stress in elderly subjects: the
prospective investigation of the vasculature in Uppsala Seniors
(PIVUS) Study. Lipids. 43:133–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schulte-Altedorneburg G, Droste DW, Haas
N, Kemény V, Nabavi DG, Füzesi L and Ringelstein EB: Preoperative
B-mode ultrasound plaque appearance compared with carotid
endarterectomy specimen histology. Acta Neurol Scand. 101:188–194.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ostling G, Hedblad B, Berglund G and
Gonçalves I: Increased echolucency of carotid plaques in patients
with type 2 diabetes. Stroke. 38:2074–2078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabetai MM, Tegos TJ, Nicolaides AN,
Dhanjil S, Pare GJ and Stevens JM: Reproducibility of
computer-quantified carotid plaque echogenicity: can we overcome
the subjectivity? Stroke. 31:2189–2196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kyriacou EC, Petroudi S, Pattichis CS,
Pattichis MS, Griffin M, Kakkos S and Nicolaides A: Prediction of
high-risk asymptomatic carotid plaques based on ultrasonic image
features. IEEE Trans Inf Technol Biomed. 16:966–973. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Östling G, Persson M, Hedblad B and
Gonçalves I: Comparison of grey scale median (GSM) measurement in
ultrasound images of human carotid plaques using two different
softwares. Clin Physiol Funct Imaging. 33:431–435. 2013.PubMed/NCBI
|
|
34
|
Mathiesen EB, Bønaa KH and Joakimsen O:
Echolucent plaques are associated with high risk of ischemic
cerebrovascular events in carotid stenosis: the tromsø study.
Circulation. 103:2171–2175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ariyoshi K, Okuya S, Kunitsugu I,
Matsunaga K, Nagao Y, Nomiyama R, Takeda K and Tanizawa Y:
Ultrasound analysis of gray-scale median value of carotid plaques
is a useful reference index for cerebro-cardiovascular events in
patients with type 2 diabetes. J Diabetes Investig. 6:91–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|