Introduction
Osteosarcoma is one of the most common primary
malignant neoplasms in children, adolescents, and young adults
(1). The introduction of
chemotherapy has lead to a significant improvement in the prognosis
of patients with localized osteosarcoma, and long-term survival
rates of <20% have been observed to improve to >65% after the
use of multiagent chemotherapy regimens (2). However, patients with osteosarcoma who
present with metastasis continue to have poor prognosis, which is
associated with a strong resistance to chemotherapy (3,4).
Consequently, it is imperative that novel treatment strategies are
developed for such patients.
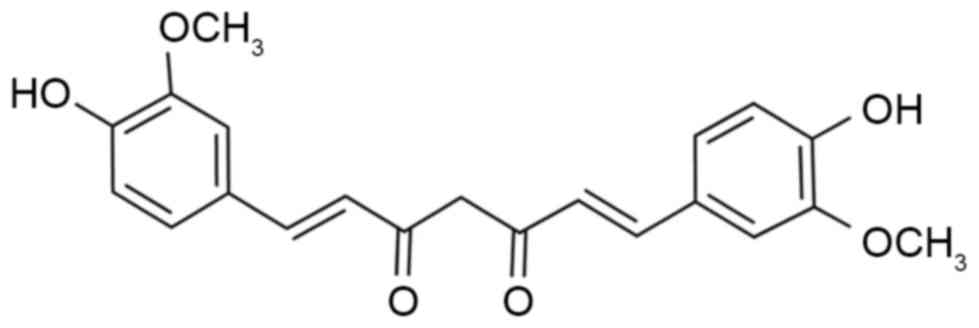
Curcumin (Fig. 1) is
derived from the rhizome of the East Indian plant Curcuma
longa. Over the past three decades, curcumin has been widely
used as a cancer chemotherapy agent in a wide range of cancer
models, including those for colorectal, pancreatic, breast and
hematological malignancies, and has been utilized for its ability
to alleviate therapy-induced toxicities such as Mitomycin C
associated side-effects and chemotherapy-induced mucosal barrier
injury (5–10). Curcumin has been found to inhibit the
tumorigenesis and progression of various tumors, such as colorectal
cancer, lung adenocarcinoma (5).
These anti-cancer effects are predominantly mediated through the
negative regulation of various oncogenic molecules and pathways,
including activator protein 1, nuclear factor κB, peroxisome
proliferator-associated receptor gamma, signal transducer and
activator of transcription, Wnt/β-catenin, nuclear factor
(erythroid-derived 2)-like 2, tumor necrosis factor-α,
interleukins, inducible nitric oxide synthase, cyclooxygenase-2,
lipooxygenase, p38 mitogen-activated protein kinase (MAPK), c-Jun
N-terminal kinase (JNK1/2), extracellular signal-regulated kinase
1/2, growth factor induced signaling cascades, cyclin D1, p53, in
addition to intracellular adhesion molecule-1 (11). Curcumin has also been used in
combination with various other anti-cancer agents, such as
gemcitabine, docetaxel and acetylcysteine in pre-clinical cancer
studies (5,10,12).
Autophagy, which is a process involving
self-degradation and the turnover of cellular components, has a
complex role in the initiation and progression of cancer. Evidence
suggests that autophagy has anti-survival characteristics, and can
contribute to tumor suppression in different cancer cell types
(13). The predominant function of
the JNK signal transduction pathway is to induce defense mechanisms
that protect organisms against a number of stressors, including UV
irradiation and oxidative stress, which can induce apoptosis or
growth inhibition. This pathway has also been confirmed to be
associated with the molecular events involved in the regulation of
autophagy (14). The current study
aimed to determine whether curcumin was able to induce autophagy in
osteosarcoma cells. In addition, the underlying interaction between
autophagy and apoptosis was investigated.
Materials and methods
Cell lines and curcumin
The human osteosarcoma cell line MG63 was purchased
from the American Type Culture Collection (Manassas, VA, USA). All
of the cells were cultured with Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBSand
penicillin/streptomycin (both Thermo Fisher Scientific, Inc.).
Proliferation assay
Cell proliferation assays were performed using cell
counting kit (CCK)-8 (Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA) in accordance with a previously described
method (15). A total of 2,000 cells
were seeded in each well in a 96-well plate. CCK-8 solution (10 µl)
was added to 100 µl of culture media, and the optical density was
measured at 450 nm. The concentrations of z-VAD-FMK, 3-MA or
SP600125 used in the experiments were 5, 5 or 10 µM, respectively.
Three independent experiments were performed.
Apoptosis assay
Cell apoptosis assays were performed by flow
cytometry (BD FACSCalibur flow cytometer; BD Biosciences, Franklin
Lakes, NJ, USA), as previously described (16,17).
Cells at a density of 0.5×105 cells/dish in 60-mm cell
culture dishes were pre-incubated with or without agents for 24 h,
collected and stained using the Annexin V-FITC Apoptosis Detection
kit (BD Biosciences) according to the manufacturer's instructions.
In brief, cells were washed with cold PBS and resuspended in 100 µl
Annexin V binding buffer, followed by incubation with fluorescein
isothiocyanate-conjugated Annexin V and propidium iodide for 15 min
at room temperature in the dark.
Western blot analysis
Total protein (~500 mg) was extracted using lysis
reagents (Cell Signaling Technology, Inc., Danvers, MA, USA) and
quantified using a bicinchoninic acid assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Proteins (50 mg) were separated by 10%
SDS-PAGE and transferred to polyvinylidene difluoride (PVDF)
membranes. The membranes were then blocked with 5% skimmed milk for
2 h at room temperature and incubated overnight at 4°C with mouse
anti-Bcl-2 monoclonal antibody (1:1,000; sc-56015), rabbit
anti-caspase-3 polyclonal antibody (1:1,000; sc-271757), mouse
anti-β-actin monoclonal antibody (1;1,000; sc-81178; all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-Bax monoclonal
antibody (1:1,000; ab-32503), rabbit anti-ATG5 monoclonal antibody
(1:1,000; ab108327), rabbit anti-LC3-I/II polyclonal antibody
(1:1,000; ab128025), rabbit anti-p-EKR polyclonal antibody
(1:1,000; ab176660), rabbit anti-p-JNK polyoclonal antibody
(1:1,000; ab47337), rabbit anti-p-P38 polyclonal antibody (1:1,000;
ab47363) and mouse anti-p-p53 polyclonal antibody (1:1,000; ab1431)
(all Abcam, Cambridge, MA, USA) overnight at 4°C and further
incubated for 1 h with horseradish peroxidase-conjugated anti-mouse
(1:1,000; sc-2371), or anti-goat (1:1,000; sc-2350) or anti-rabbit
(1:1,000; sc-2370) secondary antibodies (all Santa Cruz
Biotechnology, Inc.). Immune complexes were then detected by
incubating the PVDF membranes with HRP-conjugated secondary
antibody for 2 h at room temperature, followed by exposure of the
membrane to enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.). Quantification of the protein bands was
performed using ImageJ 1.48 software (National Institutes of
Health, Bethesda, MD, USA).
Autophagy assay
Monodansylcadaverine (MDC; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), a specific marker for autophagic
vacuoles was used in order to further confirm whether curcumin was
able to induce autophagy. MG63 cells were labeled with 0.05 mM MDC
in PBS at 37°C for 10 min, washed three times with PBS and
immediately analyzed under an AV300-ASW confocal microscope
(Olympus Corp., Tokyo, Japan) with a ×60 oil lens. The amount of
LC3 puncta per cell was quantified. A minimum of 10 cells in five
independent experiments were analyzed at random.
Statistical analysis
Statistical analysis was performed using SPSS
statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA).
Student's t-test was used to analyze all other data. All tests were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference.
Results
Curcumin suppresses osteosarcoma cell
proliferation
To determine the cytotoxic effects of curcumin
(Fig. 1) on osteosarcoma cell lines,
a CCK-8 assay was performed to evaluate the proliferation of human
osteosarcoma MG63 cells. As shown in Fig. 2A, curcumin treatment markedly
decreased the proliferation of MG63 cells in a dose- and
time-dependent manner. Furthermore, the exposure of MG63 cells to
curcumin at various concentrations for 24 h dose-dependently
increased the number of cytolytic cells (Fig. 2B). The IC50 value for
curcumin was 9.2 µM in MG63 cells.
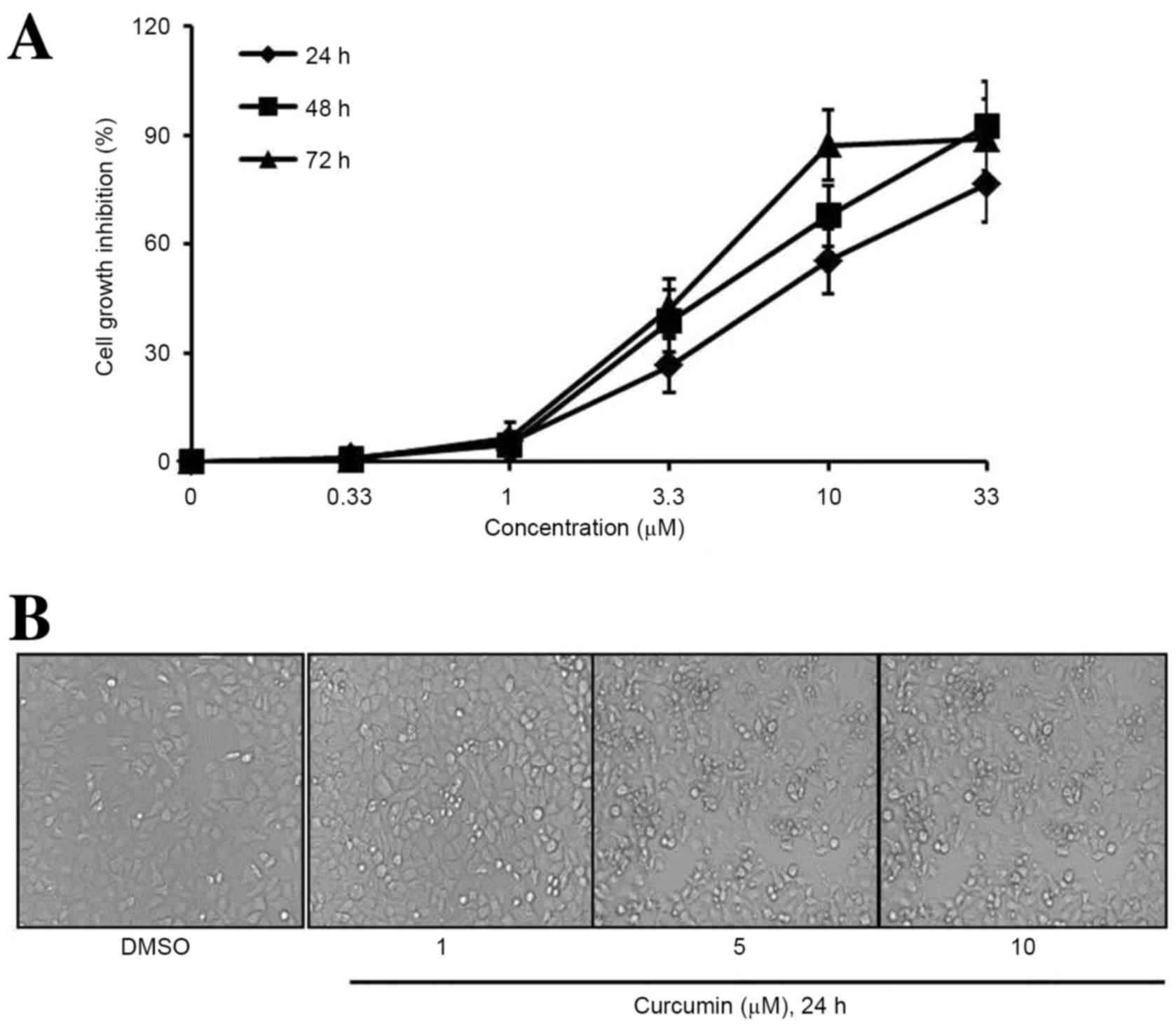 | Figure 2.Curcumin decreases the viability of
osteosarcoma cells. (A) Following treatment with 0, 0.33, 1, 3.3,
10 or 33 µM curcumin for 24, 48 or 72 h, cell viability was
determined by a cell counting kit-8 assay. Curcumin decreased cell
viability in a time- and concentration-dependent manner. (B)
Following treatment with DMSO, 1, 5 or 10 µM curcumin for 24 h,
cells were observed by a phase contrast microscope. Data are
represented as the mean ± standard deviation, n=3. |
Curcumin promotes apoptosis in
osteosarcoma cells
Flow cytometry was employed to investigate the
anti-cancer effects of curcumin on the apoptosis of MG63 cells.
Following treatment with different concentrations of curcumin (1, 5
or 10 µM) for 24 h, the apoptosis rate of cells was markedly
increased in a dose-dependent manner (Fig. 3A). The Annexin V positive cell ratios
(%) for the concentrations of 1, 5 or 10 µM curcumin were 4.6, 23.8
and 41.9%, respectively in MG63 cells. The results showed that 5 or
10 µM curcumin could significantly induce MG63 cell apoptosis
compared with the DMSO group (Fig.
3B; P<0.05 and P<0.01, respectively). To further
investigate the potential mechanisms of curcumin-induced apoptosis
in MG63 cells, the expression levels of apoptosis signaling
proteins were detected by western blot analysis. The results
indicated that the protein expression levels of cleaved caspase-3
and Bax were increased, while the expression of anti-apoptosis
proteins Bcl-2 and caspase-3 were reduced after treatment (Fig. 3C). These results indicate that the
mechanism underlying curcumin-induced apoptosis may involve the
activation of the caspase-3 pathway in MG63 cells.
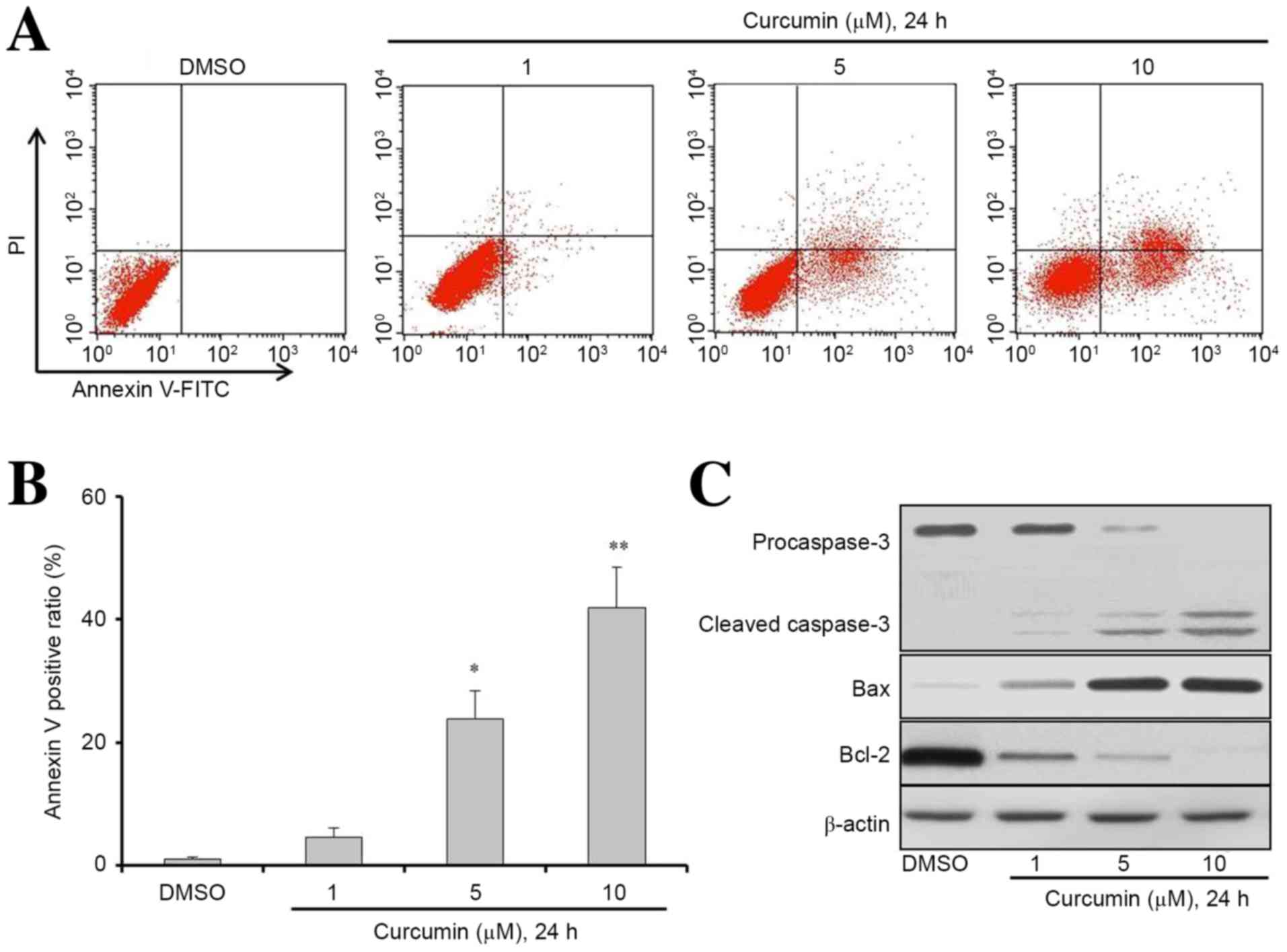 | Figure 3.Curcumin induces osteosarcoma cells
apoptosis. (A and B) MG63 cells were seeded at a density of
0.5×105 cells/dish in 60-mm cell culture dishes and
incubated for 24 h. Cells were then treated with DMSO, 1, 5 or 10
µM curcumin for 24 h. Apoptosis was determined by a FITC-labeled
Annexin V/PI apoptosis detection kit and flow cytometry.
Representative results from flow cytometry are shown. (C) Western
blot analysis was used to determine the expression levels of
apoptosis-associated proteins in cells treated with DMSO or
curcumin at different concentrations as indicated for 24 h. Cell
lysates were extracted for western blot analysis to determine the
expression of procaspase-3, cleaved-caspase-3, Bcl-2, Bax, and
β-actin (loading control). Data are presented as the mean ±
standard deviation, n=3. *P<0.05 and **P<0.01 vs. the DMSO
group. DMSO, dimethyl sulfide; Bcl-2, B-cell lymphoma 2; FITC,
fluorescein isothiocyanate; PI, propidium iodide. |
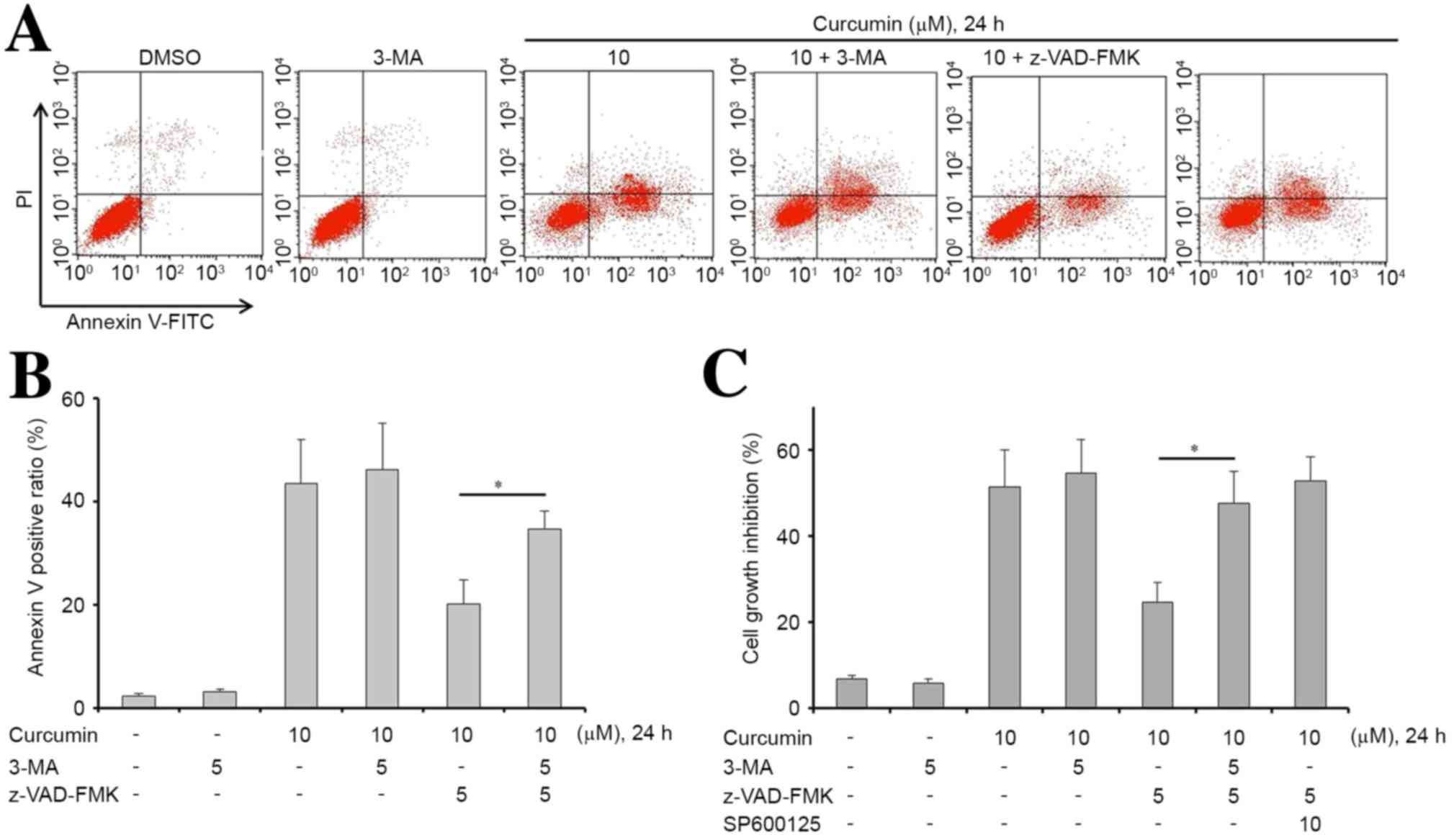
Inhibition of apoptosis enhances
curcumin-induced autophagy through the JNK signaling pathway
The current study subsequently explored whether
curcumin was able to alter the level of autophagy, which would
consequently affect cell apoptosis. The auto-fluorescent substance
MDC was used as a marker to detect the level of autophagy in MG63
cells. Following incubation with curcumin for 24 h, the percentage
of MDC-positive cells significantly increased in a dose-dependent
manner, particularly in the curcumin combined with z-VAD-FMK group
(Fig. 4A; P<0.05). These results
suggest that z-VAD-FMK was able to promote curcumin-induced
autophagy in MG63 cells.
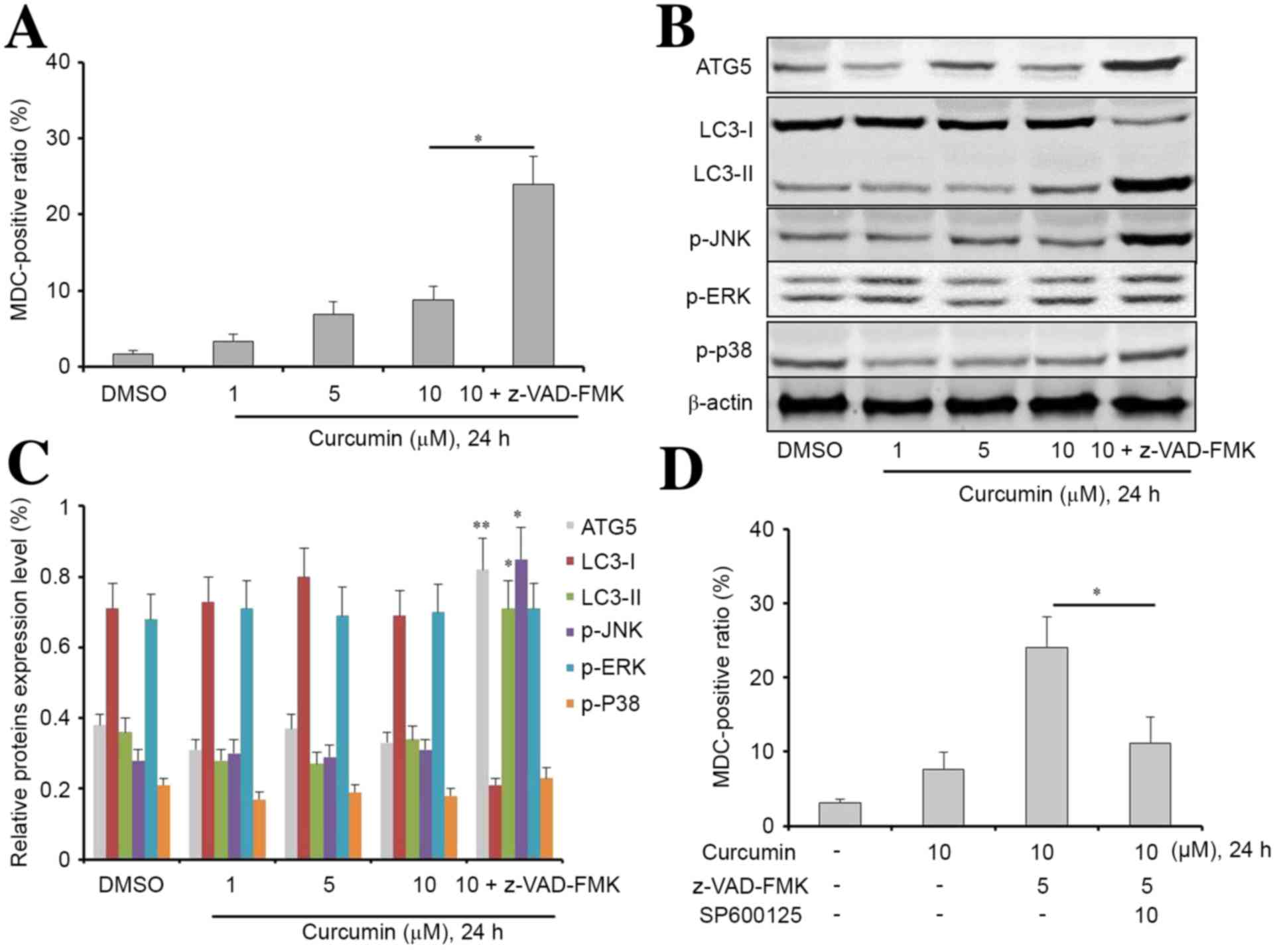 | Figure 4.Z-VAD-FMK is beneficial to the
induction of autophagy of curcumin-treated MG63 cells. (A)
Following treatment with 1, 5, 10 µM curcumin or 10 µM curcumin
combined with z-VAD-FMK for 24 h, cell autophagy was determined by
MDC assay. Curcumin increased cell autophagy in a
concentration-dependent manner. In addition, z-VAD-FMK increased
curcumin-induced autophagy in MG63 cells. (B and C) Western blot
analysis was used to determine the expression levels of
autophagy-associated proteins in cells treated with DMSO, curcumin
at different concentrations or curcumin combined with z-VAD-FMK (as
indicated) for 24 h. Cell lysates were extracted for western blot
analysis to assess the expression of ATG5, LC3-I, LC3-II, p-JNK,
p-ERK, p-p38. β-actin was used as a loading control. *P<0.05 and
**P<0.01 vs. the DMSO group. (D) p-JNK inhibitor SP600125
turnover z-VAD-FMK increased curcumin-induced autophagy in MG63
cells. Data are presented as the mean ± standard deviation, n=3.
*P<0.05 and **P<0.01. MDC, monodansylcadaverine; ATG5,
autophagy related 5; LC3-I, light chain 3-I; LC3-II, light chain
3-II; p-JNK, phosphorylated c-Jun N-terminal kinase 3; p-ERK,
phosphorylated extracellular signal-regulated kinase. |
Next, we extracted the total protein from MG63 cells
incubated with different concentrations of curcumin or with the
combination treatment for 24 h. The expression levels of ATG5 LC3-I
LC3-2, p-JNK, p-ERK, and p-P38 were analyzed by western blotting.
As shown in Fig. 4B and C, compared
with the other groups, there was a significant increase in the
expression levels of ATG5 (P<0.01), LC3-II (P<0.05), and
p-JNK (P<0.05) in the curcumin combined with z-VAD-FMK group. In
addition, there was a significant reduction in the expression
levels of LC3-I in the curcumin combined with z-VAD-FMK group
compared with the other groups. Notably, JNK inhibitor SP600125
effectively reversed combination treatment-induced autophagy in
MG63 cells, suggesting that JNK pathway signaling may have an
important role in curcumin-induced autophagy (Fig. 4D).
Inhibition of autophagy enhances
curcumin-induced apoptosis in MG63 cells
Subsequently, in order to examine whether the
inhibition of autophagy sensitizes MG63 cells to curcumin, cells
were treated with curcumin, z-VAD-FMK or in combination with 3-MA,
which is an autophagy inhibitor. Treatments with these drugs,
either alone or in combination, significantly affected the
apoptosis of MG63 cells (P<0.05). The results showed that 3-MA
alone did not induce apoptosis or inhibit proliferation in MG63
cells (Fig. 5). However, an
accumulation of apoptotic cells was observed upon treatment with
curcumin, z-VAD-FMK and 3-MA, compared with treatment with curcumin
and z-VAD-FMK. This result indicated that curcumin-induced
apoptosis was efficiently increased via inhibition of autophagy
(Fig. 5A and B). Furthermore, 3-MA
or SP600125 effectively reversed z-VAD-FMK-induced proliferation in
curcumin-treated MG63 cells (Fig.
5C).
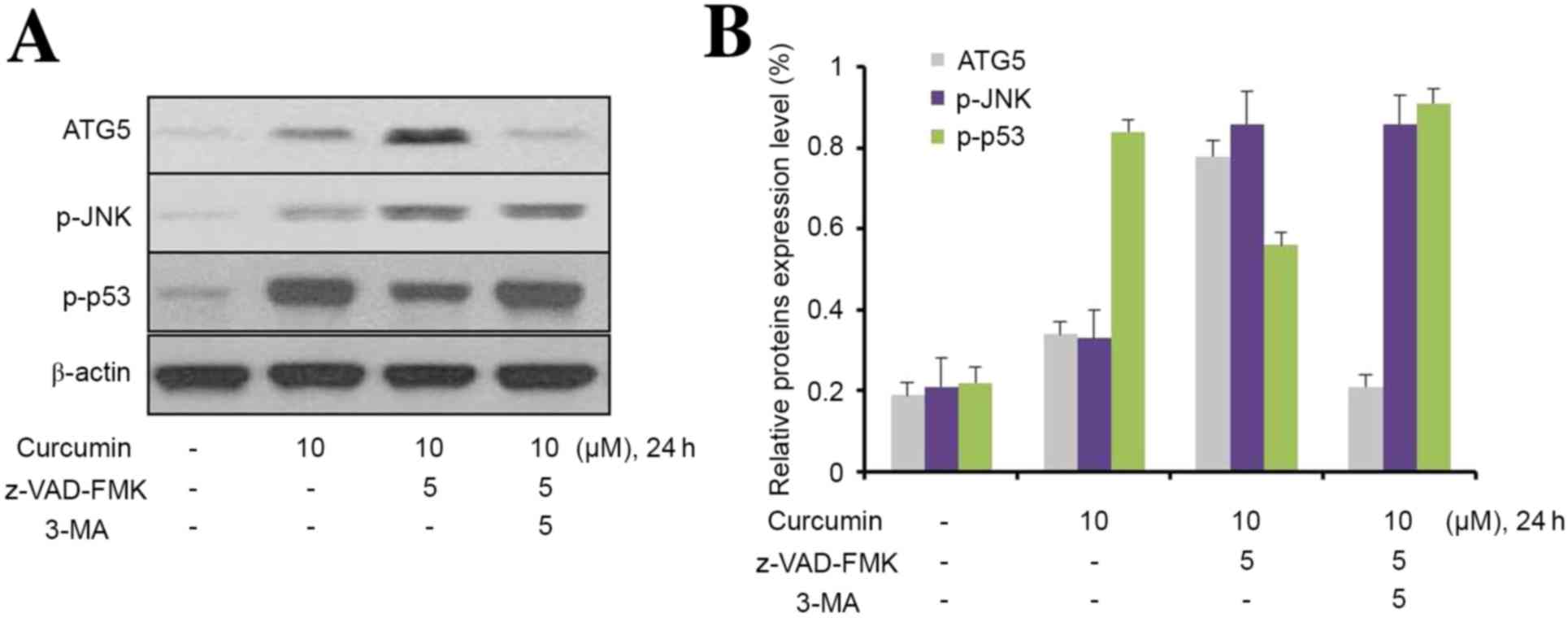
3-MA inhibits curcumin-induced
hyperactive of p-JNK/autophagy pathway
To further explore the role of JNK in
curcumin-induced autophagy, MG63 cells were used to investigate the
effect of 3-MA on autophagic activity. As shown in Fig. 6, curcumin upregulated ATG5, p-JNK,
and p53 expression levels in MG63 cells; however, z-VAD-FMK only
upregulated the expression of ATG5 and p-JNK in 3-MA-treated cells.
Furthermore, 3-MA effectively prevented the upregulation of ATG5
and p-JNK in MG63 cells induced by the combination of curcumin and
z-VAD-FMK. Notably, z-VAD-FMK markedly downregulated p-P53
expression in curcumin-treated MG63 cells, and this phenomenon was
inhibited by 3-MA.
Discussion
Given that osteosarcoma is characterized by adjuvant
chemotherapy resistance and high rates of recurrence after curative
resection (18), it is necessary to
develop novel therapeutic agents to achieve improved patient
prognoses. Curcumin is a natural compound derived from turmeric
(Curcuma longa) and exhibits an effective antitumor effect
on various cancers, including osteosarcoma (19). In addition, curcumin can reverse
chemotherapy resistance in various types of human cancer (7,20,21).
Therefore, curcumin may be a promising agent for the treatment of
osteosarcoma.
Several studies have confirmed that chemotherapy
agents, including tamoxifen, 5-fluorouracil, and rapamycin, are
able to induce autophagy (22–24).
However, the mechanisms underlying autophagy in cancer therapy are
complex and remain controversial. It has been reported that forced
autophagy may be an apoptotic enhancer or a survival enhancer,
depending on the experimental conditions (22,25).
Attempts have been made to elucidate the potential mechanisms
involved in autophagy, so that it may be exploited as a target for
cancer therapy. The current study showed that the expression levels
of the anti-apoptotic protein Bcl-2 were significantly decreased in
MG63 cells incubated with curcumin. The results of the present
study suggest that inhibition of the Bcl-2-mediated anti-apoptotic
pathway may contribute to curcumin-induced apoptosis in MG63
cells.
Autophagy has been confirmed to have two contrasting
roles in cancer, in that it is both a tumor suppressor and a tumor
protector (26). Recently, autophagy
has been reported as a mechanism by which osteosarcoma cells
develop resistance to anti-tumor agents, including cisplatin and
doxorubicin (27,28). Dysregulation of the p38 MAPK
signaling pathway has an important role in tumor development and
progression (29). In addition, p38
MAPK also mediates autophagy in response to anti-cancer agents in
the treatment of cancer. Furthermore, p38 MAPK acts both as a
positive and negative regulator in the autophagy process (29). JNK is initially activated in response
to various stress signals and has been observed to participate in
numerous cellular events including autophagy (30). The current study demonstrated that
treatment with curcumin is correlated with increased
phosphorylation of JNK in MG63 cells. JNK displays both
tumor-promoting and tumor-suppressive functions depending on the
genetic context of the tumor cells (31). The JNK pathway has been shown to be
important in enforcing autophagy and apoptosis (32). Previous studies have further revealed
that elevated JNK signaling is also associated with autophagy
induction (32,33). The present study also found that JNK
inhibitor SP600125 was able to reverse curcumin-induced autophagy.
These results suggest that the JNK pathway has a critical role in
curcumin-induced autophagy.
The ERK signaling pathway has an important role in
cancer development and progression. In addition, ERK activity has
been confirmed to participate in autophagy and autophagic cell
death (34). Notably, in human
ovarian cancer cells, cytoplasmic sequestration of ERK by PEA-15
has been confirmed to promote autophagy (34). Furthermore, forced ERK activation by
overexpression of activated MEK can promote autophagy without any
other stimuli (35). However, there
was no apparent change in p-ERK expression in osteosarcoma MG63
cells treated with curcumin, suggesting curcumin does not affect
the ERK signaling pathway.
Autophagy and apoptosis are well-regulated
biological processes that have important roles in tumor development
and progression. The role of the anti-apoptotic protein Bcl-2 in
autophagy has been explored, and it has been proposed to be a major
binding partner of Beclin-1, or to directly inhibit components of
the autophagic pathway proteins, Bax and Bak (36,37). As
previously demonstrated, p53 also participates in the regulation of
autophagy. Nuclear p53 can induce autophagy through transcriptional
effects, while cytoplasmic p53 may act as a master repressor of
autophagy (38). It has been
confirmed that p53 stimulates autophagy by transactivation of
damage-regulated autophagy modulator or through the inhibition of
the mTOR signaling pathway via activation of the AMP kinase
(39,40). Furthermore, the present results
revealed that the autophagy inhibitor 3-MA was able to increase
curcumin-induced apoptosis in MG63 cells. The present study also
confirmed that 3-MA was able to reverse curcumin-induced
upregulation of p-JNK and ATG5, a gene that is essential to the
process of autophagy. In addition, 3-MA also upregulated
curcumin-induced p53 expression. These results also ascertained
that the JNK pathway has a critical role in curcumin-induced
autophagy.
In conclusion, the present study demonstrated that
curcumin inhibited the growth and induced the apoptosis of human
osteosarcoma cells. The effects of curcumin-induced apoptosis in
osteosarcoma cells were associated with caspase-3 activation and
reduced the levels of Bcl-2 expression. In addition, curcumin
induces both apoptosis and autophagy in osteosarcoma cells.
Furthermore, curcumin-induced autophagy has an anti-apoptotic role
in osteosarcoma cells. These results provide important insight into
the interaction between apoptosis and autophagy in osteosarcoma
cells, and provides further information regarding the clinical
treatment strategies using curcumin.
Glossary
Abbreviations
Abbreviations:
|
JNK
|
c-Jun N-terminal kinase
|
|
3-MA
|
3-methyladenine
|
|
MDC
|
monodansylcadaverine
|
|
ATG5
|
autophagy related 5
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
LC3
|
microtubule associated protein 1 light
chain 3
|
References
|
1
|
Anderson P and Salazar-Abshire M:
Improving outcomes in difficult bone cancers using multimodality
therapy, including radiation: Physician and nursing perspectives.
Curr Oncol Rep. 8:415–422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dieudonne FX, Marion A, Hay E, Marie PJ
and Modrowski D: High Wnt signaling represses the proapoptotic
proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res.
70:5399–5408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shakibaei M, Kraehe P, Popper B, Shayan P,
Goel A and Buhrmann C: Curcumin potentiates antitumor activity of
5-fluorouracil in a 3D alginate tumor microenvironment of
colorectal cancer. BMC Cancer. 15:2502015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao F, Kang J, Cao Y, Fan S, Yang H, An Y,
Pan Y, Tie L and Li X: Curcumin attenuates palmitate-induced
apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and
mitochondrial survival pathways. Apoptosis. 20:1420–1432. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang
S and Su S: Curcumin improves the tumoricidal effect of mitomycin C
by suppressing ABCG2 expression in stem cell-like breast cancer
cells. PLoS One. 10:e01366942015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van't Land B, Blijlevens NM, Marteijn J,
Timal S, Donnelly JP, de Witte TJ and M'Rabet L: Role of curcumin
and the inhibition of NF-kappaB in the onset of
chemotherapy-induced mucosal barrier injury. Leukemia. 18:276–284.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yosifov DY, Kaloyanov KA, Guenova ML,
Prisadashka K, Balabanova MB, Berger MR and Konstantinov SM:
Alkylphosphocholines and curcumin induce programmed cell death in
cutaneous T-cell lymphoma cell lines. Leuk Res. 38:49–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou QM, Wang XF, Liu XJ, Zhang H, Lu YY,
Huang S and Su SB: Curcumin improves MMC-based chemotherapy by
simultaneously sensitising cancer cells to MMC and reducing
MMC-associated side-effects. Eur J Cancer. 47:2240–2247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmukler E, Kloog Y and Pinkas-Kramarski
R: Ras and autophagy in cancer development and therapy. Oncotarget.
5:577–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou YY, Li Y, Jiang WQ and Zhou LF:
MAPK/JNK signalling: A potential autophagy regulation pathway.
Biosci Rep. 35:pii: e001992015.
|
|
15
|
Zhang P, Zhang P, Zhou M, Jiang H, Zhang
H, Shi B, Pan X, Gao H, Sun H and Li Z: Exon 4 deletion variant of
epidermal growth factor receptor enhances invasiveness and
cisplatin resistance in epithelial ovarian cancer. Carcinogenesis.
34:2639–2646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma H, Chen H, Guo X, Wang Z, Sowa ME,
Zheng L, Hu S, Zeng P, Guo R, Diao J, et al: M phase
phosphorylation of the epigenetic regulator UHRF1 regulates its
physical association with the deubiquitylase USP7 and stability.
Proc Natl Acad Sci USA. 109:pp. 4828–4833. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hui B, Shi YH, Ding ZB, Zhou J, Gu CY,
Peng YF, Yang H, Liu WR, Shi GM and Fan J: Proteasome inhibitor
interacts synergistically with autophagy inhibitor to suppress
proliferation and induce apoptosis in hepatocellular carcinoma.
Cancer. 118:5560–5571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wunder JS, Gokgoz N, Parkes R, Bull SB,
Eskandarian S, Davis AM, Beauchamp CP, Conrad EU, Grimer RJ, Healey
JH, et al: TP53 mutations and outcome in osteosarcoma: A
prospective, multicenter study. J Clin Oncol. 23:1483–1490. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang Z, Xing J and Yu X: Curcumin induces
osteosarcoma MG63 cells apoptosis via ROS/Cyto-C/Caspase-3 pathway.
Tumour Biol. 35:753–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu WD, Qin Y, Yang C, Li L and Fu ZX:
Effect of curcumin on human colon cancer multidrug resistance in
vitro and in vivo. Clinics (Sao Paulo). 68:694–701. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye MX, Zhao YL, Li Y, Miao Q, Li ZK, Ren
XL, Song LQ, Yin H and Zhang J: Curcumin reverses cis-platin
resistance and promotes human lung adenocarcinoma A549/DDP cell
apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine.
19:779–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Wu D, Shen J, Zhou M and Lu Y:
Rapamycin induces autophagy in the melanoma cell line M14 via
regulation of the expression levels of Bcl-2 and Bax. Oncol Lett.
5:167–172. 2013.PubMed/NCBI
|
|
24
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salazar M, Carracedo A, Salanueva IJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pandolfi PP: Breast cancer-loss of PTEN
predicts resistance to treatment. N Engl J Med. 351:2337–2338.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao XD, Cao L, Zhang Q, Hu XY and Zhang
Y: Effect of PI3K-mediated autophagy in human osteosarcoma MG63
cells on sensitivity to chemotherapy with cisplatin. Asian Pac J
Trop Med. 8:731–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang Z, Huo L, Li K, Wu Y and Hu Z:
Blocked autophagy by miR-101 enhances osteosarcoma cell
chemosensitivity in vitro. ScientificWorldJournal. 2014:7947562014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyriakis JM, Banerjee P, Nikolakaki E, Dai
T, Rubie EA, Ahmad MF, Avruch J and Woodgett JR: The
stress-activated protein kinase subfamily of c-Jun kinases. Nature.
369:156–160. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang C, Chen K, Xia Y, Dai W, Wang F, Shen
M, Cheng P, Wang J, Lu J, Zhang Y, et al: N-acetylcysteine
attenuates ischemia-reperfusion-induced apoptosis and autophagy in
mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS One.
9:e1088552014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 03:239–252. 2000. View Article : Google Scholar
|
|
34
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corcelle E, Nebout M, Bekri S, Gauthier N,
Hofman P, Poujeol P, Fénichel P and Mograbi B: Disruption of
autophagy at the maturation step by the carcinogen lindane is
associated with the sustained mitogen-activated protein
kinase/extracellular signal-regulated kinase activity. Cancer Res.
66:6861–6870. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lindqvist LM, Heinlein M, Huang DC and
Vaux DL: Prosurvival Bcl-2 family members affect autophagy only
indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci USA.
111:pp. 8512–8517. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tasdemir E, Maiuri M Chiara, Morselli E,
Criollo A, D'Amelio M, Djavaheri-Mergny M, Cecconi F, Tavernarakis
N and Kroemer G: A dual role of p53 in the control of autophagy.
Autophagy. 4:810–814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:pp. 8204–8209. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|