Introduction
Human bocavirus (HBoV) is classified in the
Bocavirus genus within the Parvoviridae family, and was
first identified in children with respiratory diseases (1). HBoV infections have been observed
worldwide not only in respiratory tract secretions but also in
urine, fecal and serum samples (2,3). Newly
identified HBoV including HBoV2, HBoV3 and HBoV4 were identified in
human stool samples. Previous findings suggest that HBoV is
associated with human diseases and notable pathogenesis; however,
there is an inadequate amount of evidence to support this due to
the limited establishment of in vitro HBoV culture systems
and animal models (4). HBoV encodes
three nonstructural proteins including: NP1, NS1 and NS1-70, in
addition to two structural proteins, VP1 and VP2. HBoV NP1 is a
nuclear protein, which has an important role in the DNA replication
process of HBoV within the nuclei of infected cells (5,6). It has
been reported that NP1 is able to cause cell cycle arrest at G2/M
phase followed by apoptosis, via the mitochondrion pathway in HeLa
cells (7). Infection with Bocavirus
minute virus of canines (MVC) has also been demonstrated to induce
apoptosis, dependent on the replication of the viral genome and
arrest G2/M phase in Walter Reed/3873D (WRD) canine cells (8).
The multiple integration of biological and
pathological progresses, such as proliferation, differentiation,
apoptosis and metabolism, is able to affect the pathogenesis of
human diseases. Advanced understanding of the cellular and
molecular mechanism of these progresses is important for developing
novel diagnostic and therapeutic targets. Previous studies have
indicated the cellular bases and roles of autophagy in human health
and disease world-wide (9,10). Autophagy is responsible in the in
vivo protection against human pathogens that are degraded in
vitro by bacteria, viruses and parasites (11,12).
Autophagy-related proteins (ATGs), are conserved in mammalian cells
and have been indicated to be essential components of the
autophagic progress (13). The
ubiquitin-like conjugation of ATG5-ATG12 contributes to
autophagosome formation and induces LC3 lipidation (LC3I) (10,14). The
conversion of cytosolic LC3I to phosphatidylethanolamine-conjugated
form (LC3II), a key marker for the autophagosome, indicates the
formation of autophagosome (15).
SQSTM1, also referred to as p62 protein, is decreased in the
presence of autophagy and accumulated in the absent of autophagy,
suggesting SQSTM1 may be a marker for autophagy (16).
The molecular mechanism by which HBoV induces
apoptosis and autophagy is not yet well understood. Previous
studies have demonstrated that celecoxib and caffeine induced
autophagy through inhibition of the PI3K/ATK signaling pathway
(17,18). The PI3K/AKT signaling pathway is
widely utilized in normal and abnormally activated cells, such as
in cancer cells (19). Activation of
AKT triggers phosphorylation of downstream targets which impacts
cell proliferation, apoptosis, migration and autophagy progresses
(20). In the present study, human
bronchial epithelial cells (HBECs) were used to examine the effect
of HBoV on cell proliferation, apoptosis and autophagy, as well as
the mechanism involved. We propose a model of positive regulation
of autophagy as part of the host response to HBoV infection in
HBEC.
Materials and methods
Cell culture
Human bronchial epithelial cells (HBECs; Institute
of Biochemistry and Cell Biology, Shanghai, China) at
1×105 cells/well were grown on rat-tail collagen
I-coated dishes and incubated at 37°C in a humidified chamber
containing 5% CO2 for 12 h. Subsequently, HBEC were
changed to grow in bronchial epithelial cell basal medium
(Clonetics, Co., San Diego, CA, USA) supplemented with 100 mg/ml
penicillin G and 50 µg/ml streptomycin, 0.5 µg/l human epidermal
growth factor and 50 mg/l bovine pituitary extract (all obtained
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
in tissue culture flasks and expanded in the same growth medium.
All HBECs were cultured using an incubator and maintained in a
humidified atmosphere containing 5% CO2 at 37°C.
Lentiviral production and
transduction
The HBoV coding sequence was cloned into a
pBluescript SKII vector donated from Yi Li from the Wuhan
Engineering Institute (Wuhan, China). The HBoV recombination
expression vector was referred to as pWHL-1. Constructs were
subsequently transfected into HEK 293T cells using Lipofectamine
2000 according to the manufacturer's instructions. Viruses were
collected following 48 h transfections and HBECs were infected.
HBECs without transfection were used as a control and HBEC with the
empty PLKO.1-EGFP vector transfection was used as the negative
control (NC).
Cell proliferation assay
HBECs (3×103 cells/well) transfected with
or without 50 nM pWHL-1 were harvested and plated in 96-well
plates. A total of 10 µl Cell Counting assay kit-8 (CCK-8) solution
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocol, was added to each well and the
absorbance was measured at 450 nm using a microplate reader.
Apoptosis assay
HBECs (3×103 cells/well) transfected with
or without 50 nM pWHL-1 were harvested and plated in 96-well
plates. A cell fixation and permeabilization kit (cat. no.
ab185917; Abcam, Cambridge, UK) was used to fix the HBECs in
suspension and then permeabilizing the cell membranes, according to
the manufacturer's protocol. Subsequently, cells were washed with
PBS for 5 min at 25°C, harvested and stained with 195 µl Annexin
V-FITC and 5 µl propidium iodide (PI; BD Biosciences, San Jose, CA,
USA) for 15 min in the dark at room temperature followed by flow
cytometry analysis using BD Accuri™ C6, version
1.0.264.21 software (BD Biosciences, San Jose, CA, USA).
Immunohistochemistry
HBECs transfected with or without 50 nM pWHL-1 were
fixed with 10% formaldehyde for 48 h at 25°C and blocking of
endogenous peroxidases was completed by soaking slides in a
solution of 90% methanol/3% H2O2 for 10 min
at 37°C. For antigen retrieval, the HBECs were microwaved in 10 mM
citrate buffer (pH 6.0) at 95°C for 10 min. HBECs were then
incubated with proliferating cell nuclear antigen (PCNA) rabbit
monoclonal antibody (cat. no. ab18197; Abcam; 1:1,000) for 1 h at
room temperature after blocking non-specific binding with 10%
normal goat serum (cat. no. 005-000-121; Qcbio Science &
Technologies Co., Ltd., Shanghai, China) in PBS at 37°C for 30 min,
followed by incubation with goat anti-rabbit biotin-conjugated IgG
(cat. no. ab181744; Abcam; 1:1,000) at 25°C for 30 min. HBECs were
stained with 3,3′-diaminobenzidine (Shanghai Long Island Biotec.,
Co., Ltd., Shanghai, China) and hematoxylin staining
(Sigma-Aldrich; Merck KGaA). Immunohistochemical signals were
calculated with the positive staining of cells using a light
microscope (CX41RF; Olympus Corporation, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HBECs transfected with
or without pWHL-1 using TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific Inc.). A total of 1 µg RNA was reverse transcribed to
synthesize cDNA using Primescript RT Reagent (Takara Biotechnology
Co., Ltd., Dalian, China). DNaseI treatment was used to remove
genomic DNA. RNA-Primer Mix (12 µl), 5xRT Reaction Buffer (5 µl),
25 mM dNTPs (1 µl), 25 U/µl RNase Inhibitor (1 µl), 200 U/µl M-MLV
Rtase (1 µl), Oligo(dt)18 (1 µl) and ddH2O
(DNase-free; 4 µl). SYBR-Green qPCR Master Mix (X2; Fermentas;
Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) was used to
Real-time PCR performed on an ABI 7500 Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). SYBRGreen Mix (12.5 µl), forward primer (0.5 µl), reverse
primer (0.5 µl), ddH2O (9.5 µl), cDNA (2 µl). The PCR
cycling conditions were as follows: 95°C for 10 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 45 sec, and a final
extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec
and 60°C for 15 sec. The primers are listed in Table I. GAPDH mRNA was used as internal
control. mRNA expression levels were calculated by the comparative
ΔΔCq method and the fold changes were analyzed by
2−ΔΔCq (21).
The experiment was repeated three times.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene | Primer sequences |
|---|
| LC3I-forward |
5′-TCCGACCGGCCTTTCAAGCAG-3′ |
| LC3I-reverse |
5′-GAGAACCTGACCAGAACTCCCAG-3′ |
| LC3II-forward |
5′-GGAAAGCAGCAGTGTACC-3′ |
| LC3II-reverse |
5′-CTTTAAGCCGGAAGGCAG-3′ |
| ATG5-forward |
5′-GGCTGAGTGAACATCTGAG-3′ |
| ATG5-reverse |
5′-CCCAGTTGCCTTATCTGAC-3′ |
| SQSTM1-forward |
5′-GGAGTCGGATAACTGTTC-3′ |
| SQSTM1-reverse |
5′-GATTCTGGCATCTGTAGG-3′ |
| GAPDH-forward |
5′-CACCCACTCCTCCACCTTTG-3′ |
| GAPDH-reverse |
5′-CCACCACCCTGTTGCTGTAG-3′ |
Western blot analysis
Total proteins were extracted from HBEC transfected
with or without pWHL-1 using RIPA buffer containing 50 mM Tris-HCl,
(pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 2 mM
phenylmethylsulfonyl fluoride, phosphatase and protease inhibitor
cocktail (CalbioChem; Merck KGaA) at 4°C for 20 min, followed by
centrifugation at 12,000 × g for 1 min at 25°C. A total of 30 µl
protein was separated using 12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Membranes were blocked in
fat-free milk overnight at 4°C following three washes with
Tris-buffered saline with Tween-20 (Amresco, LLC, Solon, OH, USA)
for 5 min at 25°C and subsequently incubated with primary
antibodies at 4°C overnight. Antibodies used in western blot
analysis were as follows: Rabbit monoclonal antibodies for LC3 I/II
(cat. no. 4108; 1:1,000; CST Biological Reagents Company Limited,
Shanghai, China), SQSTM (cat. no. ab109012; 1:10,000), ATG5 (cat.
no. ab109490; 1:1,000), caspase-3 (cat. no. ab32042; 1:1,000),
Bcl-2 (cat. no. ab32124; 1:1,000), Bax (cat. no. ab320503; 1:500),
p-p53 (cat. no. ab1431; 1:1,000), p-AKT (cat. no. ab38449;
1:1,000), AKT (cat. no. ab8805; 1:500; all purchased from Abcam),
GAPDH (cat. no.5174; CST Biological Reagents Company Limited;
1:1,500), and mouse monoclonal antibody for p53 (cat. no. ab1101;
Abcam; 1:1,000). Membranes were subsequently washed three times
with Tris-buffered saline with Tween-20 (Amresco, LLC), and the
peroxidase-conjugated goat anti-rabbit/mouse secondary antibody
(1:1,000; cat. no. A0208 and A0216; Beyotime Institute of
Biotechnology, Haimen, China) was incubated at 37°C for 1 h and
washed three times with Tris-buffered saline with Tween-20
(Amresco, LLC). Immunoreactivity was detected with enhanced
chemiluminescence (Merck Millipore; Merck KGaA) and signals were
quantified by densitometry (Quantity One software version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). GAPDH mRNA was used
as internal control and the experiment was repeated in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation.
The paired, two-tailed Student's t-test was used to analyze the
significance of difference between groups. Each experiment was
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
HBoV suppresses HBEC
proliferation
To investigate the biological significance of HBoV
in HBEC, HBoV stably expressing cell lines of HBEC were established
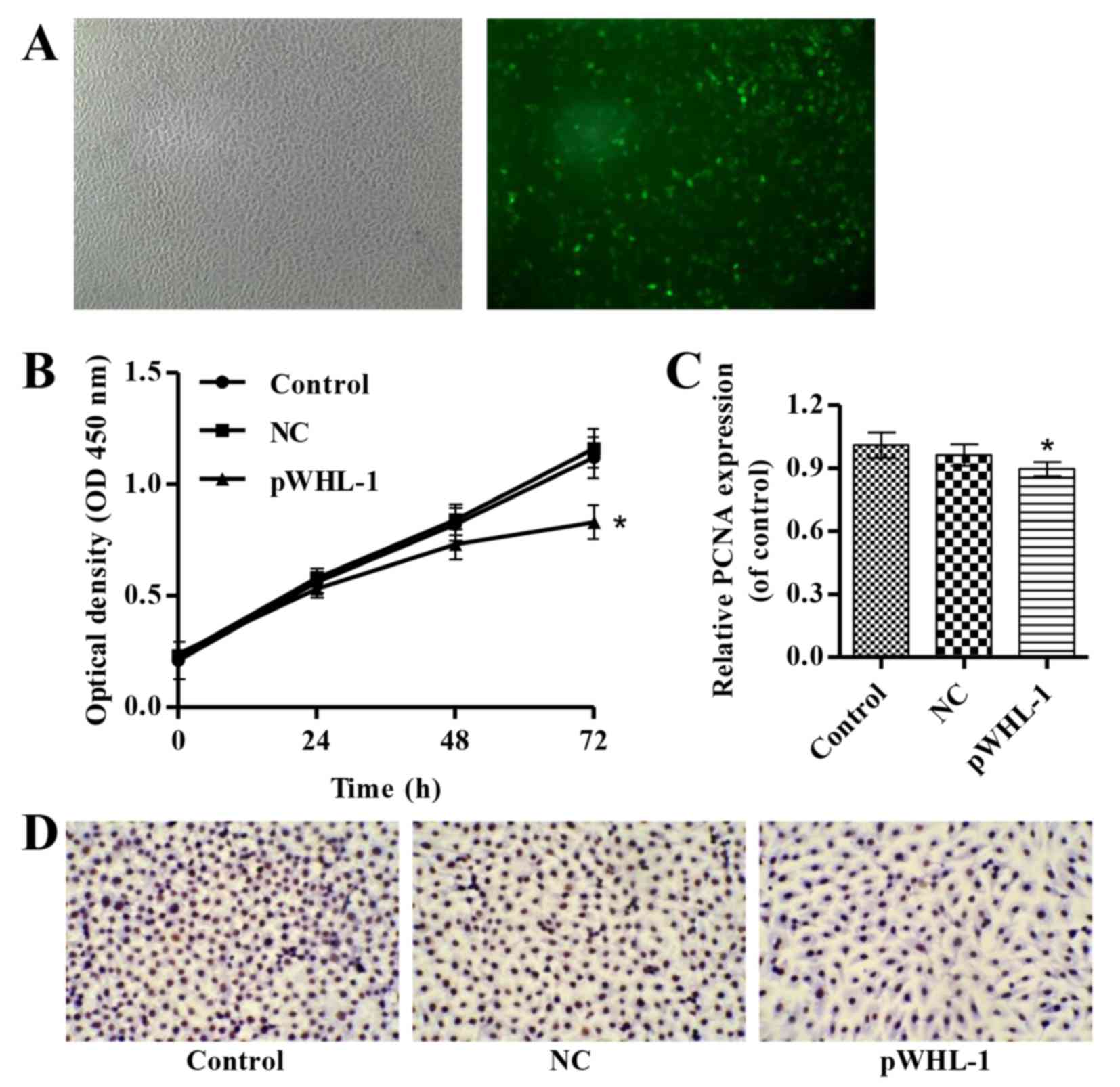
by pWHL-1 transfection. As indicated in Fig. 1A, fluorescence microscopy evaluation
demonstrated a transfection efficiency in HBECs following
transfection with pWHL-1. Furthermore, pWHL-1 transfection
significantly reduced the proliferation of HBEC when compared with
the control and NC groups (P<0.05; Fig. 1B). Immunohistochemistry analysis of
PCNA, a marker of cell proliferation, indicated that PCNA exhibits
significantly decreased levels in pWHL-1 transfected HBEC when
compared with the NC (P<0.05; Fig. 1C
and D).
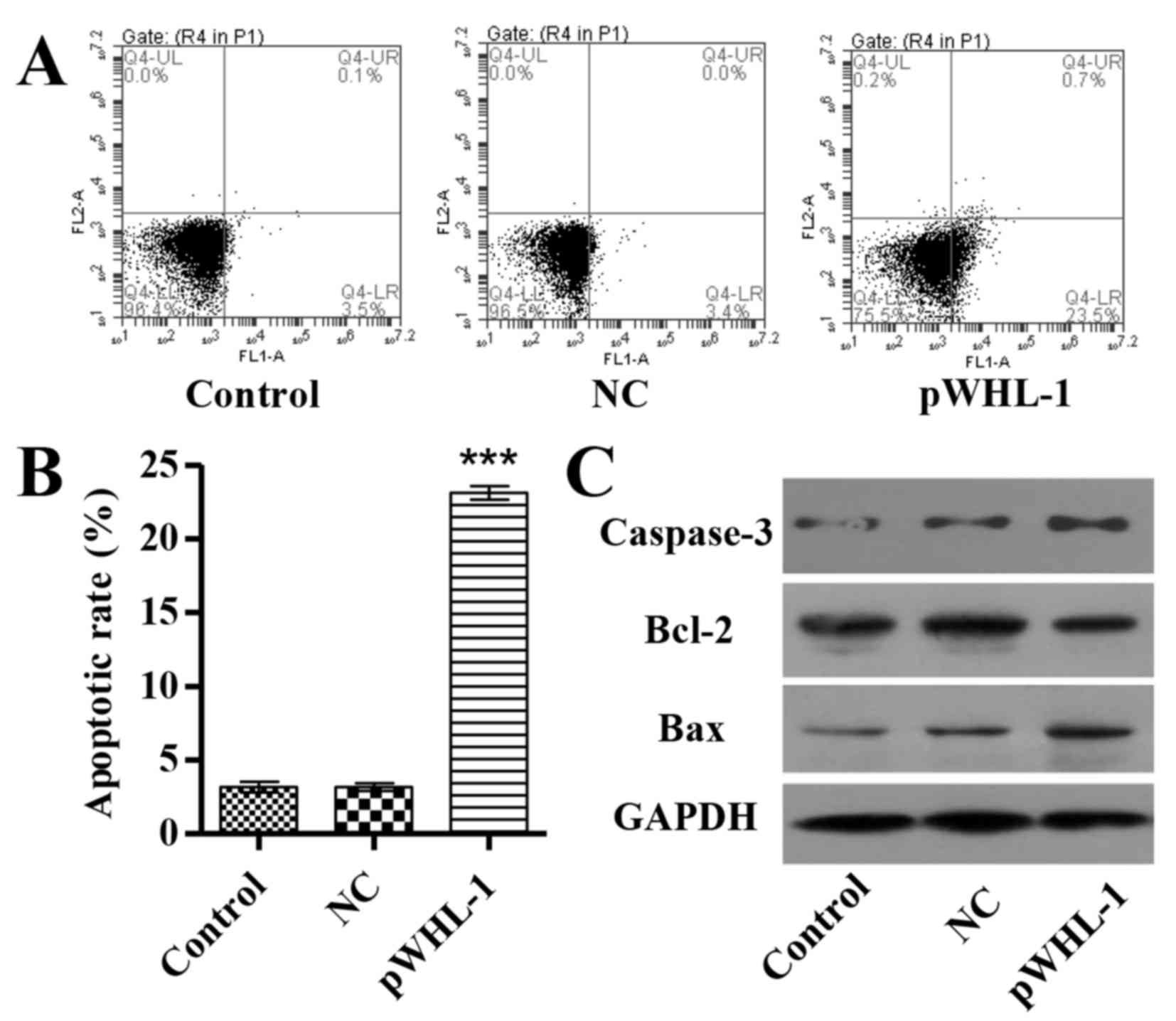
HBoV promotes HBEC apoptosis
A total of 48 h after transfection, apoptosis of
HBEC was investigated by Annexin V-FITC/PI staining and flow
cytometry analysis. The results revealed that pWHL-1 transfection
significantly induced HBEC apoptosis by 6.3-fold when compared with
the NC groups (P<0.001; Fig. 2A and
B). To further investigate the mechanism of HBoV associated
with apoptosis of HBEC, three apoptosis associated proteins were
also detected in HBEC. Furthermore, the protein expression level of
B cell lymphoma (Bcl-2) was significantly decreased (P<0.01),
whereas the protein expression levels of Bcl-2 associated X (Bax,
P<0.01) and caspase-3 were significantly increased in HBEC with
pWHL-1 transfection, whereas in pWHL-1 transfected HBEC (P<0.01;
Fig. 2C).
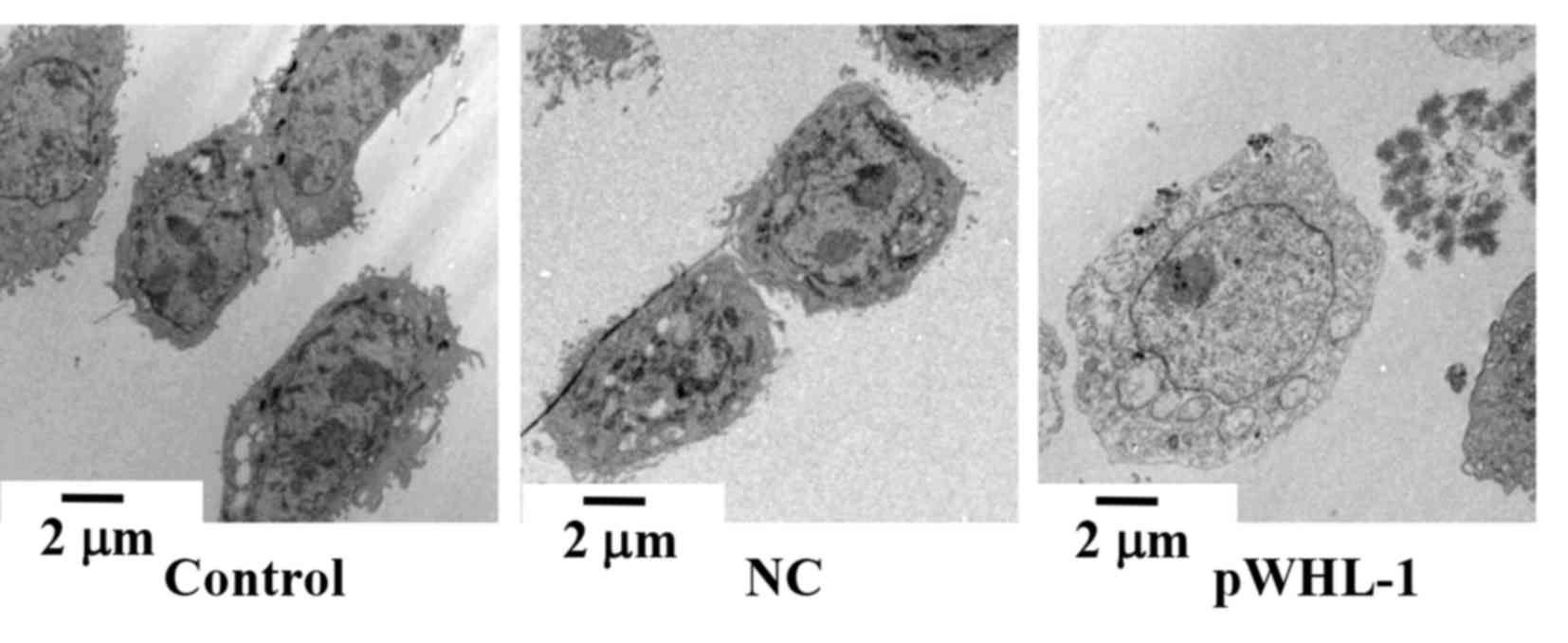
HBoV induces the autophagy progress in
HBEC
To investigate the effect of HBoV on autophagy of
HBEC, a transmission electron microscope was used to observe the
cellular morphology of HBEC. The results indicated that pWHL-1
transfected HBEC possessed disordered nuclei, damage of the
cellular membrane of organelles, including the mitochondria, and a
large number of autophagic vacuoles and autophagosomes in
cytoplasm. In comparison with the pWHL-1 transfected HBEC, the
control and NC groups showed normally ordered nuclei, abundant and
complete cellular membrane of organelles, such as mitochondria, and
a fewer number of autophagic vacuoles and autophagosome in
cytoplasm (Fig. 3).
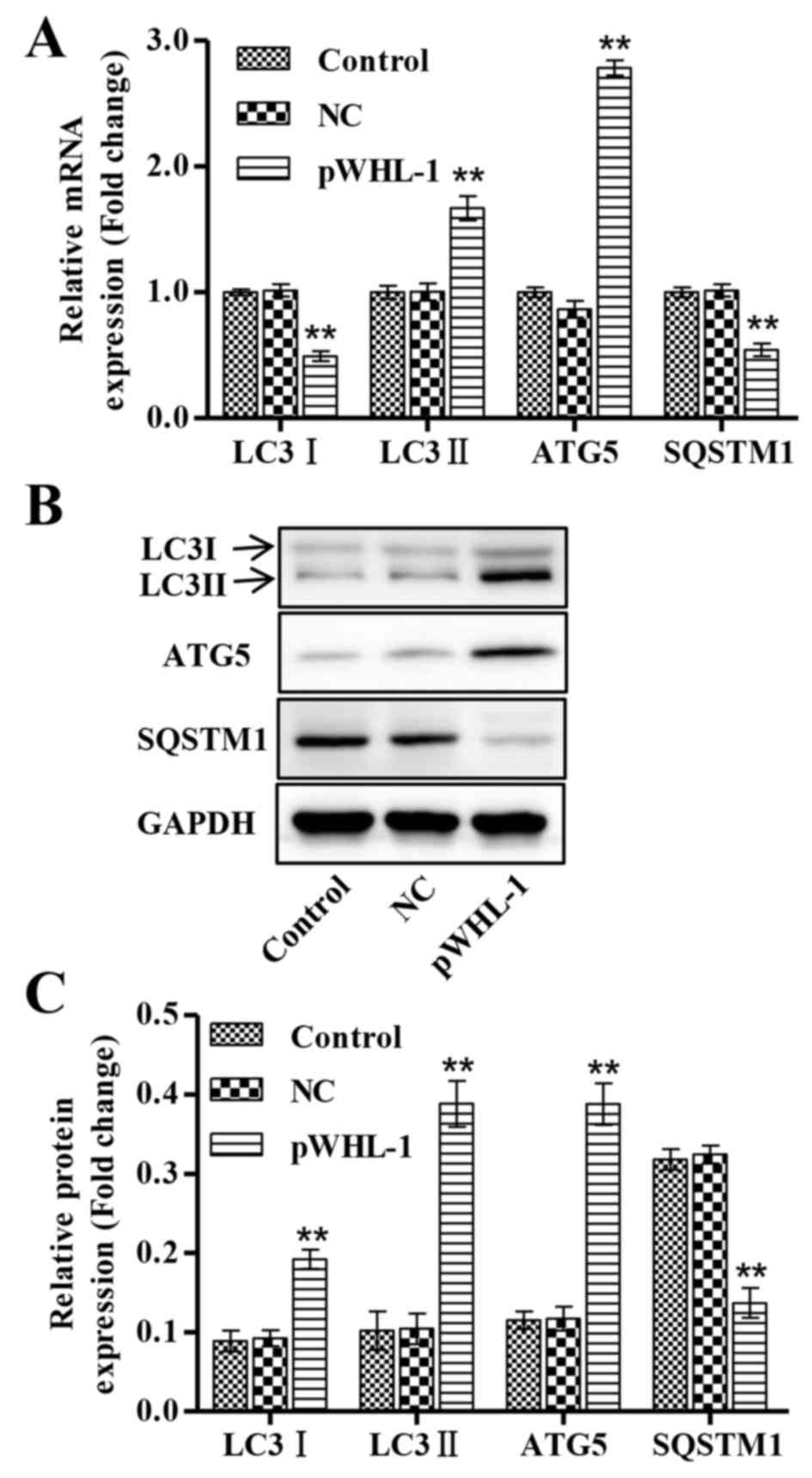
Subsequently it considered whether HBoV functionally
regulates the autophagy-associated protein in HBEC. To address this
question, RT-qPCR and western blot analysis was performed to detect
the mRNA and protein expression levels of core proteins involved in
the autophagy progress, respectively. These results showed that the
mRNA expression levels of LC3I and SQSTM1 were significantly
decreased in pWHL-1 transfected HBEC when compared with the control
and NC groups, whereas the mRNA expression levels of LC3II and ATG5
were significantly increased in pWHL-1 transfected HBEC when
compared with the control and NC groups (P<0.01; Fig. 4A). Similarly, the protein expression
levels of LC3I and SQSTM1 were significantly decreased in pWHL-1
transfected HBEC when compared with the control and NC groups,
whereas the protein expression levels of LC3II and ATG5 were
significantly increased in pWHL-1 transfected HBEC when compared
with the control and NC groups (P<0.01; Fig. 4B and C).
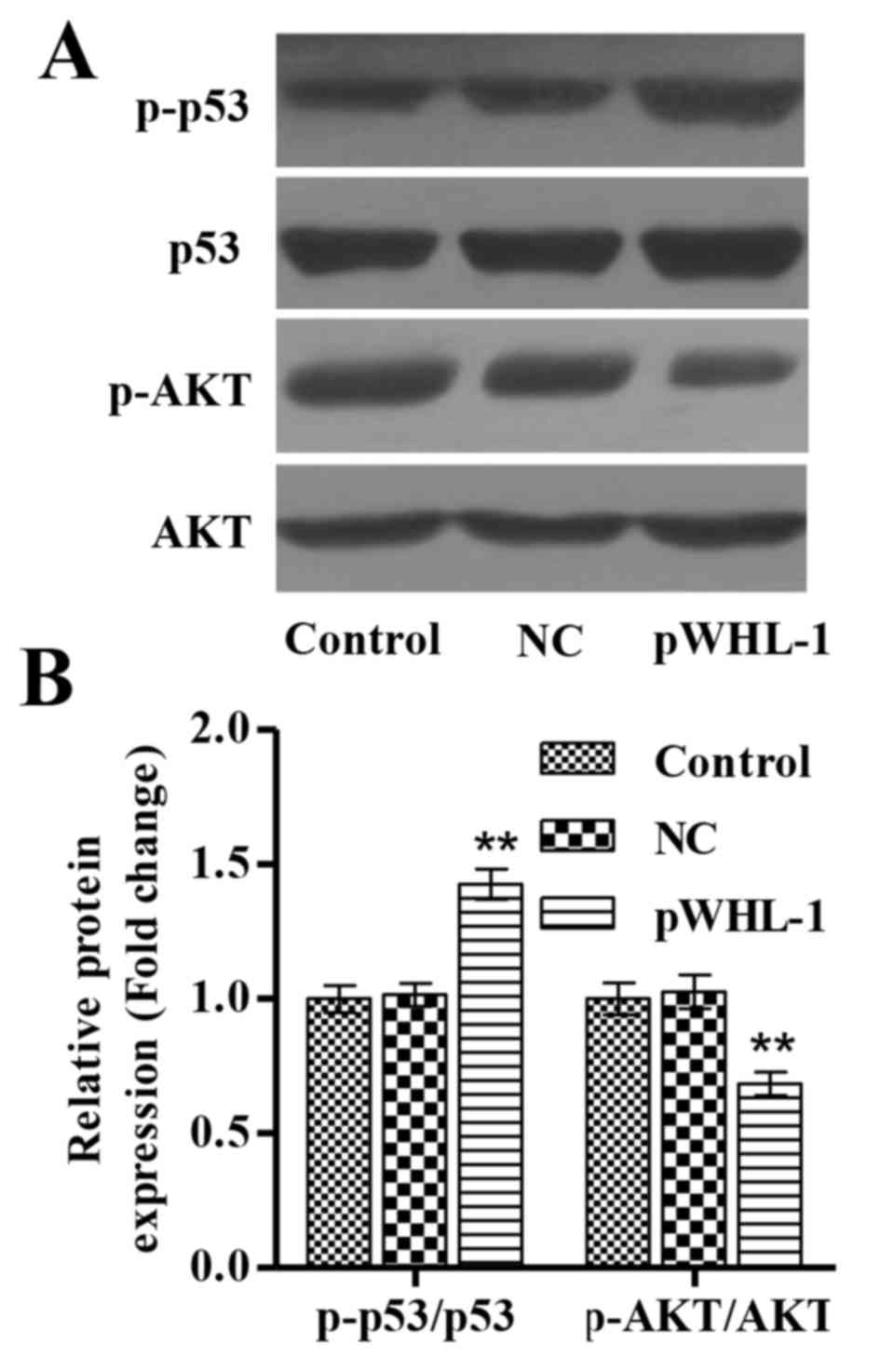
HBoV inhibits activation of p53 and
AKT
It has been widely recognized that signaling
pathways such as p53 and PI3K/AKT pathways are often activated in
tumor cells and promote cell proliferation and repress apoptosis
and autophagy (19,20). To examine the role of HBoV on p53 and
AKT in HBECs, the activation of these proteins was assessed by
western blot analysis. In NC HBECs, the P-53/p53 and p-AKT/AKT
ratios were unchanged compared with the control cells. In HBECs
transfected with pWHL-1, p53 phosphorylation was significantly
increased, while AKT phosphorylation was significantly decreased,
compared with the NC cells (P<0.01; Fig. 5A and B).
Discussion
Human bocavirus (HBoV) has been widely regarded to
induce apoptosis and autophagy, which is associated with viral
pathogenesis (22). In a previous
report, human airway epithelia infected with HBoV were revealed to
result in cilia loss and airway epithelial cell hypertrophy;
however this was not associated with apoptotic or necrotic cell
death (23). Similarly, a previous
study demonstrated that HBoV nonstructural protein failed to cause
cell death (5). However, in the
present study, we demonstrated that HBoV recombination expressing
vector (pWHL-1) induced proliferation inhibition, apoptosis and
autophagy in HBECs.
To investigate the effect of HBoV recombination
expressing vector transfection of HBEC, the proliferation of HBEC
was measured using aCCK-8 assay. The results showed that pWHL-1
transfection significantly inhibited the proliferation of HBEC when
compared with the control and NC groups. It has been reported that
HBoV is able to arrest the cell cycle at S phase; however, cell
cycle arrest occurs predominantly at the G2/M phase, followed by
cell apoptosis and proliferation suppression (8). The expression of PCNA was detected in
pWHL-1 transfected HBEC by immunohistochemistry assay. The
expression of PCNA was significantly decreased in
pWHL-1-transfected HBECs when compared with the control and NC
groups, which is consisted with the decreased proliferation
observed following pWHL-1 transfection in HBECs.
Previous studies have demonstrated that HBoV induced
apoptosis in several cell types, including HeLa, Walter Reed/3873D
canine and various types of epithelial cells (7,8).
Therefore, the predominant focus of the present study was on the
effect of pWHL-1 on HBEC apoptosis. The present findings identified
that pWHL-1 significantly induced apoptosis of HBECs and regulated
the protein expression of caspase-3, Bax and Bcl-2. Western blot
analysis demonstrated that the protein expression levels of
caspase-3 and Bax were increased, whereas the protein expression
levels of Bcl-2 were decreased in pWHL-1-transfected HBECs compared
with control and NC groups, suggesting that HBoV may induce
apoptosis through a mitochondrion-mediated pathway and increase the
ratio of Bax/Bcl-2. In agreement with our findings, a previous
study reported that HBoV also induced cell apoptosis through the
activation of caspase-3 and caspase-9 and an increase of Bax/Bcl-2
ratio was observed (7).
Autophagy is a crucial component of the cellular
stress adaptation response that maintains mammalian homeostasis
(24). Autophagosome formation
proceeds through a series of stages and has various roles in cancer
development and progression, and is involved in the proliferation
of normal cells (25). In the
present study, pWHL-1 infection was indicated to induce autophagy,
as evidenced by the presence of disordered nuclei, damage of the
cellular membrane of organelles, such as mitochondria, and large
numbers of autophagic vacuoles and autophagosome observed in
cytoplasm. In addition, specific autophagy associated proteins were
also detected in HBEC. RT-qPCR and western blot analysis
demonstrated that the mRNA and protein expression of LC3II and ATG5
were significantly increased, whereas LC3I and SQSTM1 mRNA and
protein expression levels were significantly decreased in
pWHL-1-transfected HBECs. An increased ratio of LC3II/LC3I is a
marker of an enhancement in autophagosomes formation, whereas
SQSTM1 expression is negatively correlated with autophagy (26). ATG5 is involved in the early stage of
autophagosome formation and has multiple functions in various
physiological contexts (27).
The molecular mechanism by which HBoV induces
autophagy in HBEC is not yet fully understood. Autophagy and
apoptosis may coadjust through p53 and PI3K/AKT signaling (28,29). The
p53 and PI3K/AKT signaling pathway are two well-known pathways
involved in the regulation of autophagy (30). Both pathways are associated with
tumorigenesis and activated in a number of cancers (19). Yersinia pestis infection of
HBECs has been associated with the negative regulation of autophagy
via the observed decrease exhibited of p53 cytoplasmic localization
and PI3K/AKT activation (31).
Furthermore, plumbagin has been demonstrated to induce autophagy
via the inhibition of the PI3K/AKT pathway in human non-small cell
lung cancer cells (32) In the
present study, the activation of p53 was significantly increased;
however, AKT protein activation via phosphorylation was
significantly decreased in pWHL-1-transfected HBECs when compared
with the control and NC groups, indicating that HBoV induced HBEC
autophagy predominantly through enhancing p53 activation and
blocking AKT activation.
In conclusion, the present study demonstrated that
HBoV promoted the inhibition of proliferation, apoptosis and
autophagy in HBEC and the apoptosis and autophagy were associated
with the regulation of p53 and AKT. The present study may be useful
to address the precise effect of HBoV in HBEC proliferation,
apoptosis and autophagy and to delineate the molecular mechanism of
HBoV in respiratory diseases.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101306).
References
|
1
|
Allander T, Tammi MT, Eriksson M, Bjerkner
A, Tiveljung-Lindell A and Andersson B: Cloning of a human
parvovirus by molecular screening of respiratory tract samples.
Proc Natl Acad Sci USA. 102:pp. 12891–12896. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lindner J and Modrow S: Human bocavirus-a
novel parvovirus to infect humans. Intervirology. 51:116–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allander T, Jartti T, Gupta S, Niesters
HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A,
Tiveljung-Lindell A, et al: Human bocavirus and acute wheezing in
children. Clin Infect Dis. 44:904–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitui MT, Tabib SM, Matsumoto T, Khanam W,
Ahmed S, Mori D, Akhter N, Yamada K, Kabir L, Nishizono A, et al:
Detection of human bocavirus in the cerebrospinal fluid of children
with encephalitis. Clin Infect Dis. 54:964–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen AY, Cheng F, Lou S, Luo Y, Liu Z,
Delwart E, Pintel D and Qiu J: Characterization of the gene
expression profile of human bocavirus. Virology. 403:145–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Chen AY, Cheng F, Guan W, Johnson
FB and Qiu J: Molecular characterization of infectious clones of
the minute virus of canines reveals unique features of bocaviruses.
J Virol. 83:3956–3967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun B, Cai Y, Li Y, Li J, Liu K, Li Y and
Yang Y: The nonstructural protein NP1 of human bocavirus 1 induces
cell cycle arrest and apoptosis in Hela cells. Virology. 440:75–83.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen AY, Luo Y, Cheng F, Sun Y and Qiu J:
Bocavirus infection induces mitochondrion-mediated apoptosis and
cell cycle arrest at G2/M phase. J Virol. 84:5615–5626. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubinsztein DC, Codogno P and Levine B:
Autophagy modulation as a potential therapeutic target for diverse
diseases. Nat Rev Drug Discov. 11:709–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tallóczy Z, Jiang W, HW IV Virgin, Leib
DA, Scheuner D, Kaufman RJ, Eskelinen EL and Levine B: Regulation
of starvation- and virus-induced autophagy by the eIF2alpha kinase
signaling pathway. Proc Natl Acad Sci USA. 99:pp. 190–195. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burman C and Ktistakis NT: Autophagosome
formation in mammalian cells. Semin Immunopathol. 32:397–413. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohsumi Y: Molecular dissection of
autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol.
2:211–216. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A,
Brech A and Johansen T: Monitoring autophagic degradation of
p62/SQSTM1. Method Enzymol. 452:181–197. 2009. View Article : Google Scholar
|
|
17
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014.PubMed/NCBI
|
|
18
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K,
et al: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annovazzi L, Mellai M, Caldera V, Valente
G, Tessitore L and Schiffer D: mTOR, S6 and AKT expression in
relation to proliferation and apoptosis/autophagy in glioma.
Anticancer Res. 29:3087–3094. 2009.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen AY and Qiu J: Parvovirus
infection-induced cell death and cell cycle arrest. Future Virol.
5:731–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Deng X, Yan Z, Cheng F, Luo Y,
Shen W, Lei-Butters DC, Chen AY, Li Y, Tang L, et al: Establishment
of a reverse genetics system for studying human bocavirus in human
airway epithelia. PLoS Pathog. 8:e10028992012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allan LA and Clarke PR: Apoptosis and
autophagy: Regulation of caspase-9 by phosphorylation. FEBS J.
276:6063–6073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Dong Z, Huang B, Zhao B, Wang H,
Zhao J, Kung H, Zhang S and Miao J: Distinct patterns of autophagy
evoked by two benzoxazine derivatives in vascular endothelial
cells. Autophagy. 6:1115–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizushima N, Ohsumi Y and Yoshimori T:
Autophagosome formation in mammalian cells. Cell Struct Funct.
27:421–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Lin Y, Yang H, Deng Q, Chen G and
He J: The expression of p33(ING1), p53, and autophagy-related gene
Beclin1 in patients with non-small cell lung cancer. Tumor Biol.
32:1113–1121. 2011. View Article : Google Scholar
|
|
29
|
Cheng Y, Ren X, Zhang Y, Patel R, Sharma
A, Wu H, Robertson GP, Yan L, Rubin E and Yang JM: eEF-2 kinase
dictates cross-talk between autophagy and apoptosis induced by Akt
Inhibition, thereby modulating cytotoxicity of novel Akt inhibitor
MK-2206. Cancer Res. 71:2654–2663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan L, Wei S, Wang J and Liu X:
Isoorientin induces apoptosis and autophagy simultaneously by
reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38
signaling pathways in HepG2 cancer cells. J Agric Food Chem.
62:5390–5400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alem F, Yao K, Lane D, Calvert V,
Petricoin EF, Kramer L, Hale ML, Bavari S, Panchal RG and Hakami
RM: Host response during Yersinia pestis infection of human
bronchial epithelial cells involves negative regulation of
autophagy and suggests a modulation of survival-related and
cellular growth pathways. Front Microbiol. 6:502015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li YC, He SM, He ZX, Li M, Yang Y, Pang
JX, Zhang X, Chow K, Zhou Q, Duan W, et al: Plumbagin induces
apoptotic and autophagic cell death through inhibition of the
PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells.
Cancer Lett. 344:239–259. 2014. View Article : Google Scholar : PubMed/NCBI
|