Introduction
Acute lung injury (ALI) is defined as pulmonary
dysfunction caused by pathogenic factors. It may aggravate to
develop into more severe acute respiratory distress syndrome (ARDS)
(1). In the clinic, affected
patients present with intractable hypoxemia and progressive
respiratory distress. The major associatedpathologic changes are
extensive injury of pulmonary capillary endothelial cells and
alveolar epithelial cells (2).
Subsequently, pneumonedema develops and the membrane becomes
transparent (3). Major
pathophysiological changes associated with ALI and ARDS are a
decrease in lung compliance, diffusion disturbance and the
disproportionality of ventilation/perfusion. The pathogenesis of
ALI is complex and has remained to be fully elucidated (4). When ALI occurs, inflammatory cells,
including neutrophils and macrophages, adhere, accumulate and can
be activated in the lung (5).
Afterwards, inflammatory cells produce large amounts of active
oxygen and oxidase through respiratory burstand degranulation.
Consequently, the pulmonary vasculature and alveolar epithelial
cells are seriously damaged (6).
Apoptosis, also known as programmed cell death,
refers to an active type of death in which cells themselves
terminate their lives under certain physiological or pathological
conditions. It is an essential process for organisms to maintain a
dynamic equilibrium, which affects lesions and recovery of lung
tissues (7). Excessive apoptosis of
alveolar epithelial cells and vascular endothelial cells may result
in structural distortion and destruction of pulmonary tissues as
well as fibroblast proliferation. The association between apoptosis
and ALI remains a hot topic (8).
In recent years, molecular biological mechanisms
have received continuous attention. The pathway associated with
biological damage of the lung is the induction of a lung
inflammatory reaction mediated by cells and inflammation mediators
(9). Biological damage refers to an
inflammatory injury in which inflammatory mediators, cytokines and
inflammatory cells participate. Mechanical and biological damage
are connected with each other (7).
ALI causes biological damage, while biological damage may
exacerbate mechanical damage (10).
As a traditional Chinese medicine, Salvia
miltiorrhizae has been employed in Asian countries to cure and
prevent various types of disease with high efficacy (11). In recent years, two active components
with the highest phamaceutical value have been extracted, i.e.,
tanshinone IIA and salvianolic acid B (Fig. 1) (12). The latter has been reported to aid
inneural functional recovery in rats with cerebral injury (12). Furthermore, it was shown to have
neuroprotective effects in rats with focal cerebral ischemic
reperfusion (13). It has been
suggested that theneural functional recoveryeffect of salvianolic
acid B is dependent on its specific chemical structure, which are
accountable for is neuroprotective effects (12,14). The
present study assessed whether salvianolic acid B exerts a
protective effect on LPS-induced ALI.
Materials and methods
Animals, experimental groups and
LPS-induced ALI model
Adult male Sprague-Dawley (SD) rats weighing 50±2 g
were obtained from the Animal Center of Anhui Medical University
(Hefei, China), and were housed in a humidity-controlled
environment (22±2°C; 55±5% humidity) with a 12-h light/dark cycle
and allowed free access to water and standard rat chow. The rats
were anesthetized with an intraperitoneal injection of 3%
pentobarbital sodium (30 mg/kg body weight; Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China). Subsequently, 0.01% LPS (100
µg/kg body weight; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was injected into the tail vein of SD rats through a 24-gauge
catheter. All 26 adult male SD rats were randomly divided into 3
groups: The control group (sham, n=6), LPS-induced ALI model group
(model, n=10), salvianolic acid B-treated group (treated, n=10).
The rats from the salvianolic acid B-treated group were pretreated
with salvianolic acid B (1 mg/ml; 20 ml/kg body weight) 1 h prior
to LPS challenge, then 20 ml/kg of salvianolic acid B every 2 days
for 4 weeks thereafter.
Lung wet/dry weight ratio
The right edematous lung tissue from every group was
weighed to determine the tissue's wet weight. Subsequently,
theright lung edema tissue was placed in an oven at 80°C for 24 h
and weighed to determine the tissue dry weight. The tissue wet/dry
weight ratio was then calculated.
Lung histopathology
The left edematous lung tissue from every group was
fixed in 4% paraformaldehyde for 24 h and dehydrated by an ethanol
gradient. The tissue was embedded in paraffin and sectioned into
4-µm slices. Sliced tissue samples were stained with hematoxylin
and eosin.
Determination of oxidative stress and
inflammation
Lung homogenate 10% (w/v) was prepared and
centrifuged at 12,000 × g for 10 min. The supernatant was collected
and standard enzyme-linked immunosorbent assay kits were used to
measure the content of caspase-3 (DYC835-2; R&D Systems, Inc.,
Minneapolis, MN, USA), and malondialdehyde (MDA; A003-1),
superoxide dismutase (SOD; A001-1), catalase (CAT; A007-1),
glutathione peroxidase (GPx; A005; all from Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), tumor necrosis factor
(TNF)-α (ab46070) and interleukin (IL)-6 (ab100772; both from
Abcam, Cambridge, UK), according to the manufacturer's
instructions.
Western blot analysis
Lung homogenate 10% (w/vol) was prepared and
centrifuged at 12,000 × g for 10 min. The supernatant was collected
and used to measure protein contents using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology, Haimen, China). In
brief, total protein (50 µg/lane) was subjected to 10–12% SDS-PAGE
and subsequently transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
then blocked with 5% skimmed milk in Tris-buffered saline with 0.1%
Tween-20 (pH 7.4) and incubated with anti-transforming growth
factor (TGF)-β1 (1:2,000 dilution; 3709; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-α-smooth muscle actin (SMA; 1:2,000
dilution; ab5694; Abcam) anti-collagen I (1:2,000 dilution;
ab34710; Abcam) or β-actin (1:5,000 dilution; sc-7210; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The
membrane was incubated with horseradish peroxidase-conjugated
secondary antibody (sc-2357, 1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 37°C for 2 h.
Statistical analysis
Values are expressed as the mean ± standard
deviation by SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance and pairwise comparison were
performed. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
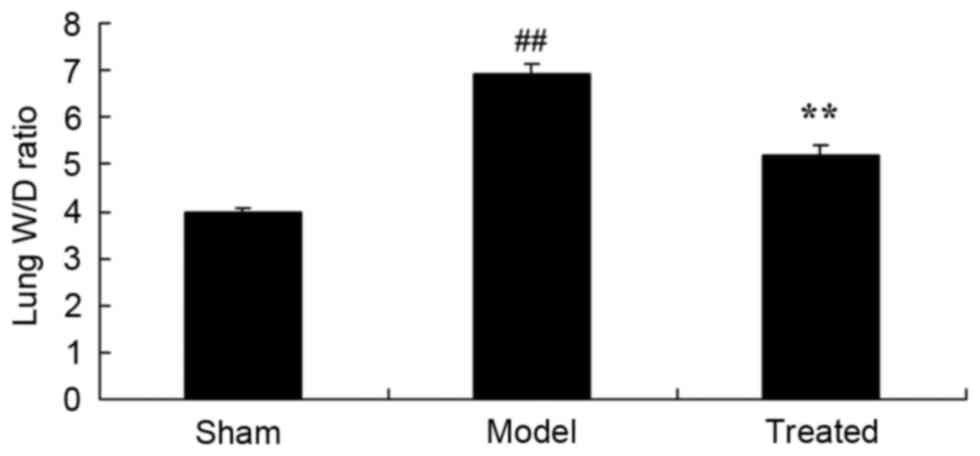
Salvianolic acid B attenuates
LPS-induced increases in the lung wet/dry weight ratio in rats
As shown in Fig. 2,
the lung wet/dry weight ratio in the model group was higher than
that in the sham group. The LPS-induced increase in the lung
wet/dry weight ratio was markedly attenuated by salvianolic acid B
pre-treatment in LPS-induced ALI rats (Fig. 2).
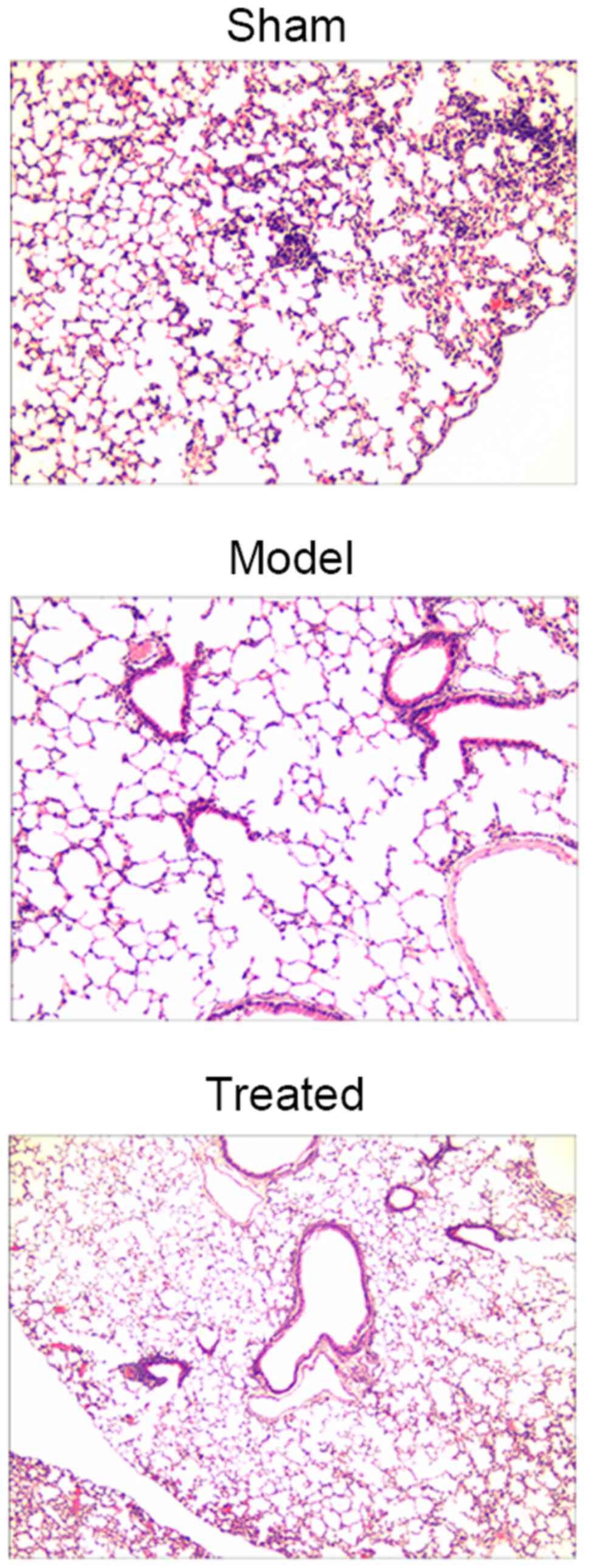
Salvianolic acid B attenuates
LPS-induced lung tissue injury in rats
As shown in Fig. 3,
sham rats had a normal lung morphology, while LPS-induced ALI rats
exhibited extensive lung damage. Of note, pre-treatment with
salvianolic acid B significantly attenuated LPS-induced lung tissue
injury in rats (Fig. 3).
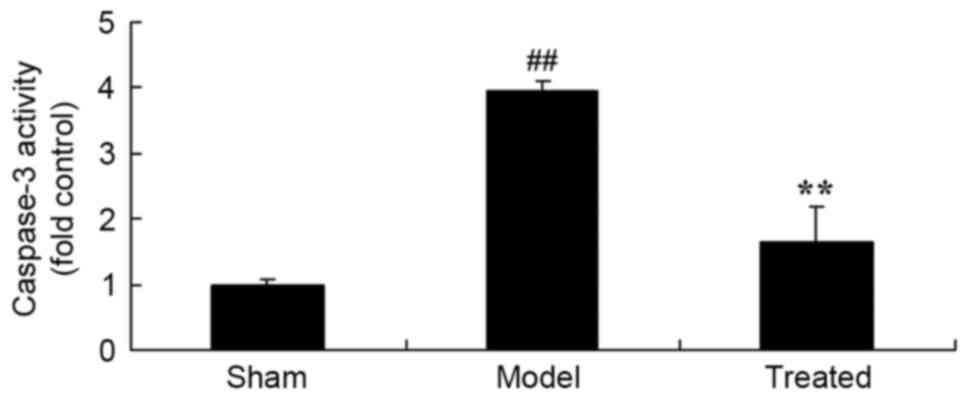
Salvianolic acid B attenuates
LPS-induced increases in the content of caspase-3 in rats
As shown in Fig. 4,
the lung tissue of LPS-induced ALI rats showed a significant
increase in caspase-3 content compared with that in the sham group.
However, treatment with salvianolic acid B significantly attenuated
LPS-induced increases in the content of caspase-3 in the rat lungs
(Fig. 4).
Salvianolic acid B attenuates
LPS-induced oxidative stress in rats
When compared to that in the sham-operated rats, the
MDA content was enhanced and the SOD, CAT and GPx content was
attenuated in LPS-induced ALI rats (Fig.
5). Of note, salvianolic acid B treatment significantly
attenuated these LPS-induced changes associated with oxidative
stress in rats (Fig. 5).
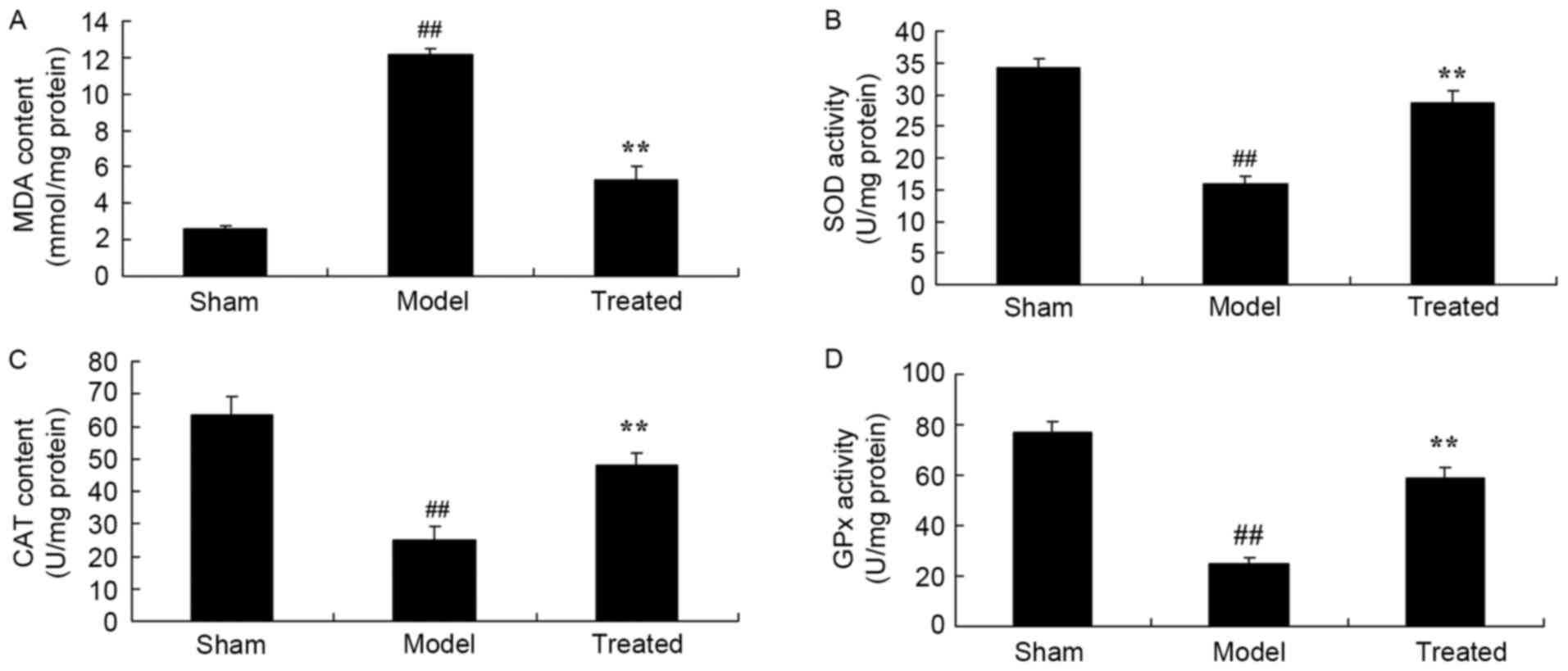 | Figure 5.Salvianolic acid B attenuates
LPS-induced oxidative stress inin the lung tissues of rats.
Treatment with salvianolic acid B inhibited LPS-induced (A)
increases in MDA as well as decreases in the (B) SOD, (C) CAT and
(D) GPx content in rats. Groups: Sham, control group; model,
LPS-induced acute lung injury model group; treated, salvianolic
acid B-treated group (1 mg/ml; 20 ml/kg body weight).
##P<0.01 vs. sham group, **P<0.01 vs. model group.
MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase;
GPx, glutathione peroxidase; LPS, lipopolysaccharide. |
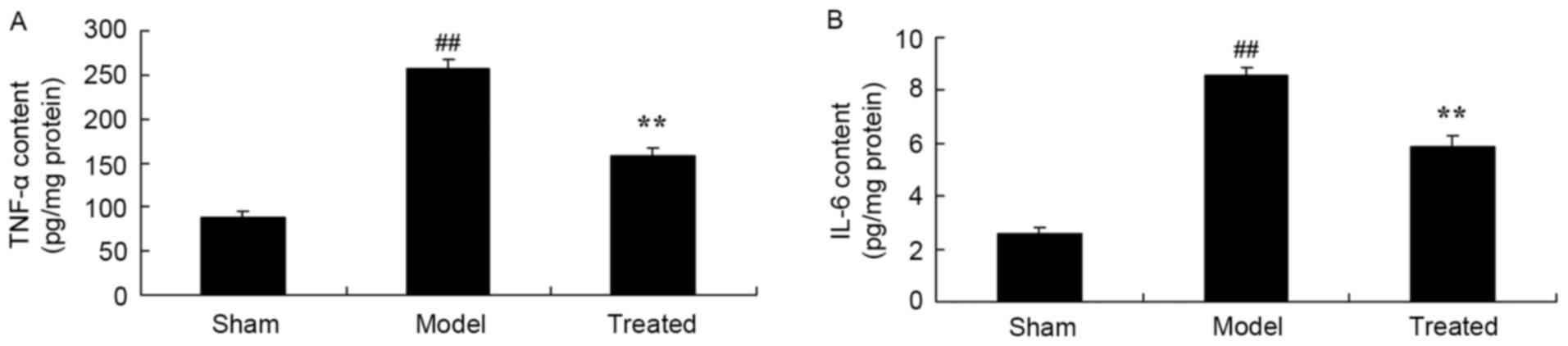
Salvianolic acid B attenuates
LPS-induced inflammation in rats
After LPS exposure, the levels of the TNF-α and IL-6
content in the rat lungs were significantly elevated compared to
those in sham-operated animals (Fig.
6). Salvianolic acid B treatment significantly attenuated
LPS-induced increases in the TNF-α and IL-6 content in rat lungs
(Fig. 6).
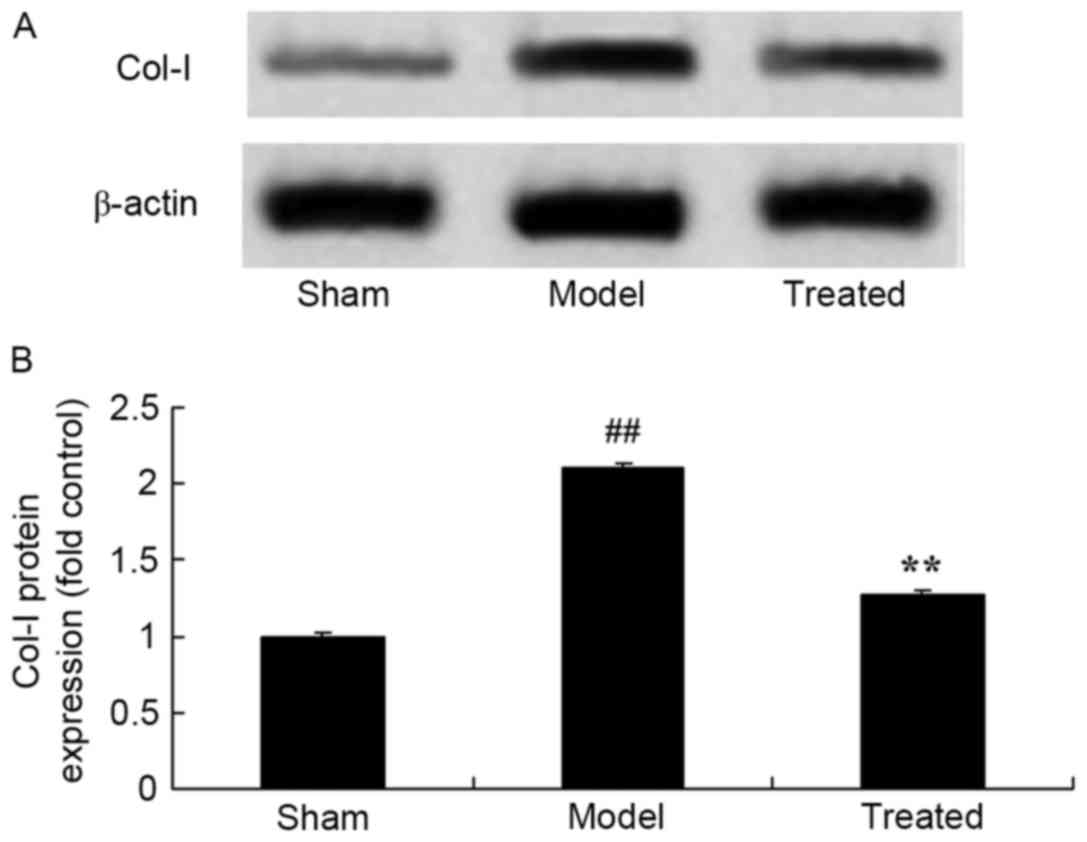
Salvianolic acid B attenuates
LPS-induced Col-I in rats
As shown in Fig. 6,
the protein expression of Col-I in the rat lungs after LPS exposure
was significantly higher than that in the sham rats (Fig. 7). The LPS-induced Col-I protein
expression in the rat lungs was significantly attenuated by
treatment with salvianolic acid B (Fig.
7).
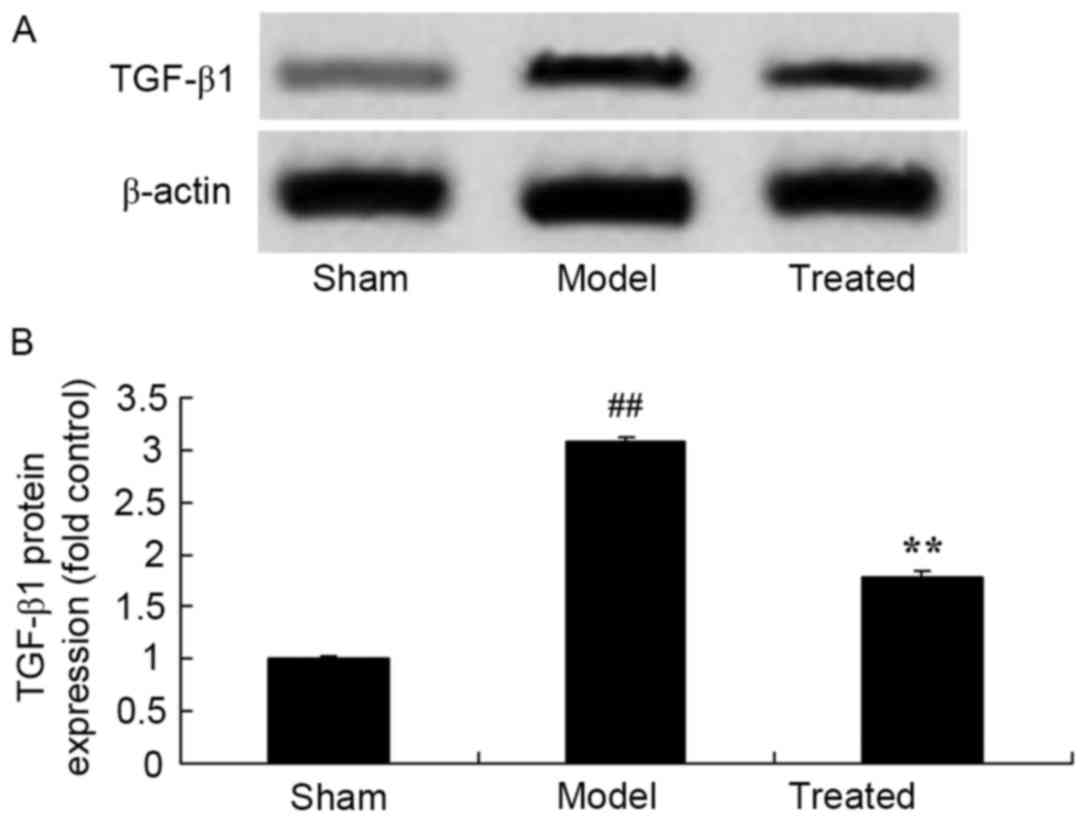
Salvianolic acid B attenuates
LPS-induced TGF-β1 in rats
As shown in Fig. 8,
TGF-β1 protein expression ws siganificantly activated in rats after
LPS exposure compared with that in sham rats. Treatment with
salvianolic acid B significantly attenuated LPS-induced TGF-β1
protein expression in ALI rats (Fig.
8).
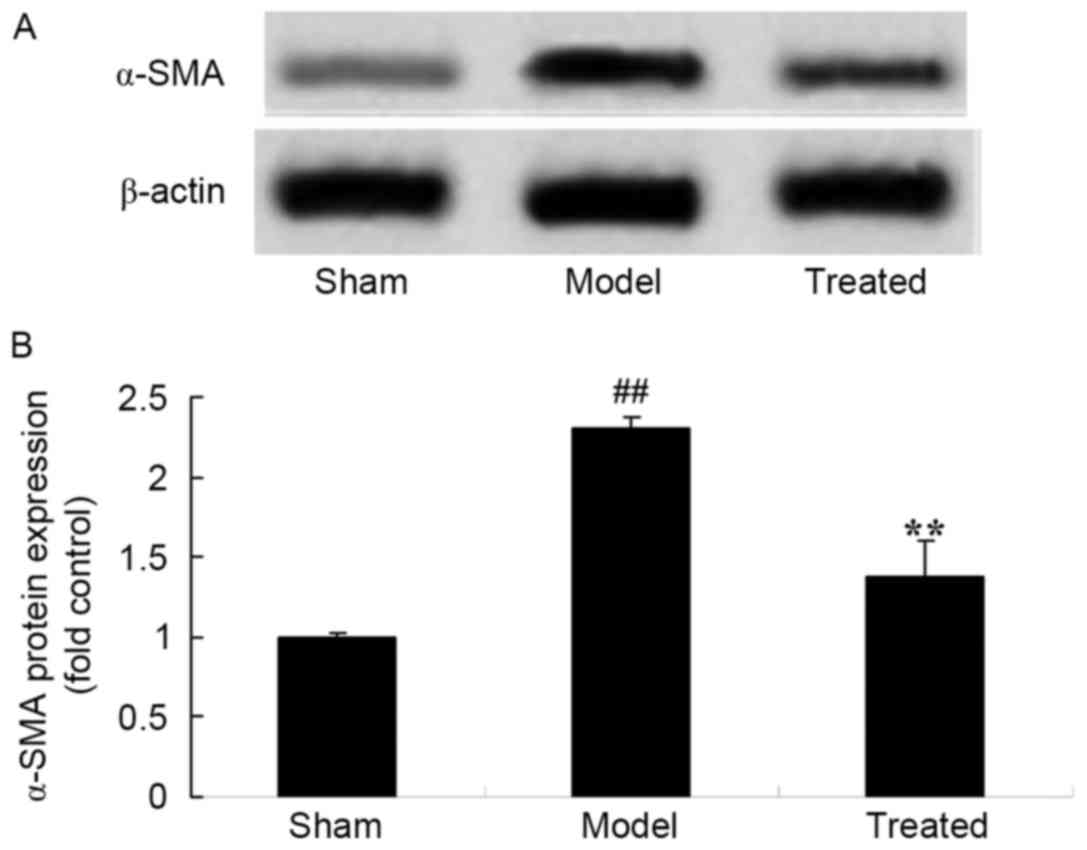
Salvianolic acid B attenuates
LPS-induced α-SMA in rats
When compared to that in the sham control rats,
α-SMA protein expression was observed to be increased in
LPS-induced ALI rats (Fig. 9). Of
note, salvianolic acid B treatment significantly attenuated
LPS-induced α-SMA protein expression in rats (Fig. 9).
Discussion
ALI is a syndrome of pulmonary inflammation and
permeability characterized by gas exchange dysfunction (15). If it aggravates, it is referred to as
ARDS in the clinic. Its pathological features are pulmonary
capillary endothelial cell damage, alveolar epithelial cell injury,
extensive aqualung, tiny pulmonary atelectasis, microthrombi and
microcirculation disturbance (16).
Common incentives include severe infection, trauma, shock and
intoxication, such as inhalation of toxic gases. While the
morbidity and fatality rates of affected patients are high, the
advance of clinical methods and comprehensive treatments have
decreased its mortality rate, which, however, remains as high as
30–40% (3). The mortality rates is
also increased in patients with accompanying multiple organ
dysfunction, with the mortality rate being 100% if more than four
organ dysfunctions are present (17). In the present study, salvianolic acid
B was shown to markedly attenuate the LPS-induced increase in the
lung wet/dry weight ratio as well as lung tissue injury in ALI
rats. Chen et al (12)
reported that salvianolic acid B attenuates traumatic brain injury
through exerting an anti-inflammatory effect in mice.
The pathogenesis of ALI is rather complex and it has
remained to be fully clarified. However, oxidative stress is
considered to be one of major pathogenic factors of ALI (18). Internally (hypoxia-ischemia or
inflammation) and externally (pressure, empyrosis), organisms
produce large amounts of reactive oxygen, which destroys the
equilibrium state of the oxidation/antioxidant system (19). Cell or tissue damage may occur to
cause associated diseases, such as tumors or diabetes (20). This process is referred to as
oxidative stress. The results of the present study indicated that
salvianolic acid B exerts protective effects against LPS-induced
oxidative stress in ALI rats. Yang et al (14) suggested that salvianolic acid B
protects against low-density lipoprotein oxidation and neointimal
hyperplasia through inhibition of reactive oxygen species
production in endothelium-denuded hypercholesterolaemic
rabbits.
The current understanding of apoptotic processes has
shifted from cell nucleus-centered to mitochondria-centered
mechanisms (8). It is thought that
mitochondria have a decisive role in apoptotic processes. As an
endogenous apoptotic pathway, the mitochondrial apoptotic pathway
is regarded as the key regulatory process (21). The primary functions of mitochondria
in cell apoptosis include the release of activity factors of
caspase, such as cytochrome c, the loss of the mitochondrial
transmembrane potential and dysfunction of oxidative
phosphorylation of mitochondria (22). During this process, cytochrome
c and caspase-3 have an essential role. Consistent with
this, the results of the present study demonstrated that treatment
with salvianolic acid B significantly attenuated LPS-induced
increases in the content of caspase-3 in the lungs of ALI rats.
Tang et al (23) suggested
that salvianolic acid B protects human endothelial progenitor cells
through inhibiting oxidative stress and caspase-3 activation.
Cells as well as body fluids participate in the
inflammatory response (24). Cells
participating in inflammation include polymorphonuclear neutrophil
(PMN), alveolar epithelial cells, vascular endothelial cells,
pulmonary vascular endothelial cells, alveolar macrophages,
pulmonary interstitial macrophages and pulmonary intravascular
macrophages, among which PMN and pulmonary intravascular
macrophageshave a pivotal role in lung injury (25). Bodily fluids include cytokines, lipid
mediators, oxygen radicals, proteases, alexin, cruor and the
fibrinolytic system (7). Consistent
with this, the present study found thats alvianolic acid B
treatment significantly attenuated LPS-induced increases in the
TNF-α and IL-6 content in the lungs of ALI rats. Xu et al
(11) suggested that salvianolic
acid B attenuates platelet-mediated inflammatory responses in
vascular endothelial cells.
Cytokines in the lung can be expressed in intrinsic
pulmonary cells, such as epithelial cells, vascular endothelial
cells and interstitial cells. Furthermore, they may be secreted by
inflammatory cells activated during lung injury processes,
including macrophages and lymphocytes, which may have biological
effects in the lung (26). In
cytokines participating in radiation-induced lung injury, TGF-β has
a predominant role and is a major cytokine, which has been most
thoroughly researched. It may induce inflammatory cells and
fibroblasts to synthesize IL-1 and IL-6 (27). Furthermore, it may self-induce the
production of more TGF-β and inhibit the degradation of
extracellular matrix (ECM). It induces fibroblasts to interact with
compound ECM and promotes the adhesion between ECM and cells. It
stimulates fibroblasts to proliferate and induces the expression of
collagen RNAI and III. The fastigium of TGF-β expression
corresponds to the vigorous stage of fibroblast proliferation and
collagen synthesis, suggesting that TGF-β and other cytokines
mediate the early inflammatory response (27). α-SMA, which is the primary molecule
of ALI, mainly exists in the cytoplasm. Studies found that TGF-β1
induces the transformation of fibroblasts and pulmonary epithelial
cells to generate ALI, which increases the expression of α-SMA
(28). The present study found that
salvianolic acid B attenuated LPS-induced increases in Col-I,
TGF-β1 and α-SMA protein expression in the lung tissues of ALI
rats. Li et al (29)
indicated that salvianolic acid B attenuated hepatic fibrosis
through Col-I, α-SMA and TGF-β signaling in rats. Lv and Xu
(30) reported that salvianolic acid
B inhibits TGF-β1 in the stimulated human hepatic stellate cell
line LX-2.
In conclusion, the results of the present study
indicated that salvianolic acid B markedly attenuated LPS-induced
increases in the lung wet/dry weight ratio as well as lung tissue
injury in ALI model rats via inhibition of apoptosis, oxidative
stress and inflammation. These findings suggested that the clinical
applicability of salvianolic acid B in the prevention and treatment
of ALI warrants further exploration.
References
|
1
|
Krupa A, Fol M, Rahman M, Stokes KY,
Florence JM, Leskov IL, Khoretonenko MV, Matthay MA, Liu KD, Calfee
CS, et al: Silencing Bruton's tyrosine kinase in alveolar
neutrophils protects mice from LPS/immune complex-induced acute
lung injury. Am J Physiol Lung Cell Mol Physiol. 307:L435–L448.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stapleton RD, Martin TR, Weiss NS, Crowley
JJ, Gundel SJ, Nathens AB, Akhtar SR, Ruzinski JT, Caldwell E,
Curtis JR, et al: A phase II randomized placebo-controlled trial of
omega-3 fatty acids for the treatment of acute lung injury. Crit
Care Med. 39:1655–1662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calfee CS, Gallagher D, Abbott J, Thompson
BT and Matthay MA: NHLBI ARDS Network: Plasma angiopoietin-2 in
clinical acute lung injury: Prognostic and pathogenetic
significance. Crit Care Med. 40:1731–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McAuley DF, Laffey JG, O'Kane CM, Cross M,
Perkins GD, Murphy L, McNally C, Crealey G and Stevenson M; HARP-2
investigators; Irish Critical Care Trials Group, :
Hydroxymethylglutaryl-CoA reductase inhibition with simvastatin in
acute lung injury to reduce pulmonary dysfunction (HARP-2) trial:
Study protocol for a randomized controlled trial. Trials.
13:1702012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Needham DM, Wozniak AW, Hough CL, Morris
PE, Dinglas VD, Jackson JC, Mendez-Tellez PA, Shanholtz C, Ely EW,
Colantuoni E, et al: Risk factors for physical impairment after
acute lung injury in a national, multicenter study. Am J Respir
Crit Care Med. 189:1214–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark BJ, Williams A, Feemster LM, Bradley
KA, Macht M, Moss M and Burnham EL: NHLBI ARDS Network
Investigators: Alcohol screening scores and 90-day outcomes in
patients with acute lung injury. Crit Care Med. 41:1518–1525. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou L, Zhao D, An H, Zhang H, Jiang C and
Yang B: Melatonin prevents lung injury induced by hepatic
ischemia-reperfusion through anti-inflammatory and anti-apoptosis
effects. Int Immunopharmacol. 29:462–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Armstrong SM, Wang C, Tigdi J, Si X,
Dumpit C, Charles S, Gamage A, Moraes TJ and Lee WL: Influenza
infects lung microvascular endothelium leading to microvascular
leak: Role of apoptosis and claudin-5. PLoS One. 7:e473232012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohira S, Oka N, Inoue N, Itatani K,
Hanayama N, Kitamura T, Fujii M, Takeda A, Oshima H, Tojo K, et al:
Effect of the neutrophil elastase inhibitor sivelestat on
perioperative inflammatory response after pediatric heart surgery
with cardiopulmonary bypass: A prospective randomized study. Artif
Organs. 37:1027–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv H, Yu Z, Zheng Y, Wang L, Qin X, Cheng
G and Ci X: Isovitexin exerts anti-inflammatory and anti-oxidant
activities on lipopolysaccharide-induced acute lung injury by
inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int J
Biol Sci. 12:72–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu S, Zhong A, Bu X, Ma H, Li W, Xu X and
Zhang J: Salvianolic acid B inhibits platelets-mediated
inflammatory response in vascular endothelial cells. Thromb Res.
135:137–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Liu W, Chao X, Zhang L, Qu Y, Huo
J and Fei Z: Salvianolic acid B attenuates brain damage and
inflammation after traumatic brain injury in mice. Brain Res Bull.
84:163–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang TL, Lin FY, Chen YH, Chiu JJ, Shiao
MS, Tsai CS, Lin SJ and Chen YL: Salvianolic acid B inhibits
low-density lipoprotein oxidation and neointimal hyperplasia in
endothelium-denuded hypercholesterolaemic rabbits. J Sci Food
Agric. 91:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walkey AJ and Wiener RS: Utilization
patterns and patient outcomes associated with use of rescue
therapies in acute lung injury. Crit Care Med. 39:1322–1328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kowalski S, McMullen MC, Girling LG and
McCarthy BG: Biologically variable ventilation in patients with
acute lung injury: A pilot study. Can J Anaesth. 60:502–503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin GS, Moss M, Wheeler AP, Mealer M,
Morris JA and Bernard GR: A randomized, controlled trial of
furosemide with or without albumin in hypoproteinemic patients with
acute lung injury. Crit Care Med. 33:1681–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afshari A, Brok J, Moller AM and
Wetterslev J: Inhaled nitric oxide for acute respiratory distress
syndrome and acute lung injury in adults and children: A systematic
review with meta-analysis and trial sequential analysis. Anesth
Analg. 112:1411–1421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grainge C, Brown R, Jugg BJ, Smith AJ,
Mann TM, Jenner J, Rice P and Parkhouse DA: Early treatment with
nebulised salbutamol worsens physiological measures and does not
improve survival following phosgene induced acute lung injury. J R
Army Med Corps. 155:105–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akcilar R, Akcilar A, Şimşek H, Koçak FE,
Koçak C, Yümün G and Bayat Z: Hyperbaric oxygen treatment
ameliorates lung injury in paraquat intoxicated rats. Int J Clin
Exp Pathol. 8:13034–13042. 2015.PubMed/NCBI
|
|
21
|
Ruchko MV, Gorodnya OM, Zuleta A, Pastukh
VM and Gillespie MN: The DNA glycosylase Ogg1 defends against
oxidant-induced mtDNA damage and apoptosis in pulmonary artery
endothelial cells. Free Radic Biol Med. 50:1107–1113. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren M, Wang YM, Zhao J, Zhao J, Zhao ZM,
Zhang TF, He J, Ren SP and Peng SQ: Metallothioneins attenuate
paraquat-induced acute lung injury in mice through the mechanisms
of anti-oxidation and anti-apoptosis. Food Chem Toxicol.
73:140–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Jacobi A, Vater C, Zou X and
Stiehler M: Salvianolic acid B protects human endothelial
progenitor cells against oxidative stress-mediated dysfunction by
modulating Akt/mTOR/4EBP1, p38 MAPK/ATF2 and ERK1/2 signaling
pathways. Biochem Pharmacol. 90:34–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WY, Huang YC, Yang ML, Lee CY, Chen
CJ, Yeh CH, Pan PH, Horng CT, Kuo WH and Kuan YH: Protective effect
of rutin on LPS-induced acute lung injury via down-regulation of
MIP-2 expression and MMP-9 activation through inhibition of Akt
phosphorylation. Int Immunopharmacol. 22:409–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Ge X, Xu F, Zhang Y, Liu Z, Pan J,
Song J, Dai Y, Zhou J, Feng J and Liang G: Design, synthesis and
biological evaluation of paralleled Aza resveratrol-chalcone
compounds as potential anti-inflammatory agents for the treatment
of acute lung injury. Bioorg Med Chem Lett. 25:2998–3004. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim J, Jeong SW, Quan H, Jeong CW, Choi JI
and Bae HB: Effect of curcumin (Curcuma longa extract) on
LPS-induced acute lung injury is mediated by the activation of
AMPK. J Anesth. 30:100–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manickam N, Patel M, Griendling KK, Gorin
Y and Barnes JL: RhoA/Rho kinase mediates TGF-β1-induced kidney
myofibroblast activation through Poldip2/Nox4-derived reactive
oxygen species. Am J Physiol Renal Physiol. 307:F159–F171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Hu J, Hao HZ, Yin Z, Liu G and Zou
XJ: Sodium tanshinone IIA sulfonate attenuates the transforming
growth factor-β1-induced differentiation of atrial fibroblasts into
myofibroblasts in vitro. Int J Mol Med. 35:1026–1032.
2015.PubMed/NCBI
|
|
29
|
Li S, Wang L, Yan X, Wang Q, Tao Y, Li J,
Peng Y, Liu P and Liu C: Salvianolic acid B attenuates rat hepatic
fibrosis via downregulating angiotensin II signaling. Evid Based
Complement Alternat Med. 2012:1607262012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lv Z and Xu L: Salvianolic acid B inhibits
ERK and p38 MAPK signaling in TGF-β1-stimulated human hepatic
stellate cell line (LX-2) via distinct pathways. Evid Based
Complement Alternat Med. 2012:9601282012. View Article : Google Scholar : PubMed/NCBI
|