Introduction
Ischemic stroke is a serious disease and threat to
the health of millions of people. It is also one of the leading
causes of disability and fatality in various industrial countries
and still has no effective therapeutic treatments (1). Cerebral ischemia results in the
disruption of blood, oxygen and glucose supplies to the brain and a
reduction in energy. The generation and accumulation of toxic
metabolites, such as glutamate and free radicals may ultimately
lead to neurodegeneration (2). The
primary method of alleviating neurologic injury caused by brain
ischemia is the restoration of the blood flow to the ischemic
region rapidly (3). However, blood
reperfusion increases the production of excitatory amino acids,
oxygen free radicals and intracellular Ca2+, as well as
gives rise to damage of the microvascular system, all of which may
induce additional damage to the brain tissue, which is termed
ischemia reperfusion injury (IRI) (4,5). Injury
caused by reperfusion may be more detrimental to health than
ischemia itself (6). Previous
studies have demonstrated that the inflammatory response is one of
the primary factors that leads to IRI (3,7,8).
Inflammation is associated with the
pathophysiological process of cerebral IRI. Reperfusion may be
promoted by the activities of microglial cells, astrocytes and
endothelial cells in the brain, which interact with each other and
generate inflammatory mediators, such as cytokines and adhesion
molecules, which damage nerve cells (5,9,10). Gene expression levels of interleukin
(IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, which are
regulated by the redox-sensitive transcription factor nuclear
factor (NF)-κB, have been demonstrated to rapidly increase the
early stage of cerebral IR, to promote an inflammatory response
(11–13) and further aggravate cerebral IR.
Accordingly, reducing the inflammatory response may have a positive
effect on the prognosis of ischemic diseases.
Heme oxygenase (HO)-1, an inducible type of heme
oxygenase, is a rate-limiting enzyme of heme metabolism, which
catalyzes heme to Fe2+, carbon monoxide and biliverdin
(BV) (14). BV is rapidly converted
to bilirubin by BV reductase (15).
A number of studies have demonstrated that HO-1 has neuroprotective
effects including antioxidant, anti-inflammatory, and
anti-apoptotic effects following cerebral IRI (16–18). In
addition, BV, which is a catalytic product of HO-1, has been
demonstrated to exhibit potent anti-inflammatory effects in various
studies, in both in vivo and in vitro models
(19–21). Previous studies have shown that
exogenous BV is able to downregulate the expression of adhesion
molecules and inhibit the aggregation of white blood cells, thereby
reducing the generation of cytokines and chemotaxis factors and the
expression of pro-inflammatory proteins, such as cyclooxygenase-2
and cytochrome P450, ultimately ameliorating inflammation (22). Furthermore, BV has been shown to
restrain the complement cascade reaction (23) and decrease the expression of
cytokines, such as TNF-α and IL-6, by reducing the reaction
activity of NF-κB in a lipopolysaccharide (LPS)-induced injury
model (24). However, the effect of
BV in cerebral IRI is still unknown, and the application of BV to
treat brain IRI is limited.
The present study aimed to investigate the effects
of exogenous BV on cerebral IRI and explored the potential
neuroprotective mechanism.
Materials and methods
Animals and grouping
A total of 135 male Sprague-Dawley rats, age 6–8
weeks, weighing 200–240 g, were provided by the Laboratory Zoology
Department at Kunming Medical University (Kunming, China). All rats
were maintained in plastic cages with soft bedding in a
temperature-controlled room at 21–25°C, with a humidity of 45–50%
and a 12 h light/dark cycle. Rats had ad libitum access to food and
water. Study protocols followed the guidelines for Laboratory
Animal Care and Safety as issued by the Unites States National
Institutes of Health. Animal care and all experimental protocols
were approved by and performed according to the Guidelines of the
Animal Care & Welfare Committee of Kunming Medical University.
Rats were randomly divided into the sham (group S; sham operation,
n=45); vehicle control (group C; IRI + normal saline, n=45); and BV
(group BV; IRI + BV treatment, n=45) groups, which are indicated in
Table I.
 | Table I.Groups and methods performed (time
after reperfusion). |
Table I.
Groups and methods performed (time
after reperfusion).
|
| Rats, n |
|---|
|
|
|
|---|
| Group | NSS Score (1–5
days) | TTC Stain (48
h) | RT-qPCR (3, 6, 12
and 24 h) | Western blotting (3
h) |
|---|
| S | 15 | 5 | 20 | 5 |
| C | 15 | 5 | 20 | 5 |
| BV | 15 | 5 | 20 | 5 |
Animal transient middle cerebral
artery occlusion model (tMCAO)
According to the Zea-Longa method (25), rats with tMCAO model of cerebral IRI
were established. Briefly, rats were anesthetized with 3.6% chloral
hydrate (350 mg/kg; Tianjin Guangfu Fine Chemical Research
Institute, Tianjin, China) intraperitoneally and the right common
carotid artery was exposed and carefully separated. The middle
cerebral artery (MCA) was obturated by inserting a nylon thread
coated with polylysine (diameter, 0.24 mm) into the internal
carotid artery, which was advanced further until it approached the
starting point of the MCA. The inserting length was 18±2 mm. Group
S rats underwent the same procedures without inserting the nylon
thread. After 2 h of tMCAO, cerebral blood flow was recovered by
removing the nylon thread. When they recovered from anesthesia,
rats were scored according to the Zea-Longa scoring system, as
reported previously (26). The
scoring was as follows: 0 points, no symptoms of neurologic
impairment; 1 point, side front paw could not stretch completely; 2
points, paw rotated inwards when walking; 3 points, paw tilted
inwards when walking; and 4 points, failed to spontaneously walk or
loss of consciousness. According to the experimental requirements,
rats that scored 0 or 4 points were excluded from the present
study; scores of 1, 2 and 3 points indicated the model was
successful.
BV treatment
BV hydrochloride (Frontier Scientific, Inc., Logan,
UT, USA) was dissolved in 0.2 M NaOH solution and adjusted to pH
7.4 with HCl. After diluting with saline, BV (35 mg/kg in 2 ml) was
intraperitoneally administered to rats 15 min prior to reperfusion,
once again 4 h after reperfusion and twice a day thereafter for 5
days. In group C, the same volume of saline was administered using
the same method.
Neural behavioral test
Rats in groups C and BV were scored using the
previously reported Neurological Severity Scores (NSS) scoring
system (27) at days 1–5 after
reperfusion, respectively. Group S were also scored at the same
time points. The NSS included four aspects: Study of feeling
(sensory tests including visual, tactile and proprioceptive test; 2
points were awarded), movement (motor tests including raising rat
by the tail and placing rat on the floor; 6 points were awarded),
reflection (reflexes absent and abnormal movements; 4 points were
awarded) and balance (beam balance tests; 6 points were awarded).
Scoring was defined as follows: 1–6, mild injury; 7–12, moderate
injury; and 13–18, severe injury.
Mensuration of cerebral infarct volume
by 2,3,5-triphenyltetrazolium chloride (TTC) staining
To evaluate the infarct volume of the ischemic
cerebral hemisphere, brains (n=5 for each group) were removed 2
days after reperfusion and cut into five coronal sections of 2-mm
thickness. Sections were incubated in 2% TTC solution
(Sigma-Aldrich; Merk KGaA, Darmstadt, Germany) at 37°C for 20 min.
After staining, the sections were washed in phosphate-buffered
saline three times for 1 min each and fixed in 4% paraformaldehyde
at room temperature for 24 h. Color images of these sections were
directly obtained with a stereomicroscope and (magnification, ×5)
then the infarct areas of each section was measured using Image J
software version 1.43 (National Institutes of Health, Bethesda, MA,
USA). In order to exclude the interference of cerebral edema after
cerebral IRI, the infarct volume percentage in the ischemic
cerebral hemisphere was calculated using the following equation:
(contralateral hemisphere volume-volume of non-ischemic ipsilateral
hemisphere)/the contralateral hemisphere, as previously reported
(28).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to detect the relative mRNA
expression levels of TNF-α, IL-6, IL-1β, iNOS and HO-1 at 3, 6, 12
and 24 h following reperfusion. Each group contained five samples
and was repeated three times. β-actin was used as internal control.
The brain cortex of ischemia region was homogenized in TRIzol
reagent (Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and total RNA was extracted from the sample
according to the manufacturer's protocol. The concentration of the
total RNA was determined with spectrophotometric optical density
measurement. Reverse transcription reactions were then performed
using the RevertAid First Strand cDNA Synthesis kit (cat. no.
K1622; Thermo Fisher Scientific, Inc.). Each reaction tube
contained 3 µg total RNA in a reaction mixture containing 1 µl
OLigDT, 4 µl 5X reaction buffer, 1 µl ribonuclease inhibitor, 2 µl
10 mM dNTP mix, 1 µl RevertAid M-MuLV Reverse Transcriptase and
diethylpyrocarbonate-treated water to a final volume of 20 µl.
Reverse transcription reactions were performed using a DNA thermal
cycler (T100; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at
42°C for 60 min and 75°C for 5 min. PCR was performed in a DNA
thermal cycler (CFX96; Bio-Rad Laboratories, Inc.) according to the
following standard protocol: One cycle of 94°C for 5 min; 40 cycles
of 94°C for 10 sec, annealing for 10 sec (TNF-α, IL-6 and β-actin:,
52°C; IL-1β:, 51°C; iNOS, 55°C; HO-1, 53°C) and 60°C for 20 sec.
Each reaction tube contained 0.6 µl forward primer, 0.6 µl reverse
primer, 0.6 µl TaqMan probe (Sangon Biotech Co., Ltd., Shanghai,
China), 1 µl cDNA, 10 µl PCR Master Mix (Life Technologies; Thermo
Fisher Scientific, Inc.) and 7.2 µl water. The primer and probe
sequences used are listed in Table
II. The relative mRNA expression levels were calculated with
standardization to β-actin by using the
2−ΔΔCq method (29).
 | Table II.Sequences of primers and probes. |
Table II.
Sequences of primers and probes.
| Gene | Primer
direction/probe | Sequence
(5′-3′) |
|---|
| TNF-α | Forward |
GCCCACGTCGTAGCAA |
|
| Reverse |
GTCTTTGAGATCCATGCCAT |
|
| Probe |
CTCACGCCACTCCAGCTGCTC |
| IL-6 | Forward |
AGAAGACCAGAGCAGATTTT |
|
| Reverse |
GAGAAAGAGTTGTGCAATG |
|
| Probe |
CCAGTTTGGAAGCATCCATC |
| IL-1β | Forward |
GAGCTGAAAGCTCTCCACCT |
|
| Reverse |
TTCCATCTTCTTCTTTGGGT |
|
| Probe |
CCTGTGGCCTTGGGCCTC |
| iNOS | Forward |
ATCGCTGGCTACCAGATGC |
|
| Reverse |
ATGGTCACCTCCAGCACAAG |
|
| Probe |
GCCACCTTGGAGTTCACCCAGTTG |
| HO-1 | Forward |
CCCCACCAAGTTCAAACAGC |
|
| Reverse |
CAATGTTGAGCAGGAAGGCG |
|
| Probe |
CGCATGAACACTCTGGAGATGACCC |
| β-actin | Forward |
GAAGATCAAGATCATTGCTCCT |
|
| Reverse |
TACTCCTGCTTGCTGATCCA |
|
| Probe |
CTGTCCACCTTCCAGCAGA |
Western blotting
Western blotting was used to detect the relative
protein expression levels of TNF-α, IL-6, IL-1β, iNOS and HO-1.
Cortex samples of the ischemic region were harvested 3 h after
reperfusion and homogenized in ice-cold radioimmunoprecipitation
assay lysis buffer (Biosharp, Hefei, China) for 30 min. One
cocktail pill (Roche Diagnostics GmbH, Mannheim, Germany), which
consisted of protease inhibitors, was added to 50 ml buffer. After
cell lysis, samples were centrifuged at 20,392 × g at 4°C for 15
min and the supernatant was collected. The protein concentration of
the supernatant was measured using a bicinchoninic acid protein
assay reagent kit (Beyotime Institute of Biotechnology, Haimen,
China). Subsequently, protein was mixed with sample buffer and
boiled for 5 min. A total of 80 µg protein was loaded per lane and
separated using 10–15% SDS-PAGE at 90 V for 40 min and then 140 V
for 1 h. The separated proteins were transferred from the gel to
cellulose nitrate membranes (GE Healthcare Life Sciences, Chalfont,
UK) and blocked in 5% non-fat milk dissolved in 1X TBS at 37°C for
1 h. Subsequently, membranes were incubated with primary antibody
overnight at 4°C. The following primary antibodies were used:
Rabbit anti-TNF-α (1:200; ab6671; Abcam, Cambridge, UK); rabbit
anti-IL-6 (1:1,000; ab9324; Abcam); rabbit anti-IL-1β (1:500;
ab9722; Abcam); rabbit anti-iNOS (1:1,000; AB5382; EMD Millipore,
Inc., Billerica, MA, USA); rabbit anti-HO-1 (1:1,000; AB1284; EMD
Millipore, Inc.); and mouse anti-β-actin (1:1,000; ab3280; Abcam).
Membranes were washed with 1X TBST three times (10 min each).
Horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody and goat anti-mouse for β-actin (1:5,000; Zhongshan Golden
Bridge Biotechnology Co., Ltd. China) were incubated in 1X TBST for
1 h at room temperature. Membranes were rinsed in TBST three times
and were visualized using enhanced chemiluminescence detection
reagents (Beyotime Institute of Biotechnology). The mean gray value
was measured using Image J 1.43 software and the relative protein
expression was calculated against the ratio of β-actin.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). Values were expressed as mean
± standard deviation. For multiple group comparison, analysis of
variance with Tukey's post hoc test was applied and differences of
P<0.05 were considered to indicate a statistically significant
difference.
Results
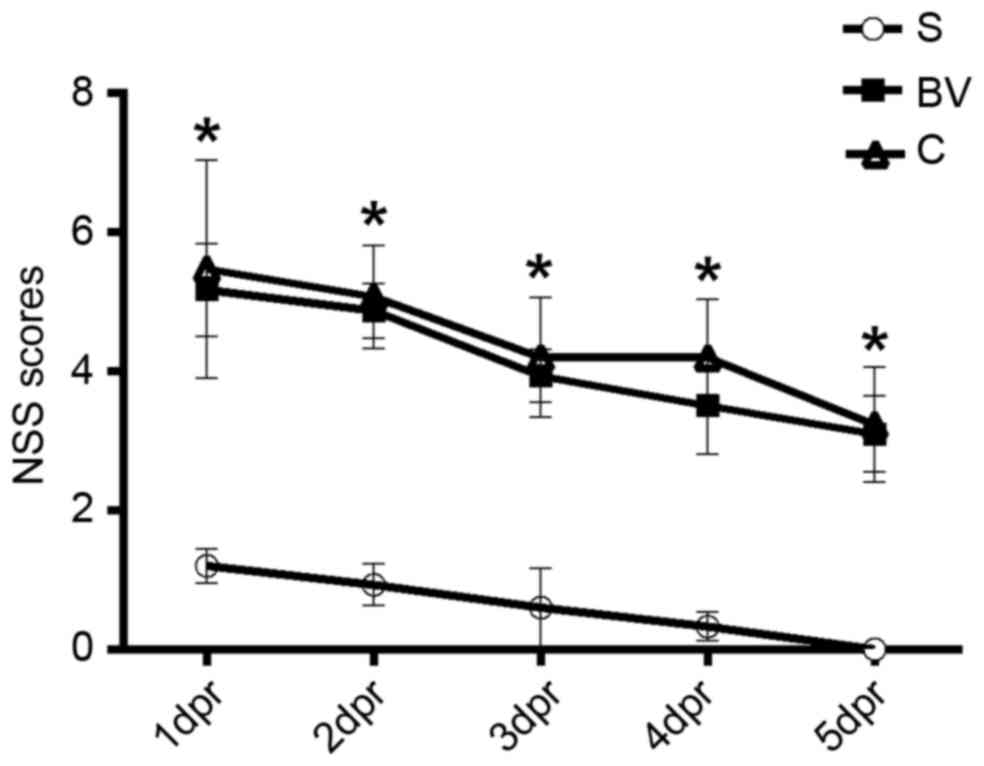
Effect of BV on the neurologic deficit
after reperfusion in rats
Neurological deficit was examined and scored with
NSS at days 1–5 after reperfusion. The scores were 1.20±0.24,
0.93±0.30, 0.60±0.56, 0.33±0.20 and 0 in group S from day 1 to 5,
respectively. Group C rats exhibited scores of 5.47±1.57,
5.07±0.74, 4.20±0.82, 4.20±0.83 and 3.23±0.82 from day 1 to 5,
respectively. Fig. 1 indicates that
group C exhibited significantly increased NSS compared with group S
at all time points (P<0.05). Compared with group C, BV treatment
markedly reduced the NSS of rats in group BV (5.17±0.67, 4.87±0.40,
3.93±0.38, 3.50±0.69 and 3.10±0.55 from day 1 to 5, respectively;
Fig. 1).
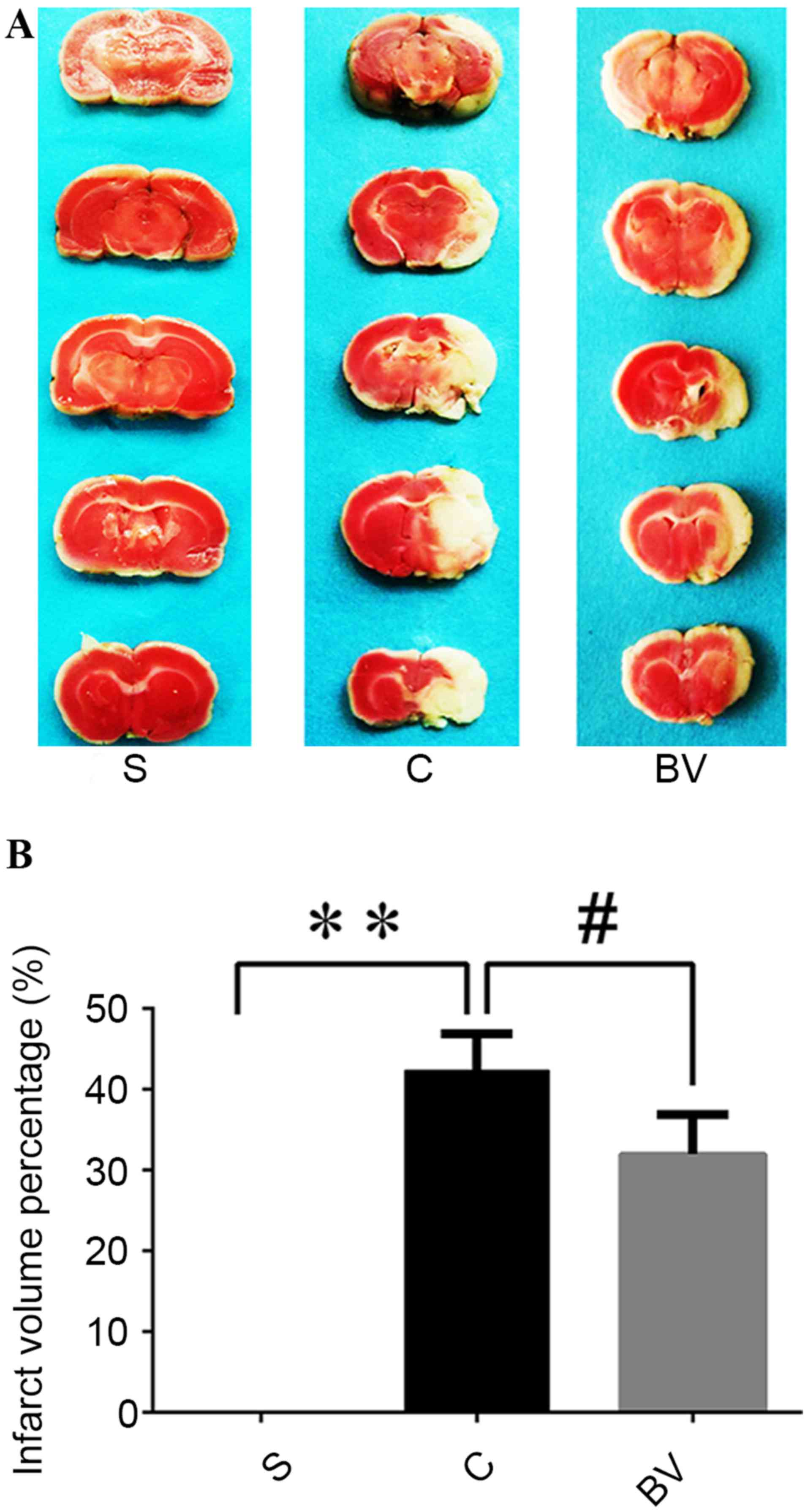
Reduction of cerebral infarction
volume in rats treated with BV
TTC staining was used to detect the infarct volume
48 h after reperfusion. As observed directly, no infarction was
detected in group S, whereas extensive infarction was developed in
the lateral cortex in group C compared with group S; BV treatment
markedly reduced the infarction compared with group C (Fig. 2A). Through quantitative analysis, in
group C, the infarct volume percentage of the ischemic cerebral
hemisphere was significantly higher than that of Group S
(42.28±4.59 vs. 0.00±0.00, respectively; P<0.01; Fig. 2B). However, BV treatment
significantly reduced the infarct volume from 42.28±4.59 to
31.95±4.88 compared with group C (P<0.05; Fig 2B).
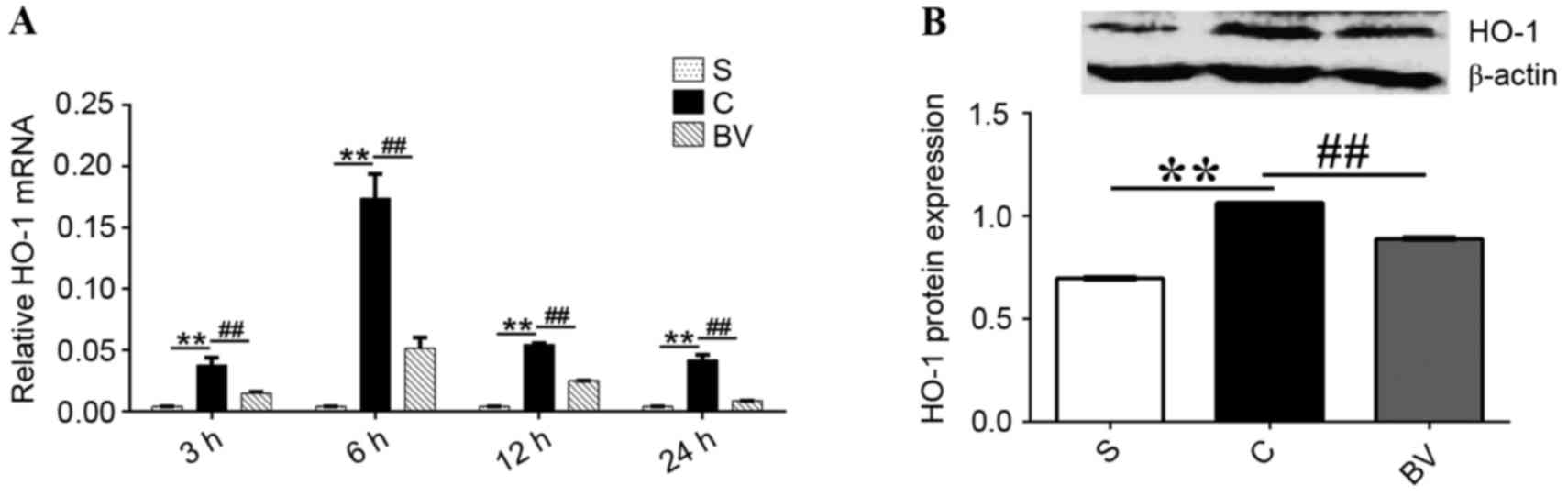
BV treatment inhibited the mRNA and
protein expression levels of HO-1
Compared with group S, the mRNA expression levels of
HO-1 were significantly upregulated at 3, 6, 12 and 24 h following
ischemia reperfusion (P<0.01; Fig.
3A). However, BV treatment significantly downregulated the mRNA
expression levels of HO-1 at these time points compared with group
C (P<0.01; Fig 3A). Additionally,
the protein expression levels of HO-1 were significantly increased
3 h following reperfusion compared with group S (P<0.01);
however, these expression levels were significantly decreased by BV
treatment compared with group C (P<0.01; Fig 3B).
mRNA expression levels of TNF-α, IL-6,
IL-1β and iNOS were downregulated by BV after cerebral IRI
The results of RT-qPCR showed that low expression
levels of TNF-α, IL-6, IL-1β and iNOS mRNA were detected in group
S. However, in group C, the mRNA expression levels of TNF-α, IL-6,
IL-1β and iNOS were significantly increased compared with group S
(P<0.01). Furthermore, TNF-α and iNOS mRNA expression levels in
group C peaked at 3 h then gradually reduced to lower levels.
However, IL-6 and IL-1β mRNA expression levels peaked at 6 h and
gradually decreased to lower levels (Fig. 4A-D). BV treatment significantly
decreased TNF-α and IL-6 mRNA expression at 3, 6, and 12 h
following reperfusion (P<0.01); however, no significant
difference was indicated at 24 h compared with group C (Fig. 4A and B); IL-1β mRNA expression levels
were significantly decreased after BV treatment at 3, 6, 12 and 24
h compared with group C (P<0.01; Fig.
4C). Furthermore, compared with group C, the expression levels
of iNOS mRNA were significantly reduced following BV treatment at 3
and 6 h (P<0.01); however, no significant difference at 12 and
24 h was indicated (P>0.05; Fig.
4D).
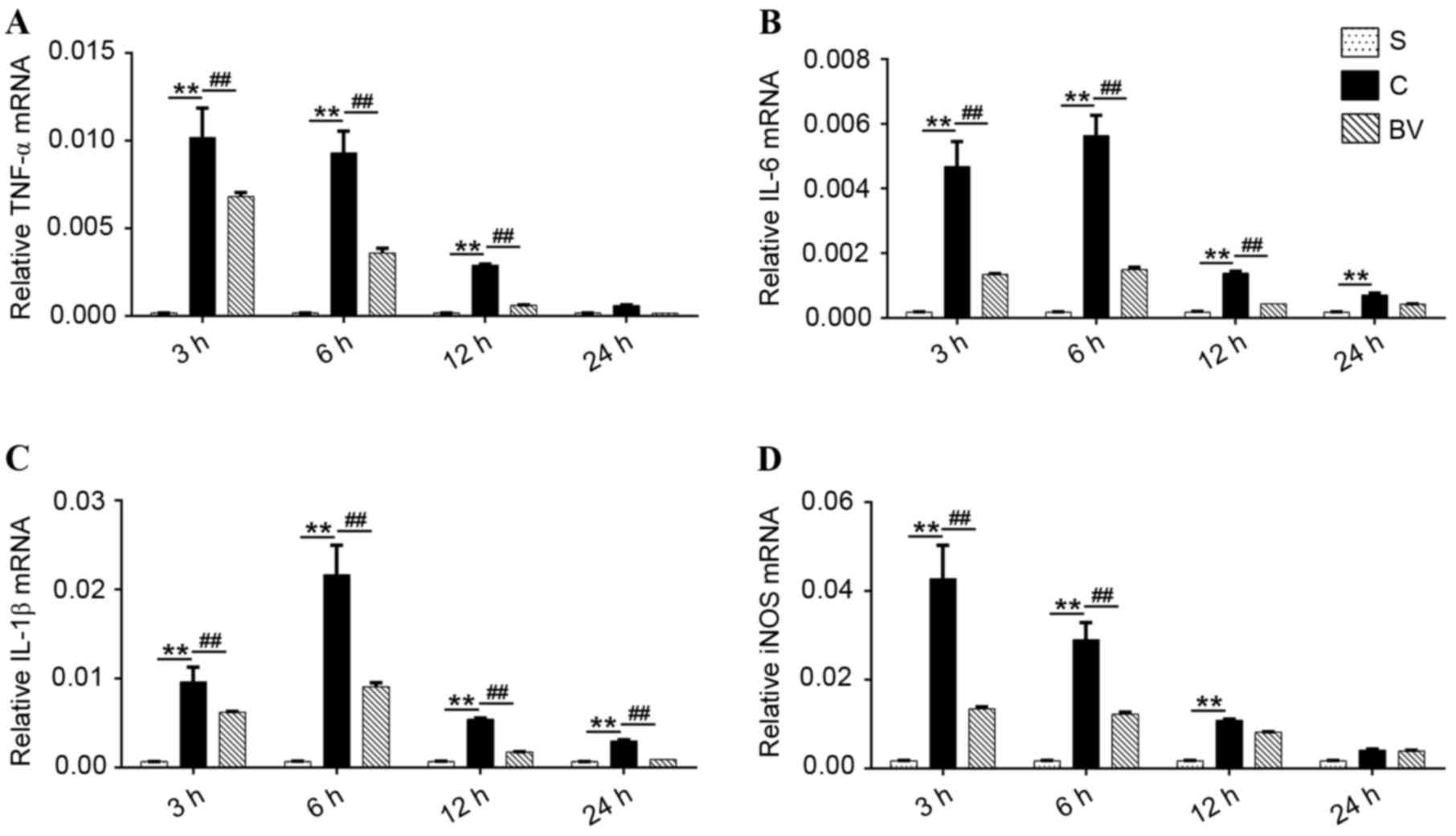 | Figure 4.Effect of BV administration on the
mRNA expression levels of TNF-α, IL-6, IL-1β and iNOS in the
ischemia cortex. (A-D) mRNA expressional changes in TNF-α, IL-6,
IL-1β and iNOS, respectively at 3, 6, 12 and 24 h after
reperfusion. Brain ischemia reperfusion injury significantly
increased the mRNA expression levels of TNF-α, IL-6, IL-1β, and
iNOS. BV administration reversed these changes. Data were presented
as mean ± standard deviation (n=5 in each group). **P<0.01;
##P<0.01. BV, biliverdin; TNF, tumor necrosis factor;
IL, interleukin; iNOS, inducible nitric oxide synthase; S, sham; C,
control; BV, biliverdin. |
BV administration downregulated the
protein expression levels of TNF-α, IL-6, IL-1β and iNOS after
cerebral IRI
Western blotting was used to investigate the effects
of BV on the protein expression levels of TNF-α, IL-6, IL-1β and
iNOS after cerebral IRI (Fig. 5A).
Western blotting analysis revealed that the protein expression
levels of TNF-α, IL-6, IL-1β and iNOS were significantly increased
at 3 h following IRI compared with group S (P<0.01); whereas,
compared with group C, BV treatment significantly decreased the
expression levels of these proteins (P<0.01; Fig. 5B-E).
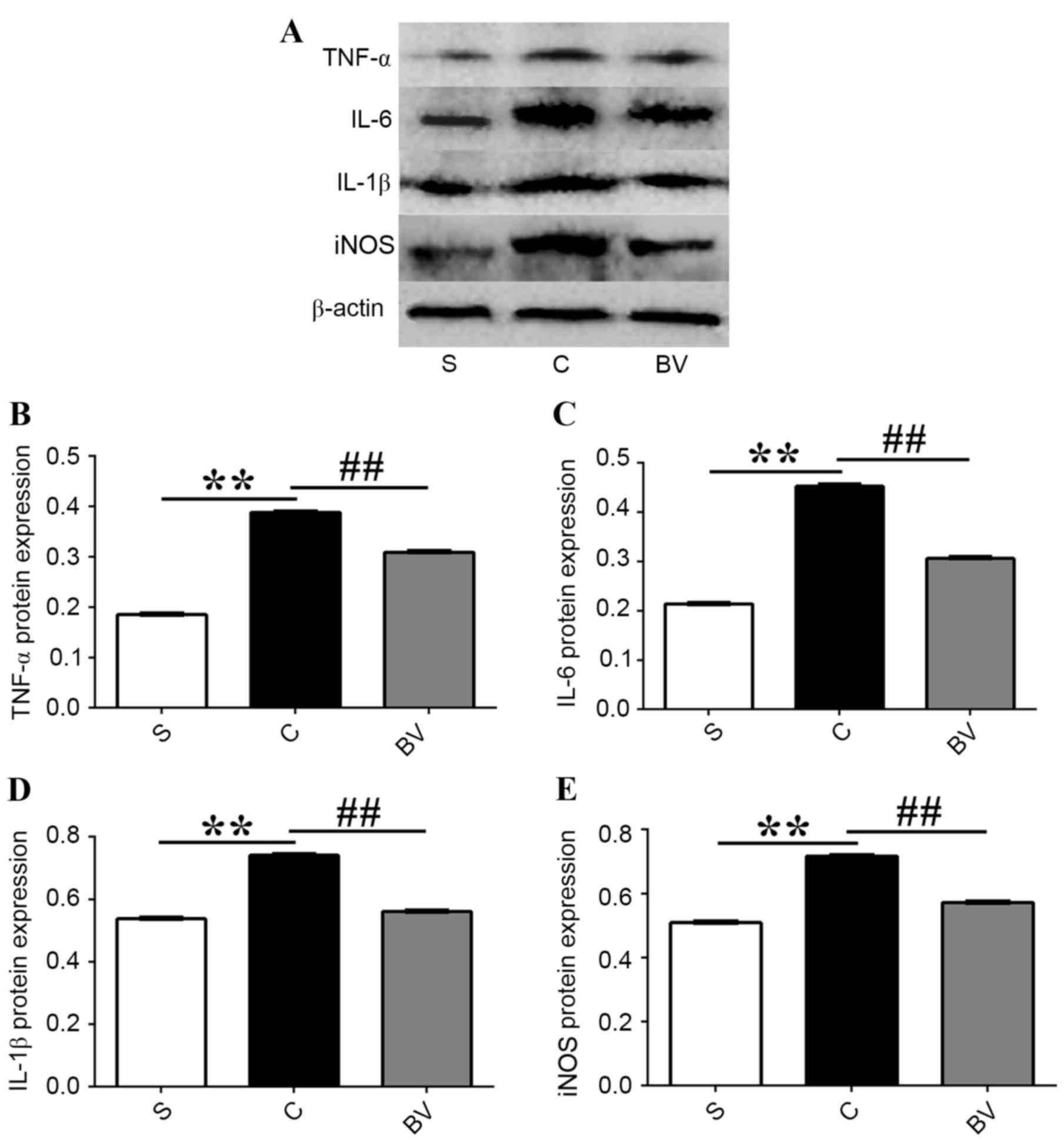 | Figure 5.Effect of BV administration on the
protein expression levels of TNF-α, IL-6, IL-1β and iNOS in the
ischemia cortex 3 h after reperfusion. (A) Western blotting of
TNF-α, IL-6, IL-1β and iNOS 3 h after reperfusion; (B) Relative
protein expression levels of TNF-α, (C) IL-6, (D) IL-1β and (E)
iNOS. Data were presented as mean ± standard deviation (n=5 in each
group). **P<0.01; ##P<0.01. BV, biliverdin; TNF,
tumor necrosis factor; IL, interleukin; iNOS, inducible nitric
oxide synthase; S, sham; C, vehicle control. |
Discussion
The present study investigated whether
intraperitoneal administration of 35 mg/kg BV was able to
ameliorate brain IRI. Furthermore, the feasible mechanism involved
in the downregulation of proinflammatory factors TNF-α, IL-6,
IL-1β, and iNOS was explored.
In the present study, tMCAO was employed to
establish a rat model of cerebral IRI. The NSS, which is a standard
for evaluation of neurological deficit in rats (27), was significantly higher in group C
than that of group S at days 1–5 after reperfusion. In addition,
TTC staining indicated significant brain infarction following IRI.
It is understood that the brain weight accounts for only 2% of body
weight; however, the oxygen consumption of the brain accounts for
20% of the total body oxygen consumption (30). The brain is intensely sensitive to
ischemia and hypoxia; therefore, ischemia following a prolonged
period of time may lead to brain infarction (31). Previous animal studies have
demonstrated that brain tissue may incur more extensive infarction
when blood flow is restored after a period of ischemia than
permanent cerebral ischemia (32,33). The
present study indicated that ischemic cerebral hemisphere
infarction volume increased significantly 48 h after reperfusion,
which revealed that cerebral infarction occurred in the injured
rats. Together, this demonstrates that the present models of tMCAO
were successful and that cerebral ischemia reperfusion damaged the
nervous function.
Results from the present study detected increased
mRNA and protein expression levels of TNF-α, IL-6, IL-1β and iNOS
were accompanied with the neurological deficit. Previous reports
have suggested that the inflammatory response has a vital role in
the process of cerebral ischemia reperfusion and aggravates IRI
(5,11,34).
Therefore, anti-inflammatory treatment may alleviate cerebral IRI
and is also expected to improve the prognosis of ischemic
stroke.
In the present study, 35 mg/kg BV was administered
intraperitoneally 15 min prior to reperfusion, once again 4 h after
reperfusion and twice a day thereafter. BV was able to ameliorate
neurological behavior and significantly reduced brain infarction
after brain ischemia/reperfusion. There are two reasons behind
choosing the 35 mg/kg dose of BV: i) The generated bilirubin after
this dose would be raised but not beyond the highest acceptable
normal range of <1 mg/dl (19);
and ii) BV has been shown to exert anti-inflammatory effects at
this dose in a liver graft IRI model (35).
HO-1 is a protective enzyme, which has a protective
effect on cerebral ischemia and traumatic brain injury (33,36,37).
Cerebral ischemia stress may significantly increase the generation
of HO-1, resulting in the effect of anti-ischemic injury (38,39). BV,
as one of the metabolites of HO-1 catalytic oxidation, has been
shown to influence the change in HO-1 expression in a study on an
inflammatory injury model of the cornea, which demonstrated that BV
treatment downregulated the expression of HO-1 mRNA (22). In addition, BV has also been
indicated to inhibit the expression levels of both HO-1 mRNA and
protein in a study of IRI of liver (40) transplantation as well as in the lungs
of rats (41), The present results
showed that HO-1 mRNA and protein expression levels were
significantly upregulated after ischemia reperfusion; however,
treatment with BV significantly downregulated the expression levels
of HO-1 protein and mRNA, which suggests that BV is able to
successfully initiate a reaction in the body, and reveals that BV
is able to regulate the expression of HO-1 by negative feedback.
Previous studies have demonstrated that BV has potent protective
roles in diverse disease models (35,41–43),
such as a syngeneic small bowel (40,44),
liver transplantation (40), cardiac
and renal transplantation (45),
liver reperfusion injury (46) and
lung reperfusion injury (19)
models. These studies were indicative of the therapeutic potential
of BV in treating clinical diseases. However, whether BV
administration may protect the brain and improve the neurological
function after cerebral IRI is still unknown, and few reports have
focused on this. This mechanism also requires further
investigating.
In the present study, RT-qPCR and western blotting
indicated that the expression levels of TNF-α, IL-6, IL-1β and iNOS
mRNA and protein were significantly upregulated after cerebral IRI.
However, BV treatment significantly downregulated the expression
levels of these factors. This finding indicated that the
inflammatory mediators TNF-α, IL-6, IL-1β and iNOS were largely
generated in brain tissue and promoted the inflammatory response
after cerebral IRI; however, BV effectively reduced the expression
of these factors and inhibited the inflammatory response, thus,
ultimately contributing to the improvement of neurological function
after cerebral IRI.
In the early stage of cerebral ischemia/reperfusion,
proinflammatory cytokines TNF-α and IL-1β are key factors that
promote the inflammatory response and initiate the process of
inflammatory reaction, promote the expression of cell adhesion
molecules and the infiltration of peripheral leukocytes, thus
aggravating brain tissue damage caused by ischemia reperfusion
(34,47–49). As
reported previously (13), TNF-α,
IL-6 and IL-1β mRNA were significantly upregulated in the ischemic
cortex from 3–72 h and peaked at 6 h after ischemia. Therefore,
TNF-α, IL-6 and IL-1β are considered three of the most important
cytokines implicated in cerebral IRI (13,50).
iNOS is an important proinflammatory mediator during the
inflammatory response and expression of iNOS has been indicated to
be significantly increased by ischemia reperfusion of the intestine
(44), heart and kidneys (45). Similarly, the present findings
revealed that the expression levels of iNOS were significantly
upregulated after focal cerebral ischemia reperfusion and that BV
administration significantly decreased iNOS expression. BV, one of
the three products of heme catabolism, has been demonstrated to
have a protective role in various models of inflammation, liver and
lung IRI and organ transplantation (43,44,46), and
it has been reported to have cytoprotective, anti-inflammatory, and
anti-oxidant effects (42). However,
the possible molecular mechanism of the protective role of BV in
cerebral IRI remains unknown. On probing the mechanism underlying
the protective effects of BV administration on brain tissue after
IRI, we discovered that BV administration repressed cerebral
IRI-induced gene and protein expression of the inflammatory
mediators TNF-α, IL-6, IL-1β and iNOS. To the best of our
knowledge, the present study is the first to suggest that the
protective effects of BV administration on cerebral IRI may occur
by targeting some elements within the anti-inflammatory pathway.
The present study has provided novel insights into the
anti-inflammatory effects of BV on a defined model of cerebral IRI
(tMCAO) (Fig. 6).
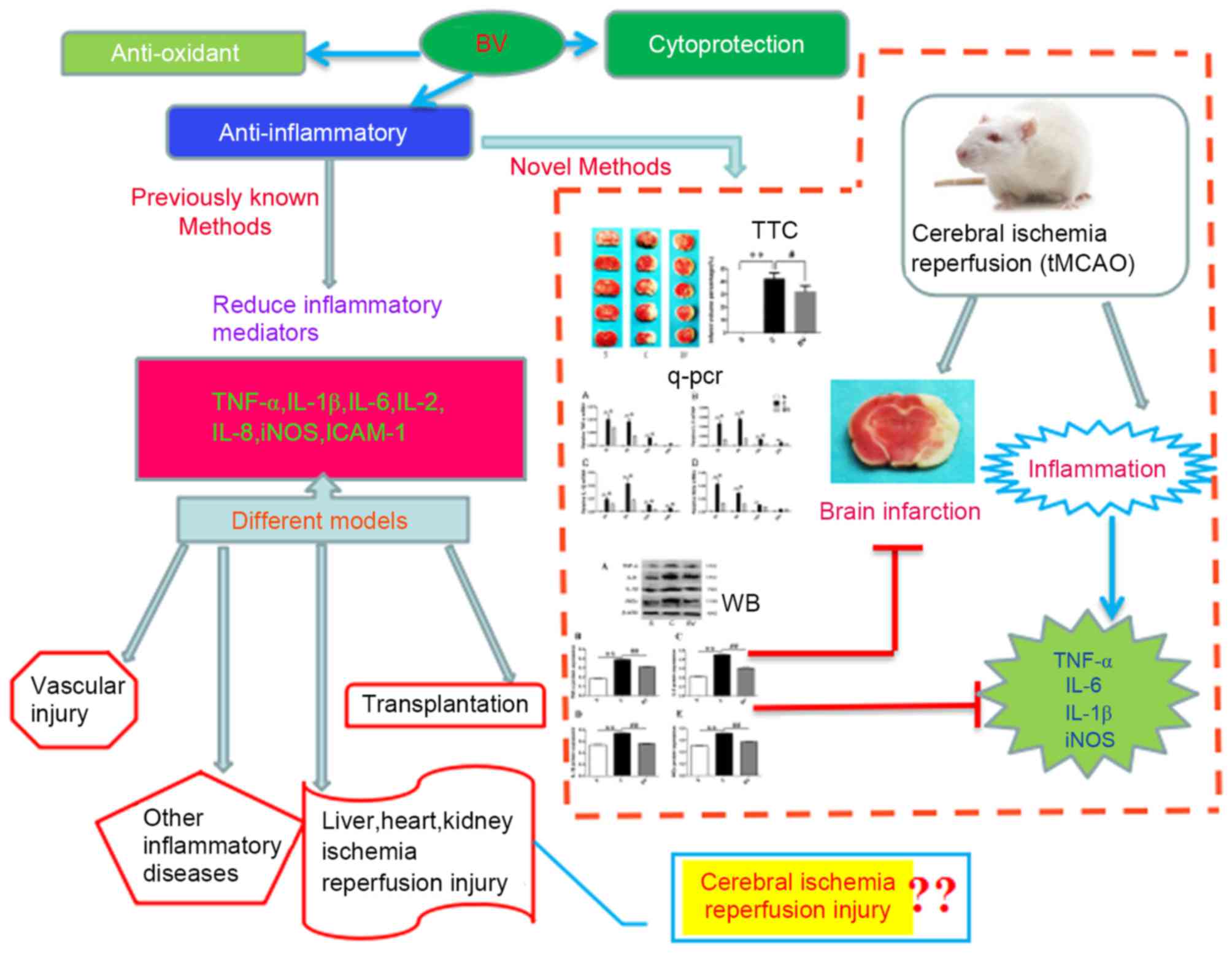 | Figure 6.Summaries for the protective role and
related mechanisms of BV on the disease models. As previously
reported, BV administration may have a protective role in diverse
disease models, such as vascular injury, organ transplantation,
ischemia reperfusion injury of liver, heart and kidney and other
inflammatory diseases. The underlying mechanisms were associated
with cytoprotection, anti-inflammation and antioxidant production.
In addition, the present study concluded that BV has a protective
role in the cerebral ischemia reperfusion injury by promoting
downregulation of TNF-α, IL-6, IL-1β and iNOS, indicating BV may be
applied in the treatment of cerebral ischemia reperfusion injury
and reduce inflammation. BV, biliverdin; TNF, tumor necrosis
factor; IL, interleukin; iNOS, inducible nitric oxide synthase;
ICAM-1, intercellular adhesion molecule 1; tMCAO, transient middle
cerebral artery occlusion; WB, western blotting; qPCR, quantitative
polymerase chain reaction; TTC, 2,3,5-triphenyltetrazolium
chloride. |
In conclusion, the present study indicated that
cerebral IRI induced inflammation, as indicated by the increase in
gene and protein expression levels of inflammatory mediators TNF-α,
IL-6, IL-1β and iNOS. BV treatment appeared to promote the
downregulation of these inflammatory mediators and reduced the
extent of cerebral infarction. Together, these findings suggested
that BV suppressed IRI-induced brain injury, at least in part,
through anti-inflammatory mechanisms.
Acknowledgements
The present study was supported by a grant from The
Cultivation of Youth Leaders in Academic and Technical Talents
Foundation of Yunnan Province, China (CN) (grant no. 2012HB030).
The authors also would like to thank the Medical University of
Kunming (Kunming, China) for its technical support.
References
|
1
|
Zhang M, Wang S, Mao L, Leak RK, Shi Y,
Zhang W, Hu X, Sun B, Cao G, Gao Y, et al: Omega-3 fatty acids
protect the brain against ischemic injury by activating Nrf2 and
upregulating heme oxygenase 1. J Neurosci. 34:1903–1915. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Y, Fu B, Zhang X, Zhao T, Chen L,
Zhang J and Wang X: Paeonol pretreatment attenuates cerebral
ischemic injury via upregulating expression of pAkt, Nrf2, HO-1 and
ameliorating BBB permeability in mice. Brain Res Bull. 109:61–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanderson TH, Reynolds CA, Kumar R,
Przyklenk K and Huttemann M: Molecular mechanisms of
ischemia-reperfusion injury in brain: Pivotal role of the
mitochondrial membrane potential in reactive oxygen species
generation. Mol Neurobiol. 47:9–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tuma RF and Steffens S: Targeting the
endocannabinod system to limit myocardial and cerebral ischemic and
reperfusion injury. Curr Pharm Biotechnol. 13:46–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishibashi N, Prokopenko O, Reuhl KR and
Mirochnitchenko O: Inflammatory response and glutathione peroxidase
in a model of stroke. J Immunol. 168:1926–1933. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan J, Konstas AA, Bateman B, Ortolano GA
and Pile-Spellman J: Reperfusion injury following cerebral
ischemia: Pathophysiology, MR imaging, and potential therapies.
Neuroradiology. 49:93–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai F, Li CR, Wu JL, Chen JG, Liu C, Min
Q, Yu W, Ouyang CH and Chen JH: Theaflavin ameliorates cerebral
ischemia-reperfusion injury in rats through its anti-inflammatory
effect and modulation of STAT-1. Mediators Inflamm. 2006:304902006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amantea D, Nappi G, Bernardi G, Bagetta G
and Corasaniti MT: Post-ischemia brain damage: Pathophysiology and
role of inflammatory mediators. FEBS J. 276:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beech JS, Reckless J, Mosedale DE,
Grainger DJ, Williams SC and Menon DK: Neuroprotection in
ischemia-reperfusion injury: An antiinflammatory approach using a
novel broad-spectrum chemokine inhibitor. J Cereb Blood Flow Metab.
21:683–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Wang L, Zhang X, Cui L, Xing Y,
Dong L, Liu Z, Li Y, Zhang X, Wang C, et al: The protection by
octreotide against experimental ischemic stroke: Up-regulated
transcription factor Nrf2, HO-1 and down-regulated NF-κB
expression. Brain Res. 1475:80–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frangogiannis NG: Chemokines in ischemia
and reperfusion. Thromb Haemost. 97:738–747. 2007.PubMed/NCBI
|
|
12
|
Wong CH and Crack PJ: Modulation of
neuro-inflammation and vascular response by oxidative stress
following cerebral ischemia-reperfusion injury. Curr Med Chem.
15:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berti R, Williams AJ, Moffett JR, Hale SL,
Velarde LC, Elliott PJ, Yao C, Dave JR and Tortella FC:
Quantitative real-time RT-PCR analysis of inflammatory gene
expression associated with ischemia-reperfusion brain injury. J
Cereb Blood Flow Metab. 22:1068–1079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill-Kapturczak N, Jarmi T and Agarwal A:
Growth factors and heme oxygenase-1: Perspectives in physiology and
pathophysiology. Antioxid Redox Signal. 9:2197–2207. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharp FR, Zhan X and Liu DZ: Heat shock
proteins in the brain: Role of Hsp70, Hsp 27 and HO-1 (Hsp32) and
their therapeutic potential. Transl Stroke Res. 4:685–692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang C, Cang J, Wang H and Xue Z:
Propofol attenuates cerebral ischemia/reperfusion injury partially
using heme oxygenase-1. J Neurosurg Anesthesiol. 25:311–316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Wang J, Li Y, Fan C, Jiang S, Zhao
L, Di S, Xin Z, Wang B, Wu G, et al: HO-1 Signaling activation by
pterostilbene treatment attenuates mitochondrial oxidative damage
induced by cerebral ischemia reperfusion injury. Mol Neurobiol.
53:2339–2353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Li X, Zhang L, Liu L, Jing G and
Cai H: Ginsenoside Rg1 suppressed inflammation and neuron apoptosis
by activating PPARγ/HO-1 in hippocampus in rat model of cerebral
ischemia-reperfusion injury. Int J Clin Exp Pathol. 8:2484–2494.
2015.PubMed/NCBI
|
|
19
|
Sarady-Andrews JK, Liu F, Gallo D, Nakao
A, Overhaus M, Ollinger R, Choi AM and Otterbein LE: Biliverdin
administration protects against endotoxin-induced acute lung injury
in rats. Am J Physiol Lung Cell Mol Physiol. 289:L1131–L1137. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wegiel B, Baty CJ, Gallo D, Csizmadia E,
Scott JR, Akhavan A, Chin BY, Kaczmarek E, Alam J, Bach FH, et al:
Cell surface biliverdin reductase mediates biliverdin-induced
anti-inflammatory effects via phosphatidylinositol 3-kinase and
Akt. J Biol Chem. 284:21369–21378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wegiel B, Gallo D, Csizmadia E, Roger T,
Kaczmarek E, Harris C, Zuckerbraun BS and Otterbein LE: Biliverdin
inhibits Toll-like receptor-4 (TLR4) expression through nitric
oxide-dependent nuclear translocation of biliverdin reductase. Proc
Natl Acad Sci USA. 108:pp. 18849–18854. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bellner L, Wolstein J, Patil KA, Dunn MW
and Laniado-Schwartzman M: Biliverdin rescues the HO-2 null mouse
phenotype of unresolved chronic inflammation following corneal
epithelial injury. Invest Ophthalmol Vis Sci. 52:3246–3253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagami T, Toyomura K, Kinoshita T and
Morisawa S: A beneficial role of bile pigments as an endogenous
tissue protector: Anti-complement effects of biliverdin and
conjugated bilirubin. Biochim Biophys Acta. 1158:189–193. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guha M and Mackman N: The
phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide
activation of signaling pathways and expression of inflammatory
mediators in human monocytic cells. J Biol Chem. 277:32124–32132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng YC, Ding ZY, Wang HQ, Ning LP and
Wang C: Effect of microRNA-155 on angiogenesis after cerebral
infarction of rats through AT1R/VEGFR2 pathway. Asian Pac J Trop
Med. 8:829–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao
J, Lei J, Yang L, Wang K, Chen L, et al: Therapeutic application of
gene silencing MMP-9 in a middle cerebral artery occlusion-induced
focal ischemia rat model. Exp Neurol. 216:35–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Welling LC, Welling MS, Teixeira MJ and
Figueiredo EG: Fueling the brain-a new role in lactate metabolism.
World Neurosurg. 84:611–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Xue Z and Jiang H: Effect of
propofol on pathologic time-course and apoptosis after cerebral
ischemia-reperfusion injury. Acta Anaesthesiol Scand. 52:413–419.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang GY and Betz AL: Reperfusion-induced
injury to the blood-brain barrier after middle cerebral artery
occlusion in rats. Stroke. 25:1658–1665. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aronowski J, Strong R and Grotta JC:
Reperfusion injury: Demonstration of brain damage produced by
reperfusion after transient focal ischemia in rats. J Cereb Blood
Flow Metab. 17:1048–1056. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stanimirovic D and Satoh K: Inflammatory
mediators of cerebral endothelium: A role in ischemic brain
inflammation. Brain Pathol. 10:113–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang LM, Wang YP, Wang K, Pu LY, Zhang F,
Li XC, Kong LB, Sun BC, Li GQ and Wang XH: Exogenous biliverdin
ameliorates ischemia-reperfusion injury in small-for-size rat liver
grafts. Transplant Proc. 39:pp. 1338–1344. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beschorner R, Adjodah D, Schwab JM,
Mittelbronn M, Pedal I, Mattern R, Schluesener HJ and Meyermann R:
Long-term expression of heme oxygenase-1 (HO-1, HSP-32) following
focal cerebral infarctions and traumatic brain injury in humans.
Acta Neuropathol. 100:377–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chao XD, Ma YH, Luo P, Cao L, Lau WB, Zhao
BC, Han F, Liu W, Ning WD, Su N, et al: Up-regulation of heme
oxygenase-1 attenuates brain damage after cerebral ischemia via
simultaneous inhibition of superoxide production and preservation
of NO bioavailability. Exp Neurol. 239:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aztatzi-Santillán E, Nares-López FE,
Márquez-Valadez B, Aguilera P and Chánez-Cárdenas ME: The
protective role of heme oxygenase-1 in cerebral ischemia. Cent Nerv
Syst Agents Med Chem. 10:310–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JC, Kim IH, Park JH, Ahn JH, Cho JH,
Cho GS, Tae HJ, Chen BH, Yan BC, Yoo KY, et al: Ischemic
preconditioning protects hippocampal pyramidal neurons from
transient ischemic injury via the attenuation of oxidative damage
through upregulating heme oxygenase-1. Free Radic Biol Med.
79:78–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fondevila C, Shen XD, Tsuchiyashi S,
Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski
JW and Bach FH: Biliverdin therapy protects rat livers from
ischemia and reperfusion injury. Hepatology. 40:1333–1341. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Zhou HC, Pan P, Zhang N and Li WZ:
Exogenous biliverdin improves the function of lung grafts from
brain dead donors in rats. Transplant Proc. 42:pp. 1602–1609. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kosaka J, Morimatsu H, Takahashi T,
Shimizu H, Kawanishi S, Omori E, Endo Y, Tamaki N, Morita M and
Morita K: Effects of biliverdin administration on acute lung injury
induced by hemorrhagic shock and resuscitation in rats. PLoS One.
8:e636062013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Overhaus M, Moore BA, Barbato JE, Behrendt
FF, Doering JG and Bauer AJ: Biliverdin protects against
polymicrobial sepsis by modulating inflammatory mediators. Am J
Physiol Gastrointest Liver Physiol. 290:G695–G703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakao A, Otterbein LE, Overhaus M, Sarady
JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F,
et al: Biliverdin protects the functional integrity of a
transplanted syngeneic small bowel. Gastroenterology. 127:595–606.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakao A, Neto JS, Kanno S, Stolz DB,
Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE and
Murase N: Protection against ischemia/reperfusion injury in cardiac
and renal transplantation with carbon monoxide, biliverdin and
both. Am J Transplant. 5:282–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fondevila C, Katori M, Lassman C, Carmody
I, Busuttil RW, Bach FH and Kupiec-Weglinski JW: Biliverdin
protects rat livers from ischemia/reperfusion injury. Transplant
Proc. 35:pp. 1798–1799. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stoll G, Kleinschnitz C and Nieswandt B:
Combating innate inflammation: A new paradigm for acute treatment
of stroke? Ann N Y Acad Sci. 1207:149–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maddahi A and Edvinsson L: Cerebral
ischemia induces microvascular pro-inflammatory cytokine expression
via the MEK/ERK pathway. J Neuroinflammation. 7:142010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Denes A, Thornton P, Rothwell NJ and Allan
SM: Inflammation and brain injury: Acute cerebral ischaemia,
peripheral and central inflammation. Brain Behav Immun. 24:708–723.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yaidikar L and Thakur S: Punicalagin
attenuated cerebral ischemia-reperfusion insult via inhibition of
proinflammatory cytokines, up-regulation of Bcl-2, down-regulation
of Bax, and caspase-3. Mol Cell Biochem. 402:141–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|