Introduction
Atherosclerosis is a chronic inflammatory disease,
characterized by progressive accumulation of lipids and cellular
and fibrous elements in the arterial wall (1,2).
Macrophages are involved in various aspects of atherosclerosis
(3,4). In particular, macrophage-mediated
uptake of modified low-density lipoprotein (LDL) and subsequent
transformation into foam cells serve a critical role in the
development of atherosclerosis (5).
Lipid accumulation in macrophages leads to the activation of
signaling pathways that involve activation of peroxisome
proliferator-activated receptor-γ (PPARγ) and liver X receptor α
(LXRα), which are transcription factors controlling the macrophage
cholesterol homeostasis (6).
Activated PPARγ and LXRα are synergistically implicated in the
transactivation of several genes, such as the ATP-binding cassette
transporter (ABCA1) and ABCG1, the products of which are involved
in regulating the macrophage cholesterol efflux and triggering
reverse cholesterol transport (7),
which is part of a ‘self-protection mechanism’ for macrophages.
The protein Tribbles homolog 1 (Trib1), a member of
the recently identified Tribbles protein family, is considered to
function as an adaptor or scaffold protein (8). Burkhardt et al have reported
that hepatic expression of Trib1 regulates the plasma levels of
LDL-cholesterol (LDL-C), very-LDL (VLDL), and triglyceride (TG) in
mice (9). In addition, Satoh et
al demonstrated that mice lacking Trib1 expression in
hematopoietic cells developed hypertriglyceridemia and insulin
resistance in response to a high-fat diet (10). These authors also suggested that
Trib1 is critical for adipose tissue maintenance and inhibition of
metabolic disorders by regulating the differentiation of
tissue-resident M2-like macrophages (10). The aforementioned findings indicate
that Trib1 serves an important role in lipoprotein metabolism.
However, the functions of Trib1 in cholesterol efflux remain
largely unknown.
In the present study, the THP-1 cell model was used
to explore the role of Trib1 in the formation of macrophage foam
cells during atherosclerosis. The effect of overexpression of Trib1
on lipid accumulation and cholesterol efflux was investigated in
macrophages, and associated molecular mechanisms were also
explored.
Materials and methods
Cell culture and differentiation
THP-1 human monocytic cell line was purchased from
the Shanghai Cell Institute of the Chinese Academy of Sciences
(Shanghai, China). THP-1 cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 µg/ml) at 37°C in 5% CO2. To
differentiate into macrophages, THP-1 monocytes were exposed to 160
nM phorbol-12-myristate-13-acetate (Sigma-Aldrich, St. Louis, MO,
USA) for 72 h. The differentiated macrophages were washed three
times with phosphate-buffered saline (PBS) and incubated in fresh
serum-free medium containing 50 µg/ml oxidized LDL (ox-LDL; Yiyuan
Biotechnology Co., Ltd., Guangzhou, China) for 48 h.
Cell transfection
In order to investigate the effect of Trib1
overexpression in THP-1 macrophages, the cells were transfected
with pCMV6-Entry-Trib1 (Trib1 overexpressing group) or pCMV6-Entry
vector (empty vector group) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The pCMV6-Entry-Trib1
and empty vector were purchased from OriGene Technologies, Inc.
(Rockville, MD, USA). Non-transfected THP-1 macrophages were used
as a control. For small interfering RNA (siRNA)-mediated knockdown
experiments, THP-1 macrophages were co-transfected with
pCMV6-Entry-Trib1 and LXRα siRNA or PPARγ siRNA (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA). After 24 h of
transfection, cells were incubated with ox-LDL (50 µg/ml) in
serum-free medium for an additional 24 h before conducting the
expression or functional studies.
Morphological examination
Non-transfected or transfected THP-1 macrophages
were cultured in chamber slides in serum-free medium. After 24-h
incubation, cells were washed three times in PBS and fixed with 5%
formalin solution for 30 min. Next, the cells were stained with oil
red O (Sigma-Aldrich) for 30 min, and counterstained with
hematoxylin for 5 min. Finally, cells were analyzed by light
microscopy (Axio Imager 2; Zeiss, Oberkochen, Germany). Five
selected high-power fields at a magnification of ×400 were randomly
selected for examination. Semi-quantitative analysis of oil red O
positive staining was conducted by the ImageJ software (version
1.48, National Institutes of Health, Bethesda, MD, USA).
Cholesterol efflux
In order to investigate the cholesterol efflux,
non-transfected or transfected THP-1 macrophages (5×105
cells/well) were seeded into 12-well plates, labeled with 0.5
µCi/ml [3H]-cholesterol (PerkinElmer, Waltham, MA, USA)
in media containing 0.2% bovine serum albumin for 24 h and then
washed with fresh media. Then, cells were washed with PBS and
incubated in the presence of 10 µg/ml apolipoprotein A-I (apoA-I;
EMD Millipore, Billerica, MA, USA) for 18 h. Medium and
cell-associated [3H] cholesterol were examined through
liquid scintillation counting. The percentage of cholesterol efflux
was calculated with the following equation: [Total media
radioactivity/(total cellular radioactivity + total media
radioactivity)] × 100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from THP-1 macrophages with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total
RNA concentration was measured by spectrophotometry with a NanoDrop
2000 Spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA
synthesis was performed using the PrimeScript RT reagent Kit
according to the manufacturer's instructions (catalog no. RR037A;
Takara, Shiga, Japan). qPCR analysis was then conducted using a
Light Cycler-FastStart DNA Master SYBR-Green I kit (Roche Molecular
Biochemicals, Manheim, Germany). The sequences of the primers used
in the current study are shown in Table
I. Data were analyzed with the 2−ΔΔCq relative
quantification method (11). Values
are presented as fold change relative to control cells.
 | Table I.Primers used in quantitative real-time
polymerase chain reaction analysis. |
Table I.
Primers used in quantitative real-time
polymerase chain reaction analysis.
| Gene | Forward primer | Reverse primer |
|---|
| ABCA1 |
5′-ATCTCATAGTATGGAAGAATGTGAAGCT-3′ |
5′-CGTACAACTATTGTATAACCATCTCCAAA-3′ |
| ABCG1 |
5′-AGGTCTCAGCCTTCTAAAGTTCCTC-3′ |
5′-TCTCTCGAAGTGAATGAAATTTATCG-3′ |
| LXRα |
5′-TCAGCATCTTCTCTGCAGACCGG-3′ |
5′-TCATTAGCATCCGTGGGAACA-3′ |
| PPARγ |
5′-TGAACAAAGACGGGATG-3′ |
5′-TCAAACTTGGGTTCCATGAT-3′ |
| β-actin |
5′-TCATGAAGTGTGACGTTGACATCCGT-3′ |
5′-CTTAGAAGCATTTGCGGTGCACGATG-3′ |
Western blot analysis
After the indicated treatment, cells were washed
with ice-cold PBS and harvested in lysis buffer [30 mM HEPES, pH
7.6, 30 mM NaCl, 1% Nonidet P-40 (vol/vol), 10% glycerol (vol/vol),
50 mM NaF and 10 mM Na pyrophosphate] supplemented with 5 mM Na
orthovanadate and protease inhibitors (Roche Diagnostics,
Indianapolis, IN, USA). Cell lysates were collected by
centrifugation at 14,000 × g for 5 min at 4°C, and the protein
concentration in total lysates was analyzed by the BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Next, 20 µg
total protein were subjected to 10% SDS-PAGE and transferred onto a
nitrocellulose membrane (Whatman; Sigma-Aldrich). Membranes were
blocked overnight with 5% non-fat dry milk in Tris-buffered saline
containing 0.1% Tween-20 for 1 h at room temperature. The membranes
were then probed overnight at 4°C with the following primary
antibodies: Anti-TRIB1 (ab137717; 1:200), anti-ABCA1 (ab18180;
1:200), anti-ABCG1 (ab155918; 1:200), anti-LXRα (ab82774; 1:200),
anti-PPARγ (ab24509; 1:200) and anti-β-actin (ab97379; 1:500; all
from Abcam, Cambridge, MA, USA). Subsequently, samples were
incubated with horseradish peroxidase-conjugated goat anti-mouse
IgG (ab97265; 1:2,000 dilution) or goat anti-rabbit IgG (ab97200;
1:2,000 dilution; both from Abcam) for 1 h at room temperature.
Final detection was performed using an ECL chemiluminescence system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and quantitative
results were obtained using Quantity One software (version 4.4.0;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Results were analyzed by one-way analysis of variance,
followed by the Tukey's post hoc test, using SPSS version 11.0
software (SPSS, Inc., Chicago, IL, USA). P-values of <0.05 were
considered to indicate differences that were statistically
significant.
Results
Overexpression of Trib1 inhibits lipid
accumulation and enhances cholesterol efflux in ox-LDL-stimulated
THP-1 macrophages
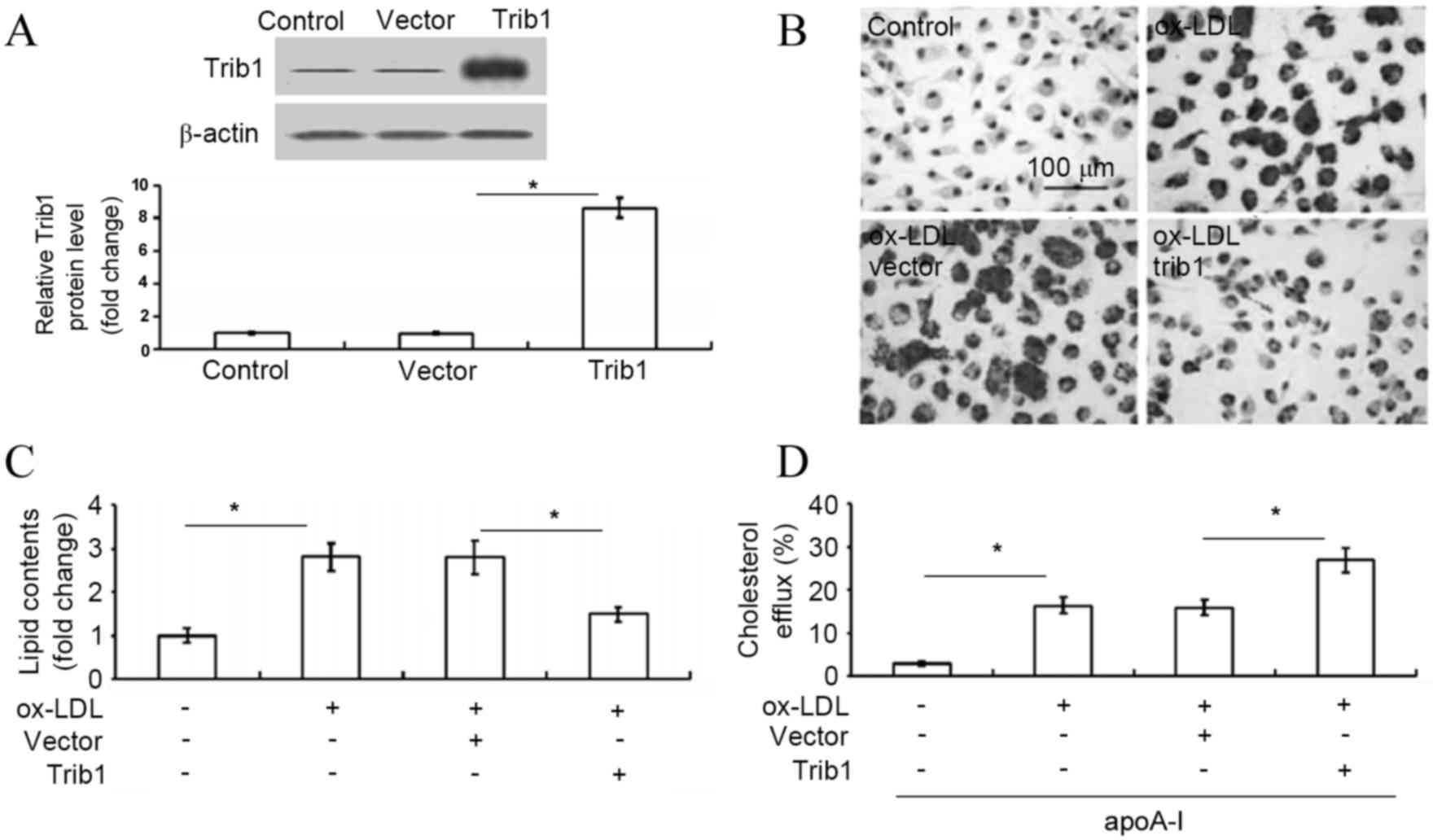
Transfection of THP-1 macrophages with
pCMV6-Entry-Trib1 resulted in significant overexpression of Trib1
(increased by ~8.6-fold), as determined by western blot analysis
(P<0.05 vs. vector-transfected cells; Fig. 1A). Subsequently, in order to explore
the intracellular lipid deposition in response to Trib1
overexpression in THP-1 macrophages, the intracellular cholesterol
levels were analyzed. Forced expression of Trib1 significantly
inhibited lipid accumulation as evidenced by oil red O staining
(P<0.05; Fig. 1B and C).
Furthermore, exogenous expression of Trib1 significantly enhanced
the apoA-I-mediated cholesterol efflux in ox-LDL-stimulated THP-1
cells (P<0.05; Fig. 1D). These
results revealed that Trib1 inhibited the intracellular lipid
deposition due to increased apoA-I-mediated cholesterol efflux in
THP-1 macrophages.
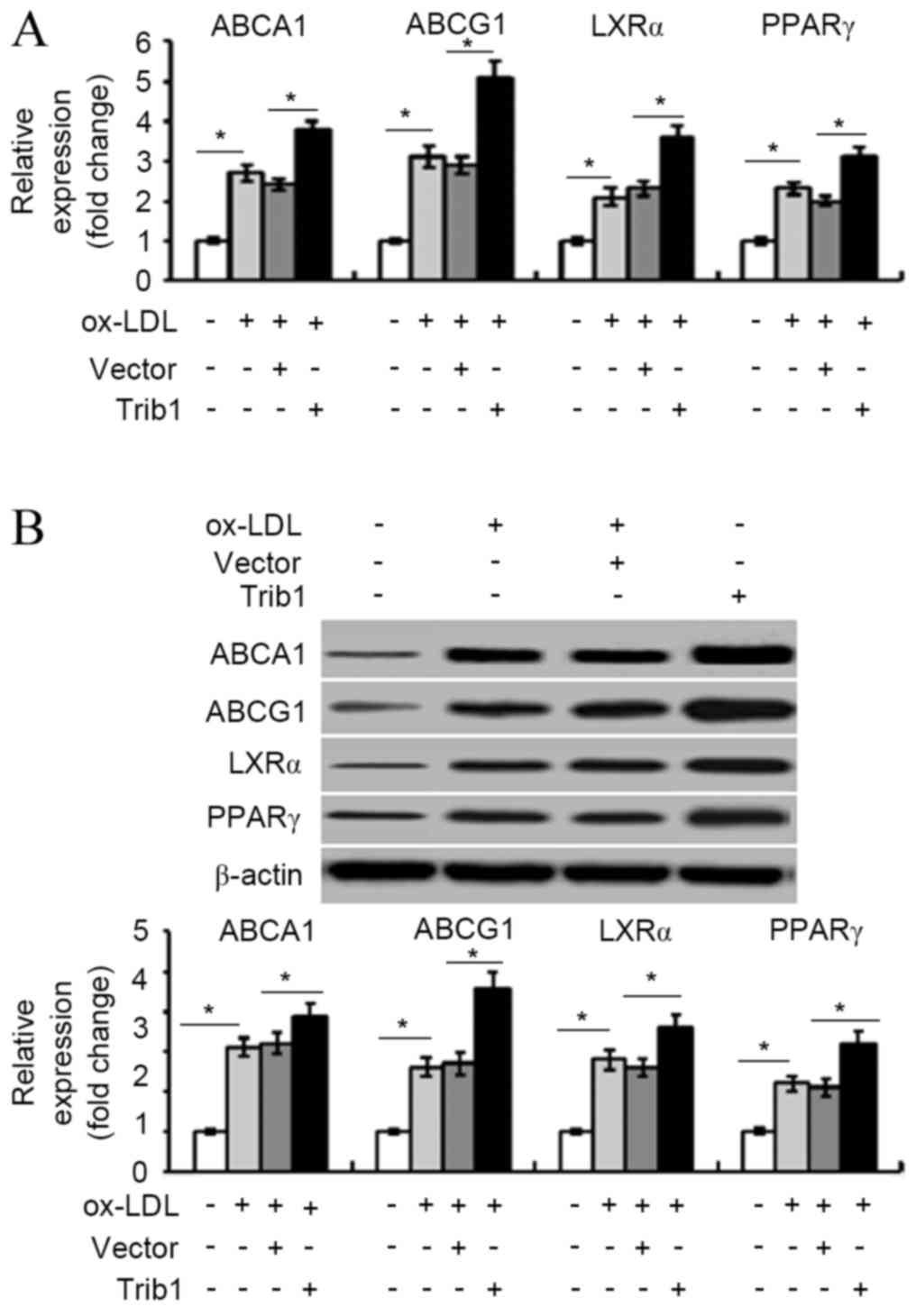
Forced expression of Trib1 increases
the expression of ABCA1, ABCG1, LXRα and PPARγ
RT-qPCR analysis demonstrated that overexpression of
Trib1 significantly increased the ABCA1, ABCG1, LXRα and PPARγ mRNA
expression levels in ox-LDL-stimulated THP-1 macrophages
(P<0.05; Fig. 2A). Similarly,
western blot analysis indicated that Trib1 overexpression led to a
significant (1.5–2-fold) increase in the levels of ABCA1, ABCG1,
LXRα, and PPARγ protein (P<0.05; Fig.
2B).
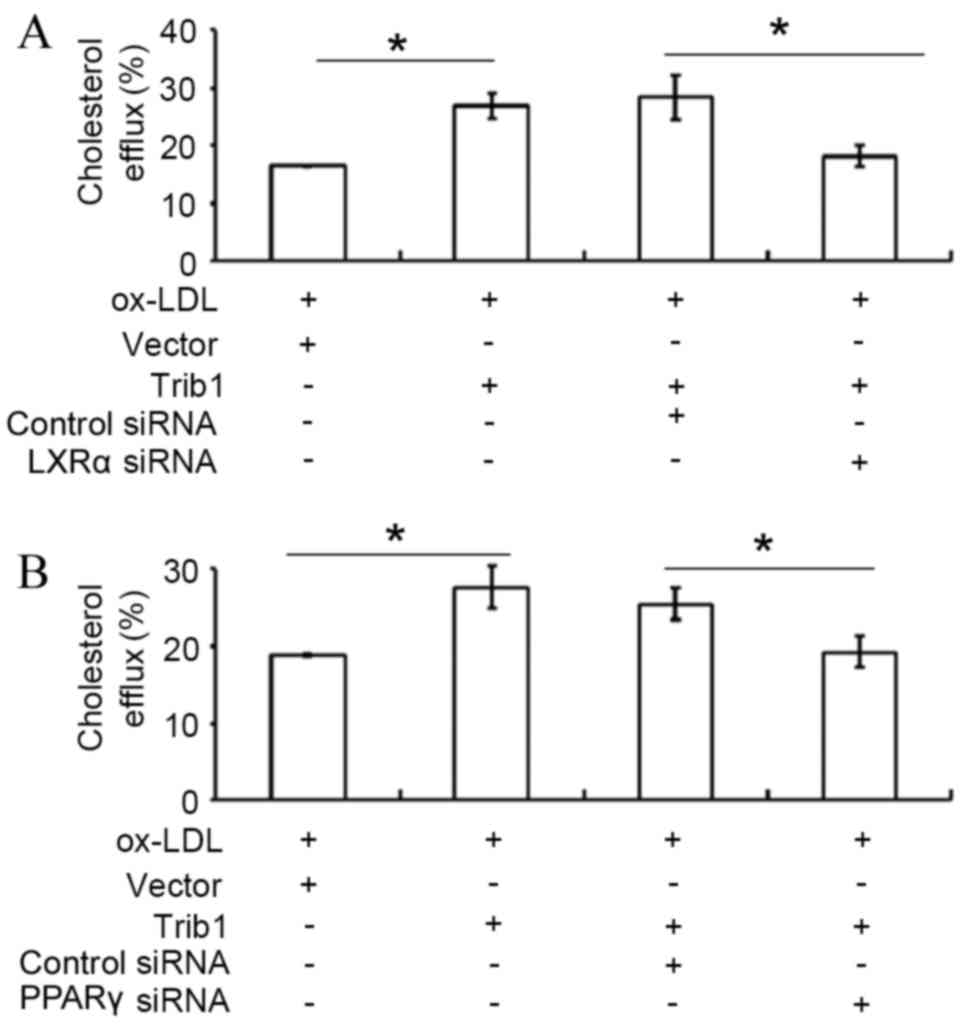
LXRα or PPARγ siRNA transfection
attenuates Trib1-induced cholesterol efflux
Silencing of LXRα by siRNA transfection
significantly decreased Trib1-induced cholesterol efflux in THP-1
macrophages transfected with pCMV6-Entry-Trib1, when compared with
the cholesterol efflux in THP-1 macrophages-transfected with
pCMV6-Entry-Trib1 and a control siRNA (P<0.05; Fig. 3A). Furthermore, knockdown of PPARγ by
siRNA transfection also significantly attenuated the Trib1-induced
cholesterol efflux compared with transfection with a control siRNA
(P<0.05; Fig. 3B).
Discussion
Genome-wide association studies identified that the
genetic locus at human chromosome 8q24 has minor alleles associated
with lower levels of plasma TG and LDL-C, as well as higher levels
of high density lipoprotein-cholesterol (12,13).
This locus contains only one annotated gene, namely Trib1 (12,13).
Burkhardt et al demonstrated that Trib1 is a regulator of
lipoprotein metabolism in mice (9),
and that hepatic-specific overexpression of Trib1 reduces the
plasma TG and cholesterol levels by reducing VLDL production
(9). Conversely, Trib1-knockout mice
exhibited elevated levels of plasma TG and cholesterol due to
increased VLDL production (9). In
addition, Satoh et al suggested that Trib1 is critical for
adipose tissue maintenance and suppression of metabolic disorders
by controlling the differentiation of tissue-resident M2-like
macrophages (10). These results
indicate that Trib1 is implicated in the regulation of lipid
metabolism. However, the underlying mechanism throughout which
Trib1 regulates lipid metabolism at the molecular level remains
unclear. In the present study, the effect of Trib1 overexpression
on lipid accumulation and intracellular cholesterol efflux was
investigated in ox-LDL-stimulated THP-1 macrophages. Transiently
transfected THP-1 macrophages were used to examine the role of
Trib1 in cholesterol homeostasis. The present results indicated
that forced expression of Trib1 decreased intracellular lipid
accumulation and enhanced cholesterol efflux in ox-LDL-exposed
THP-1 macrophages.
PPARγ is a nuclear receptor that regulates immunity
and inflammation (14,15). Upon binding with its ligands, PPARγ
activates and promotes cholesterol efflux from macrophages through
the PPARγ-LXRα-ABCA1 signaling pathway (16). LXRα is a nuclear receptor
transcription factor (17) that when
activated binds to the LXR response element in the promoter region
of the LXR target genes, such as ABCA1 and ABCG1, in order to
regulate the expression of such genes (18). The current study examined the impact
of overexpression of Trib1 on the expression of genes involved in
macrophage cholesterol efflux. After transient transfection with
pCMV6-Entry-Trib1, cellular responses were analyzed. Forced
expression of Trib1 was found to induce the expression of ABCA1,
ABCG1, LXRα and PPARγ at the mRNA and protein levels. Furthermore,
silencing of PPARγ or targeting LXRα by siRNA attenuated the
Trib1-induced cholesterol efflux, indicated that Trib1-mediated
cholesterol efflux may occur through the PPARγ-LXRα-ABCA1/ABCG1
signaling pathway. However, it is unclear how Trib1 regulates the
PPARγ-LXRα-ABCA1/ABCG1 signaling pathway. It has been suggested
that CD36 activates PPARγ through the extracellular
signal-regulated kinase (ERK)1/2-dependent cyclooxygenase 2
expression in macrophages, thereby promoting cholesterol and
phospholipid efflux from macrophages (19). Similarly, sesamin has been
demonstrated to improve macrophage cholesterol efflux through the
PPARγ1-LXRα pathway, which is dependent on mitogen-activated
protein kinase (MAPK) signaling (20). It appears that MAPK signaling serves
an important role in cholesterol efflux. Overexpression of Trib1
enhances ERK phosphorylation, reducing the apoptosis of leukemic
cells upon interleukin-3 depletion and may be a key mediator
between the RTK-MAPK pathway and the C/EBP transcription factor in
myeloid leukemogenesis (21). In the
present study, it was hypothesized that Trib1-mediated cholesterol
efflux may occur through the MAPK signaling pathway; however,
further experiments are required to test this hypothesis.
Although Trib1 overexpression leads to enhanced
cholesterol efflux from ox-LDL-loaded macrophages, it is still
unclear whether Trib1 is necessary for cholesterol efflux. The
effects of silencing Trib1 on cholesterol efflux should be
investigated further.
In conclusion, the results of the present study
indicate that Trib1 inhibits lipid accumulation and enhances
cholesterol efflux in ox-LDL-exposed THP-1 macrophages through the
PPARγ-LXRα-ABCA1/ABCG1 signaling pathway. These data provide a
rationale for investigating the potential of delivering Trib1 in
the prevention of macrophage foam cell formation and
atherosclerosis.
References
|
1
|
Moss JW, Davies TS, Garaiova I, Plummer
SF, Michael DR and Ramji DP: A Unique combination of nutritionally
active ingredients can prevent several key processes associated
with atherosclerosis in vitro. PLoS One. 11:e01510572016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narasimhulu C Aluganti, Fernandez-Ruiz I,
Selvarajan K, Jiang X, Sengupta B, Riad A and Parthasarathy S:
Atherosclerosis-do we know enough already to prevent it? Curr Opin
Pharmacol. 27:92–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conti P and Shaik-Dasthagirisaeb Y:
Atherosclerosis: A chronic inflammatory disease mediated by mast
cells. Cent Eur J Immunol. 40:380–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rojas J, Salazar J, Martínez MS, Palmar J,
Bautista J, Chávez-Castillo M, Gómez A and Bermúdez V: Macrophage
heterogeneity and plasticity: Impact of macrophage biomarkers on
atherosclerosis. Scientifica (Cairo). 2015:8512522015.PubMed/NCBI
|
|
5
|
Glass CK and Witztum JL: Atherosclerosis:
The road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bogachev O, Majdalawieh A, Pan X, Zhang L
and Ro HS: Adipocyte enhancer-binding protein 1 (AEBP1) (a novel
macrophage proinflammatory mediator) overexpression promotes and
ablation attenuates atherosclerosis in ApoE (−/−) and LDLR (−/−)
mice. Mol Med. 17:1056–1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Q, Mei Y, Shoji T, Han X, Kaminski K,
Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, et al:
Rho-associated coiled-coil-containing kinase 2 deficiency in bone
marrow-derived cells leads to increased cholesterol efflux and
decreased atherosclerosis. Circulation. 126:2236–2247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa
V, Dempsey C, Caunt JC, Oxley KM, Wyllie DH, Polgar T, Harte M, et
al: Human tribbles, a protein family controlling mitogen-activated
protein kinase cascades. J Biol Chem. 279:42703–42708. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burkhardt R, Toh SA, Lagor WR, Birkeland
A, Levin M, Li X, Robblee M, Fedorov VD, Yamamoto M, Satoh T, et
al: Trib1 is a lipid- and myocardial infarction-associated gene
that regulates hepatic lipogenesis and VLDL production in mice. J
Clin Invest. 120:4410–4414. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoh T, Kidoya H, Naito H, Yamamoto M,
Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O
and Akira S: Critical role of Trib1 in differentiation of
tissue-resident M2-like macrophages. Nature. 495:524–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kathiresan S, Melander O, Guiducci C,
Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF,
Havulinna AS, et al: Six new loci associated with blood low-density
lipoprotein cholesterol, high-density lipoprotein cholesterol or
triglycerides in humans. Nat Genet. 40:189–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willer CJ, Sanna S, Jackson AU, Scuteri A,
Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS,
Stringham HM, et al: Newly identified loci that influence lipid
concentrations and risk of coronary artery disease. Nat Genet.
40:161–169. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glass CK and Saijo K: Nuclear receptor
transrepression pathways that regulate inflammation in macrophages
and T cells. Nat Rev Immunol. 10:365–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villacorta L, Schopfer FJ, Zhang J,
Freeman BA and Chen YE: PPARgamma and its ligands: Therapeutic
implications in cardiovascular disease. Clin Sci (Lond).
116:205–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouhlel MA, Staels B and Chinetti-Gbaguidi
G: Peroxisome proliferator-activated receptors-from active
regulators of macrophage biology to pharmacological targets in the
treatment of cardiovascular disease. J Intern Med. 263:28–42.
2008.PubMed/NCBI
|
|
17
|
Huang C: Natural modulators of liver X
receptors. J Integr Med. 12:76–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zelcer N and Tontonoz P: Liver X receptors
as integrators of metabolic and inflammatory signaling. J Clin
Invest. 116:607–614. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bujold K, Rhainds D, Jossart C, Febbraio
M, Marleau S and Ong H: CD36-mediated cholesterol efflux is
associated with PPARgamma activation via a MAPK-dependent COX-2
pathway in macrophages. Cardiovasc Res. 83:457–464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majdalawieh AF and Ro HS: The
anti-atherogenic properties of sesamin are mediated via improved
macrophage cholesterol efflux through PPARγ1-LXRα and MAPK
signaling. Int J Vitam Nutr Res. 84:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yokoyama T, Kanno Y, Yamazaki Y, Takahara
T, Miyata S and Nakamura T: Trib1 links the MEK1/ERK pathway in
myeloid leukemogenesis. Blood. 116:2768–2775. 2010. View Article : Google Scholar : PubMed/NCBI
|