Introduction
Among all types of heart disease, ischemic heart
disease, also known as coronary heart disease, is the main cause of
morbidity and mortality (1).
Shortage of blood supply to the heart resulting in irreversible
cardiomyocyte death and eventually leading to myocardial infarction
is the common pathway of ischemic heart disease. Different forms of
reperfusion therapy, including coronary angioplasty, coronary
stenting and coronary revascularization, as well as pharmacological
adjuvants are widely used in clinical practice for the treatment of
this disease (2). However,
ischemia/reperfusion (I/R)-induced injury due to the restoration of
blood supply to the ischemic area occurs subsequent to reperfusion
therapy, leading to acute tissue damage (3). Present therapeutic strategies are not
efficient in preventing I/R-induced injury. Therefore, the
development of novel strategies focusing on the prevention of
I/R-induced injury is crucial for the treatment of ischemic heart
disease.
Several mediators of I/R-induced injury have been
established, including inflammatory cells (such as T-cells)
(4), proteolytic enzymes (such as
MMP2 and MMP-9) (5) and kinases
(such as Rho kinase) (6). In
addition, a number of pharmacological compounds targeting these
mediators have been reported to improve cardiac function and
attenuate I/R-induced injury (7).
Recently, an endocrine factor, fibroblast growth
factor 21 (FGF21), has received increasing attention. FGF21 belongs
to the family of 22 fibroblast growth factors, and is mainly
expressed in the liver and adipose tissue (8). FGF21, as a key metabolic regulator,
stimulates cell glucose uptake by inducing the expression of
glucose transporter-1 (GLUT1) and insulin. In addition, it has been
demonstrated that FGF21 is involved in the suppression of the
downstream signaling of apoptosis (9). Recent studies have provided evidence
supporting that endocrine FGF21 protects against myocardial
apoptosis caused by I/R-induced injury, possibly through the
fibroblast growth factor receptor (FGFR)-PIK3-AKT-caspase-3
signaling pathway (10). FGFRs are
well-known FGF-specific receptor tyrosine kinases, which serve a
fundamental role in the onset of this signaling pathway triggered
by FGF21 (11). By contrast,
antagonists of tyrosine kinases may inhibit or at least attenuate
the protective effect of FGF21. Further investigations are required
to determine the mechanism underlying the protective effect of
FGF21.
Angiopoietin-2 (Angpt2) has been examined in
different heart disease models, suggesting that Angpt2 is an
important regulator in the injury heart (12,13),
which may be a promising predictor of heart disease (14). Angpt2 is generally considered as a
natural antagonist of angiopoietin-1 (Angpt1) and TEK tyrosine
kinase, and it competitively inhibits the binding of Angpt1 and TEK
tyrosine kinase, thus disrupting the downstream signaling and
leading to endothelial damage and vessel leakage (15). Angpt2 has proinflammatory and
apoptosis-promoting abilities, further impairing the vascular
tissue (15). Furthermore, it has
been reported that Angpt2 interacts with other classes of tyrosine
kinases, such as FGFRs (16).
However, no previous studies have investigated whether Angpt2
affects the protective effect of FGF21 via inhibiting FGFR
signaling-induced GLUT1 overexpression.
Therefore, the present study sought to elucidate the
role of Angpt2 in the protective effect of FGF21 administration on
I/R-induced injury in cardiomyocytes. It was hypothesized that
upregulation of Angpt2 during I/R-induced injury may attenuate the
cardioprotective effect of FGF21 via the suppression of GLUT1
expression, and that application of Angpt2 small interfering RNA
(siRNA) along with FGF21 administration may result in more
efficient protection against I/R-induced injury.
Materials and methods
Chemicals and cell culture
Antibodies against Angpt2 (ab8452), GLUT1 (ab652)
and caspase-3 (ab13847) were purchased from Abcam (Cambridge, MA,
USA). Human recombinant protein FGF21 was purchased from Abnova
(Walnut, CA, USA; purity >95%). The H9c2 cardiomyocyte line
(obtained from American Type Culture Collection, Manassas, VA, USA)
was cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
(v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin. H9c2 cells were incubated in a 5% CO2
incubator at 37°C. After 70–80% confluence was reached, cells were
subjected to trypsinization, according to standard procedures.
Cells were then harvested in DMEM with 1% FBS and stored at −80°C
for subsequent biochemistry analysis.
Establishment of a stimulated I/R
model in H9c2 cardiomyocytes
In order to mimic I/R-induced injury in
vitro, simulated ischemia followed by reperfusion were
performed in H9c2 cells (17). Cells
were transferred to an ischemia-simulating buffer solution (137 mM
NaCl, 12 mM KCl, 0.49 mM MgCl2, 0.9 mM
CaCl2·2H2O, 4 mM HEPES and 20 mM sodium
lactate), and then placed in a sealed hypoxia chamber (containing
95% N2 and 5% CO2) for 4 h at 37°C to induce
anoxia. Cells were treated for 4, 6 and 8 h of simulated ischemia.
Subsequent to simulated ischemia, the medium was replaced by normal
medium, and cells were incubated in a 5% CO2 incubator
at 37°C for 24 h to simulate reperfusion.
siRNA transfection assay
The specific Angpt2 siRNA was synthesized
chemically, and the sequences targeting Angpt2 were
5′-GAUCGAGAUUGGAACCAGUTT-3′ (sense) and 5′-ACUGGUUCCAAUCUCGAUCTT-3′
(antisense). In addition, nonsilencing siRNA was used as a negative
control (NC), in order to determine whether there were any other
effects induced by the siRNA and transfection reagents. The
sequences of the control siRNA were 5′-UUCUCCGAACGUGUCACGUTT-3′
(sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense). Angpt2 siRNA
group was transfected with Angpt2 siRNA and control group was
transfected with non-silencing siRNA using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocols. The non-siRNA control group had similar results to the
non-silencing siRNA control group (data not shown), therefore only
the results of the non-silencing siRNA control group are
presented.
Cell counting kit 8 (CCK-8) assay
CCK-8 assay (ab65314; Abcam) was used to evaluate
cell proliferation according to the manufacturer's protocol.
Briefly, H9c2 cells were cultured at a density of of
5×104 cells/well in 96-well culture dish. After 24 h,
adherence was observed, and cells were subjected to the simulated
I/R model as indicated earlier, and treated with FGF21 and Angpt2
siRNA as appropriate. Experimental groups include: i) At normal
aerobic conditions, different incubation durations (24, 48 and 72
h) and concentrations (0, 0.25, 0.5, 1 and 2 µg/ml) of FGF21 were
tested to determine for the treatment model; ii) normal conditions
were used for the control group, and I/R group was compared with
I/R + FGF21 group; iii) I/R-treated cells with non-silencing siRNA
transfection were used as a negative control, I/R + Angpt2 siRNA
group was compared with I/R + Angpt2 siRNA + FGF21 group. At the
end of each experiment, 10 µl CCK-8 solution was added to each
well, and then the cells were further incubated for 1 h at 37°C.
The absorbance of samples at 450 nm was determined by a multi-well
plate reader.
Apoptosis assay by flow cytometry
Apoptosis was determined by Annexin V and propidium
iodide (PI) double staining (v13242; Invitrogen, Thermo Fisher
Scientific, Inc.). Briefly, following the various experimental
treatments, cells were detached with trypsin-EDTA, washed twice
with phosphate-buffered saline (PBS), resuspended in binding buffer
(10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2
and 1.8 mM CaCl2) containing FITC-Annexin V (1 g/ml) and
then further incubated for 20 min. At 10 min before the end of
incubation, PI (10 g/ml) was added to this cell suspension in order
to stain necrotic cells. Cells were analyzed with a FACScan flow
cytometer equipped with an excitation laser line at 488 nm. The PI
was analyzed using a 575 nm band pass filter.
Cell cycle analysis using flow
cytometry
After I/R treatment, cells were harvested, washed
with cold PBS, and fixed with 70% ethanol overnight at −20°C. The
fixed cells were then washed twice with cold PBS, then subjected to
centrifugation for 20 min at 15,000 × g at 4°C, and the
supernatant was discarded. Next, the cells were stained with PI
staining solution (10 µg/ml RNase A and 50 µg/ml PI) at 37°C for 30
min in the dark. The cell cycle distribution was analyzed by flow
cytometry using CellQuest software 5.1 (BD Biosciences, San Jose,
CA, USA).
Cell migration investigation by
Transwell assay
To examine cell migration, cells were transferred to
the Transwell® inserts of a 24-well plate, and subjected
to 6 h simulated ischemia followed by 24 h reperfusion. The
Transwell insert was then washed three times with PBS, and the
cells on the top surface of the insert were removed with a cotton
swab. Cells adhering to the lower surface were fixed with methanol
for 10 min, stained with 0.1% Giemsa solution for 10 min, washed
three times with PBS and air-dried. The migrating cells were
counted and images were captured using a microscope (Nikon Corp.,
Tokyo, Japan). All experiments were performed in triplicate and ten
fields of vision were counted per filter in each group.
Gene expression analysis using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA from H9c2 cells in the various
treatment groups was extracted using TRIzol kit (Invitrogen, Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The extracted RNA was measured using UVS-99
Micro-volume UV/Vis Spectrophotometer (ACTGene, Piscataway, NJ,
USA). A total of 1 µg RNA was reverse transcribed into cDNA using
oligo (dT) primer and SuperScript III reverse transcriptase
(Invitrogen, Thermo Fisher Scientific, Inc.). qPCR was performed
with an ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Target cDNA levels were determined by
SYBR-Green-based qPCR in 20-µl reactions, containing 10 µl Power
SYBR-Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primers were used: Caspase-3
forward, 5′-ATGTCGATGCAGCTAACCTC-3′, and reverse,
5′-TCCTTTTGCTGTGATCTTCC-3′; Angpt2 forward,
5′-GGCAGCGTTGATTTTCAGAGGACT-3′, and reverse,
5′-TTTAATGCCGTTGAACTTATTTGT-3′; GLUT1 forward,
5′-CTTCATCCCAGCCCTGTT-3′, and reverse, 5′-GACCTTCTTCTCCCGCATC-3′;
β-actin forward, 5′-GCACCACACCTTCTACAATG-3′, and reverse,
5′-TGCTTGCTGATCCACATCTG-3′. PCR cycling was performed as follows: 1
cycle for 2 min at 94°C, followed by 29–32 cycles of 95°C for 30
sec, 57–60°C for 45 sec, 72°C for 1 min, followed by 1 cycle of
72°C for 5 min, held at 4°C. The qPCR data were analyzed with the
2−ΔΔCq method (18) and normalized against β-actin
cDNA, used as an internal control.
Western blot analysis
Cells were harvested and stored at −80°C until
further use. For western blot analysis, frozen cells were sonicated
on ice twice for 5 sec in 50 mM lysis buffer (Invitrogen; Thermo
Fisher Scientific, Inc.), containing 3.1 mM sucrose, 1 mM DTT, 10
µg/ml leupeptin, 10 µg/ml soybean trypsin inhibitor, 2 µg/ml
aprotinin and 0.1% Triton X-100. Homogenates were centrifuged at
10,000 × g at 4°C for 20 min, and the supernatant was
collected. The total protein concentration was measured using the
Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Protein lysates (30 µg) were separated using 12% SDS-PAGE and
transferred to a polyvinylidene fluoride (PVDF) membrane. After
blocking with 5% nonfat milk, the PVDF membrane was incubated
overnight at 4°C with the monoclonal primary antibodies against
caspase-3, Angpt2, GLUT1 and GAPDH (all 1:1,000) diluted in
Tris-buffered saline with Tween-20 (TBS-T). Membranes were washed
in TBS-T (10 min, three times) and then probed with the appropriate
secondary antibody (1:10,000; Abcam). Subsequently, membranes were
developed using Versa Doc 5000 (Bio-Rad Laboratories, Inc.), and
the band densities were measured with Quantity One 4.6 software
(Bio-Rad Laboratories, Inc.). Equal protein loading was
additionally verified by measurement of GAPDH level with a
corresponding mouse monoclonal antibody (1:10,000; ab6708;
Abcam).
Statistical analysis
Statistical calculations were performed using Prism
6 (GraphPad Software, Inc., San Diego, CA, USA). Data are presented
as the mean ± standard error of the mean. Student's t-test was used
for comparisons between two groups, and one-way or two-way analysis
of variance was used for comparisons among multiple groups.
Differences with P<0.05 were considered as statistically
significant.
Results
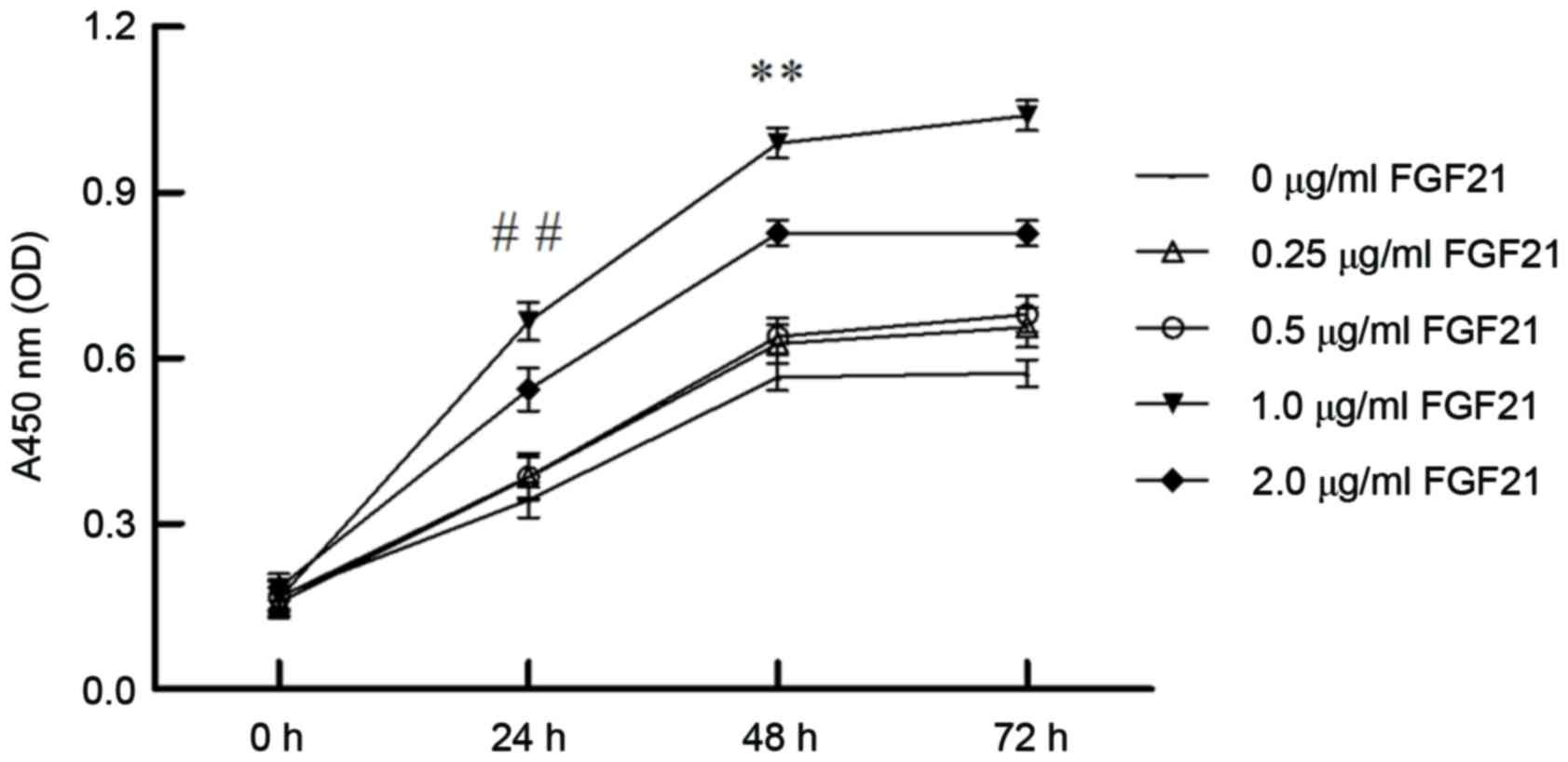
Effect of various FGF21 concentrations
and incubation durations on cell viability
The effect of incubation duration and concentration
of FGF21 on H9c2 cell viability was determined by CCK-8 assay. The
results indicated that cells treated with 1 µg/ml FGF21 for 48 h
exhibited the highest cell viability and proliferation (Fig. 1; P<0.01). Therefore, cells were
treated with the optimized FGF21 concentration of 1 µg/ml for 48 h
in subsequent experiments, as the FGF21-treatment group.
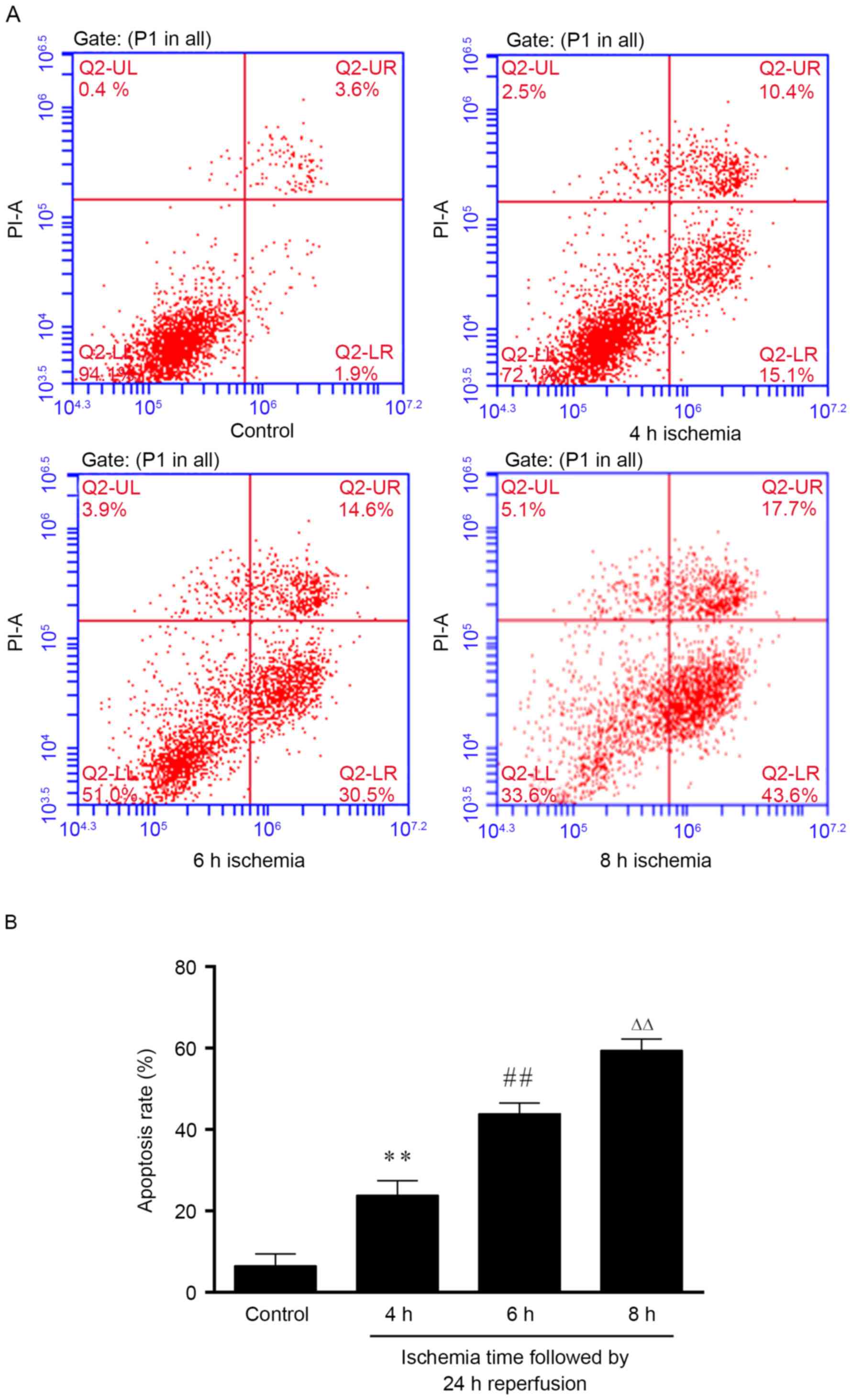
Determination of simulate I/R model
setup
The effect of the ischemia duration on the apoptosis
rate was also measured. The results indicated that 4 h of ischemia
resulted in ~20% of apoptosis (Fig.
2). With longer duration of ischemia, the cells were
significantly further damaged (P<0.01), and demonstrated
apoptosis rate of 40 and 60% for 6 and 8 h of ischemia,
respectively (Fig. 2). Based on the
apoptosis rate (~50% is appropriate for an I/R-induced injury
model), 6 h ischemia followed by 24 h reperfusion was selected for
further experiments.
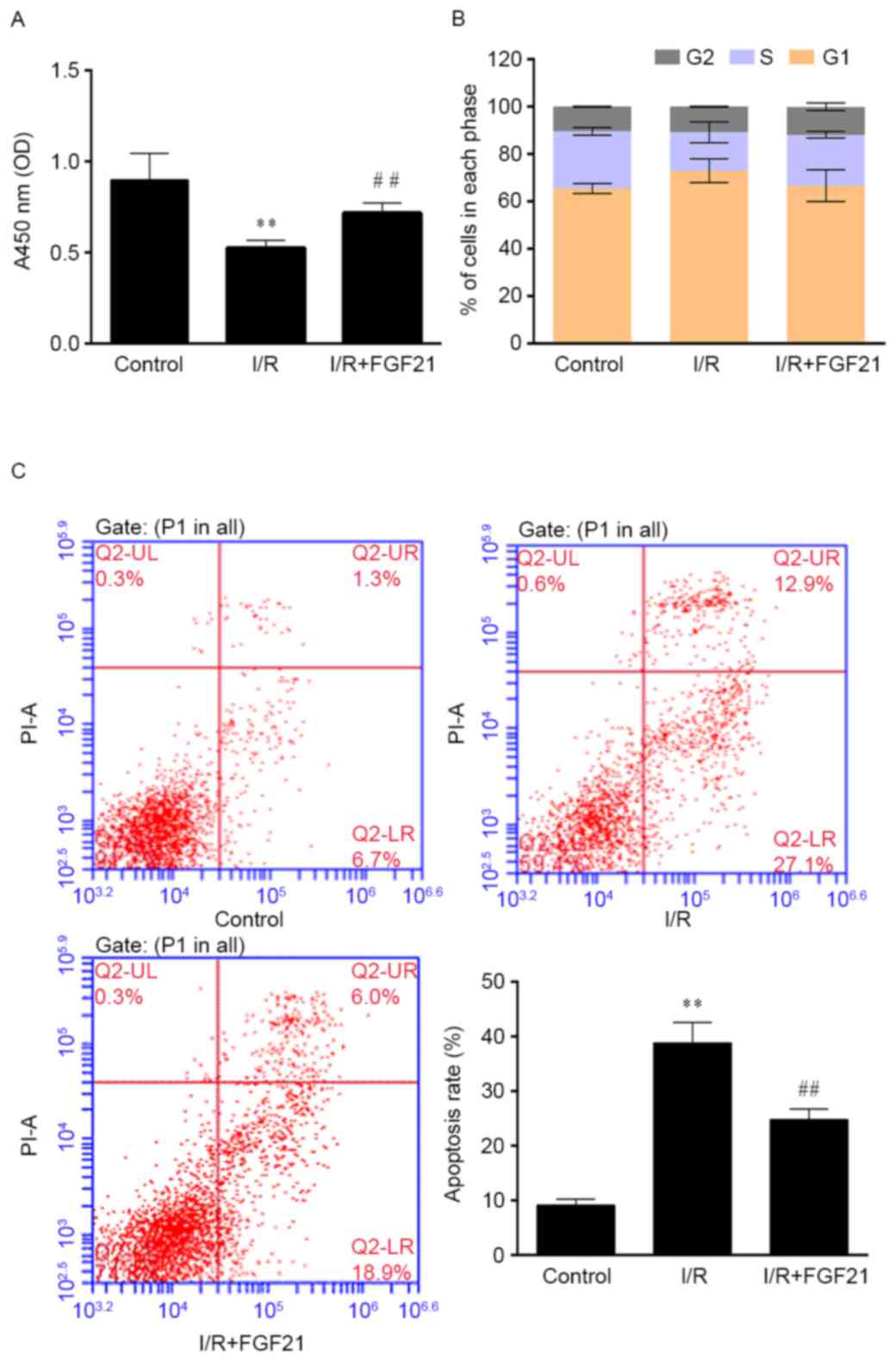
Effect of FGF21 on cell survival
The effect of simulated I/R on cell viability was
determined by CCK-8 assay. As shown in Fig. 3A, simulated I/R significantly
decreased the cell viability by ~50% in comparison with the control
group that was treated under aerobic conditions (P<0.01). By
contrast, exposure to 1 µg/ml FGF21 after simulated I/R had a
protective effect against injury, since it significantly increased
the cell viability in comparison with the I/R model group
(P<0.01).
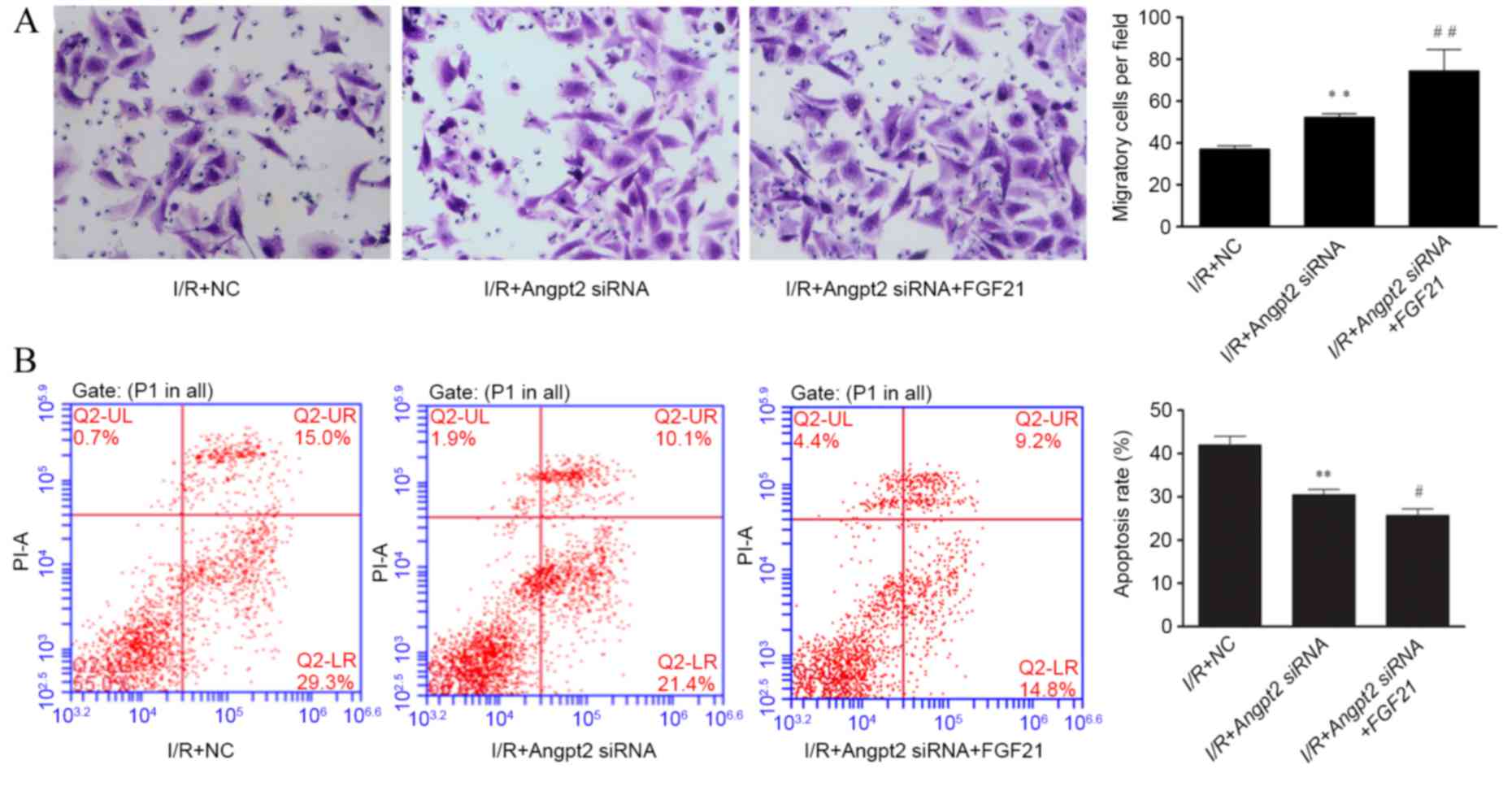
The cell cycle and apoptosis rate were subsequently
determined by flow cytometry. Cell cycle distribution was not
significantly affected by I/R and FGF21 administration (Fig. 3B). The cell apoptosis rate was
significantly increased in the simulated I/R group when compared
with that of the aerobic control group, with ~40% apoptotic cells
detected (P<0.01). However, FGF21 demonstrated an evident
apoptosis-inhibiting effect after simulated I/R, with a significant
reduction of the apoptosis rate observed in the FGF21-treated group
when compared with the I/R model group (P<0.01; Fig. 3C).
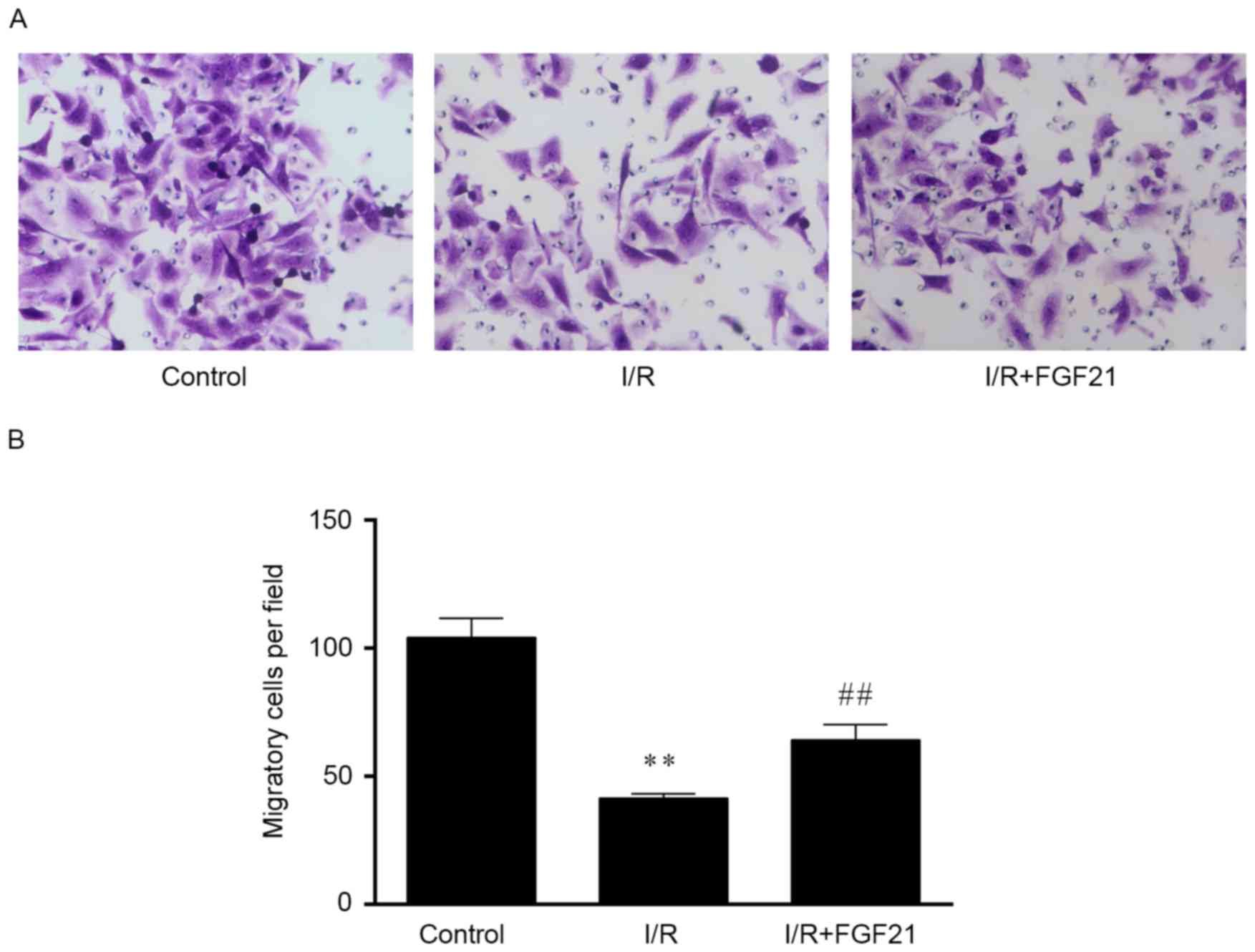
Effect of FGF21 on cell migration
Data from the Transwell migration assay demonstrated
that the cell migration ability was significantly decreased by
>50% following simulated I/R, when compared with that of the
aerobic control group (P<0.01; Fig.
4). However, FGF21 treatment enhanced the cell migration
ability during I/R-induced injury, with a significantly higher
number of migrated cells detected as compared with the I/R model
group (P<0.01).
Effect of FGF21 on the protein and
mRNA levels of caspase-3, Angpt2 and GLUT1
The protein expression levels of caspase-3, Angpt2
and GLUT1 were evaluated by western blot analysis. Measurement of
the GAPDH level was used as a control for equal protein loading. In
comparison with the aerobic control group, the protein levels of
caspase-3 and Angpt2 were markedly increased following simulated
I/R, whereas the level of GLUT1 was decreased (Fig. 5A). In the FGF21-treated group, the
expression levels of caspase-3 and Angpt2 were downregulated
following simulated I/R, while the expression of GLUT1 was
upregulated.
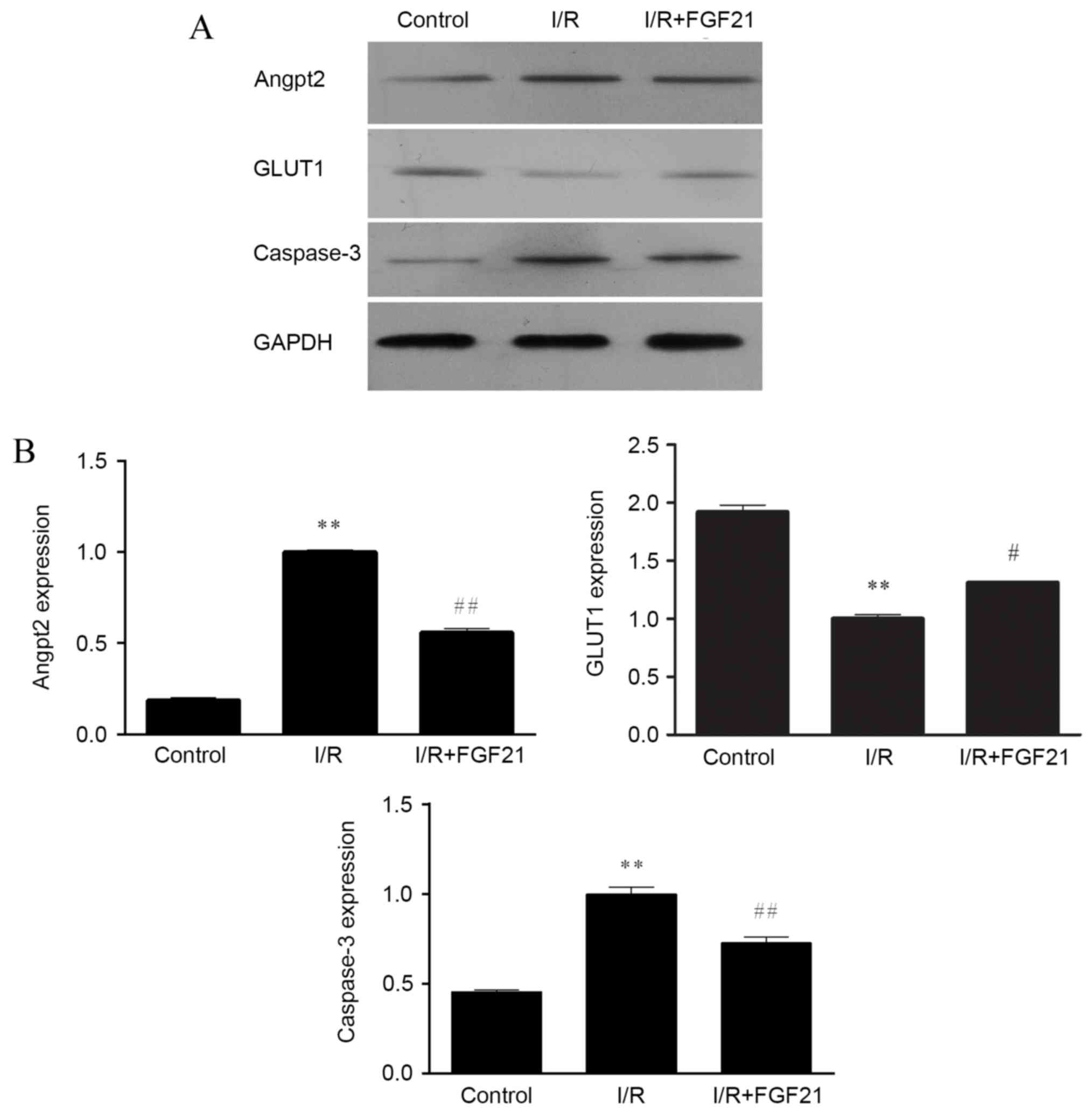 | Figure 5.Effect of FGF21 on the (A) protein
and (B) gene expression levels of Angpt2, GLUT1 and caspase-3,
determined by western blot analysis and qPCR, respectively. In
western blot analysis, equal protein loading was verified by
measurement of the GAPDH level. In qPCR, relative expression is
given as the mean of four independent experiments. **P<0.01 vs.
the control group; #P<0.05 and ##P<0.01
vs. the I/R group. FGF21, fibroblast growth factor 21; I/R,
ischemia/reperfusion; qPCR, quantitative polymerase chain reaction;
Angpt2, angiopoietin-2; GLUT1, glucose transporter 1. |
RT-qPCR was further performed to determine the gene
expression levels of caspase-3, Angpt2 and GLUT1, and the results
were similar to those obtained from western blot analysis (Fig. 5B). Significantly increased gene
expression of caspase-3 and Angpt2, and significantly decreased
gene expression of GLUT1 were observed in the simulated I/R group
compared with the control (all P<0.01). By contrast, in the
FGF21 treated group, the gene expression levels of caspase-3 and
Angpt2 were significantly downregulated in comparison with those in
the I/R model group (P<0.01) and GLUT1 was significantly
upregulated (P<0.05).
Influence of Angpt2 inhibition on the
protective effect of FGF21 against I/R injury
The results of the negative control siRNA
transfected cells and normal control cells did not differ in all
experimental groups (data not shown). Therefore, the negative
control siRNA transfected cells treated by simulated I/R were used
as a control in all siRNA transfection experiments.
Knockdown of Angpt2 expression by the Angpt2 siRNA
was evaluated by western blot analysis. Following simulated I/R,
the Angpt2 protein expression in the Angpt2 siRNA transfected group
was reduced by ~50% in comparison to control cells (Fig. 6A). A lower level of caspase-3 was
also observed in the Angpt2 siRNA transfected group, while the
level of GLUT1 was increased compared with the I/R model group
(Fig. 6A). Similar observations were
obtained for the mRNA expression levels of Angpt2, caspase-3 and
GLUT1 by RT-qPCR analysis, with significant differences observed in
the Angpt2 siRNA group compared with the control (all P<0.01;
Fig. 6B).
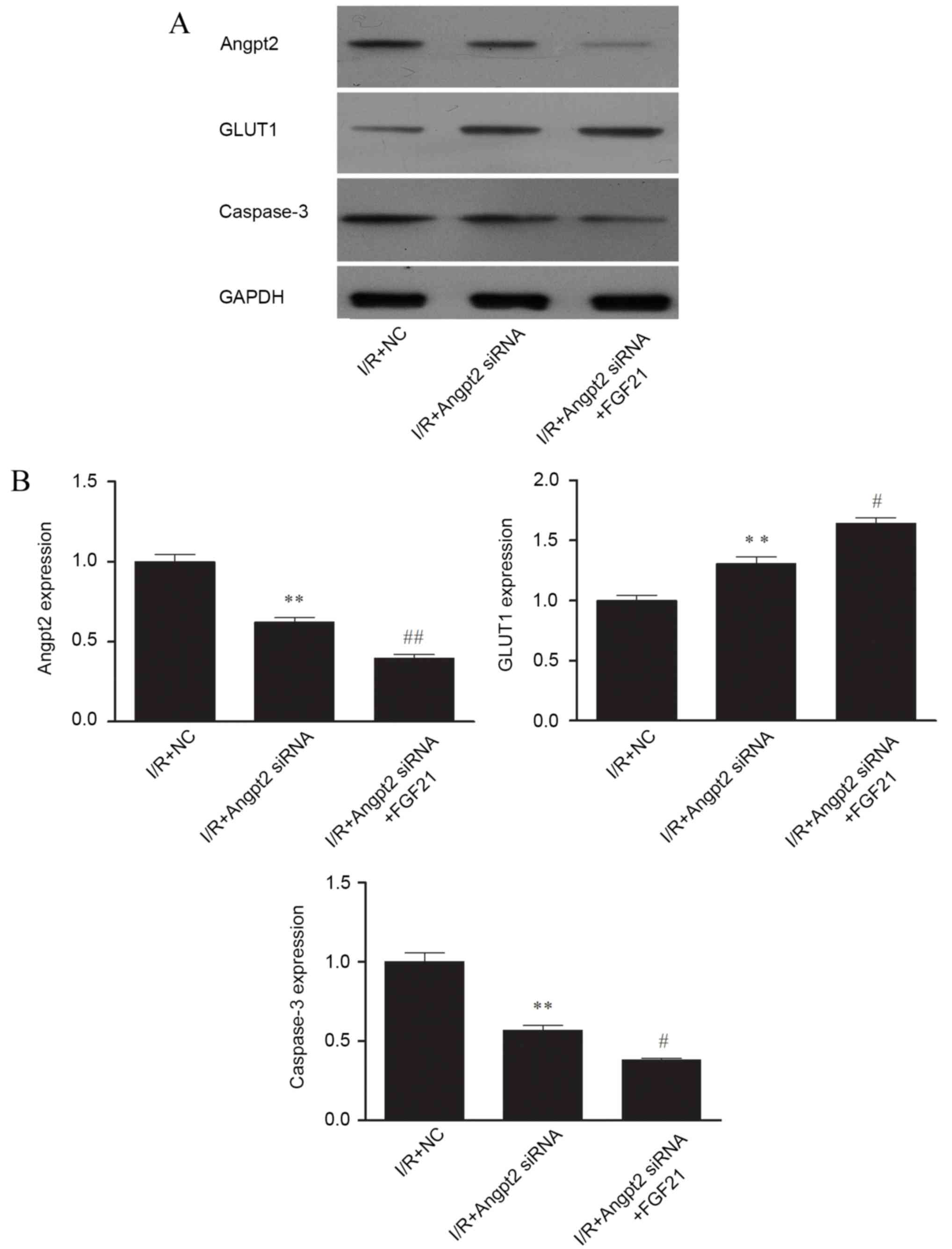 | Figure 6.Effect of Angpt2 siRNA transfection
and FGF21 treatment on the (A) protein and (B) gene expression
levels of Angpt2, GLUT1 and caspase-3, determined by western blot
analysis and qPCR, respectively. In western blot analysis, equal
protein loading was verified by measurement of the GAPDH level. In
qPCR, relative expression is given as the mean of four independent
experiments. **P<0.01 vs. the I/R + NC group;
#P<0.05 and ##P<0.01 vs. the I/R +
Angpt2 siRNA group. FGF21, fibroblast growth factor 21; I/R,
ischemia/reperfusion; qPCR, quantitative polymerase chain reaction;
Angpt2, angiopoietin-2; GLUT1, glucose transporter 1; siRNA, small
interfering RNA; NC, negative control. |
Western blot analysis also indicated that FGF21
administration with Angpt2 knockdown further inhibited the protein
expression levels of Angpt2 and caspase-3 in comparison with the
control after simulated I/R. A much larger increase of GLUT1
expression was also observed in the FGF21 + Angpt2 siRNA treated
group (Fig. 6A). Similar
observations were obtained for the mRNA expression of these genes
using RT-qPCR, indicating that the expression levels of Angpt2 and
caspase-3 were significantly reduced (P<0.01 and P<0.05,
respectively) in comparison with the I/R + Angpt2 siRNA group
(Fig. 6B).
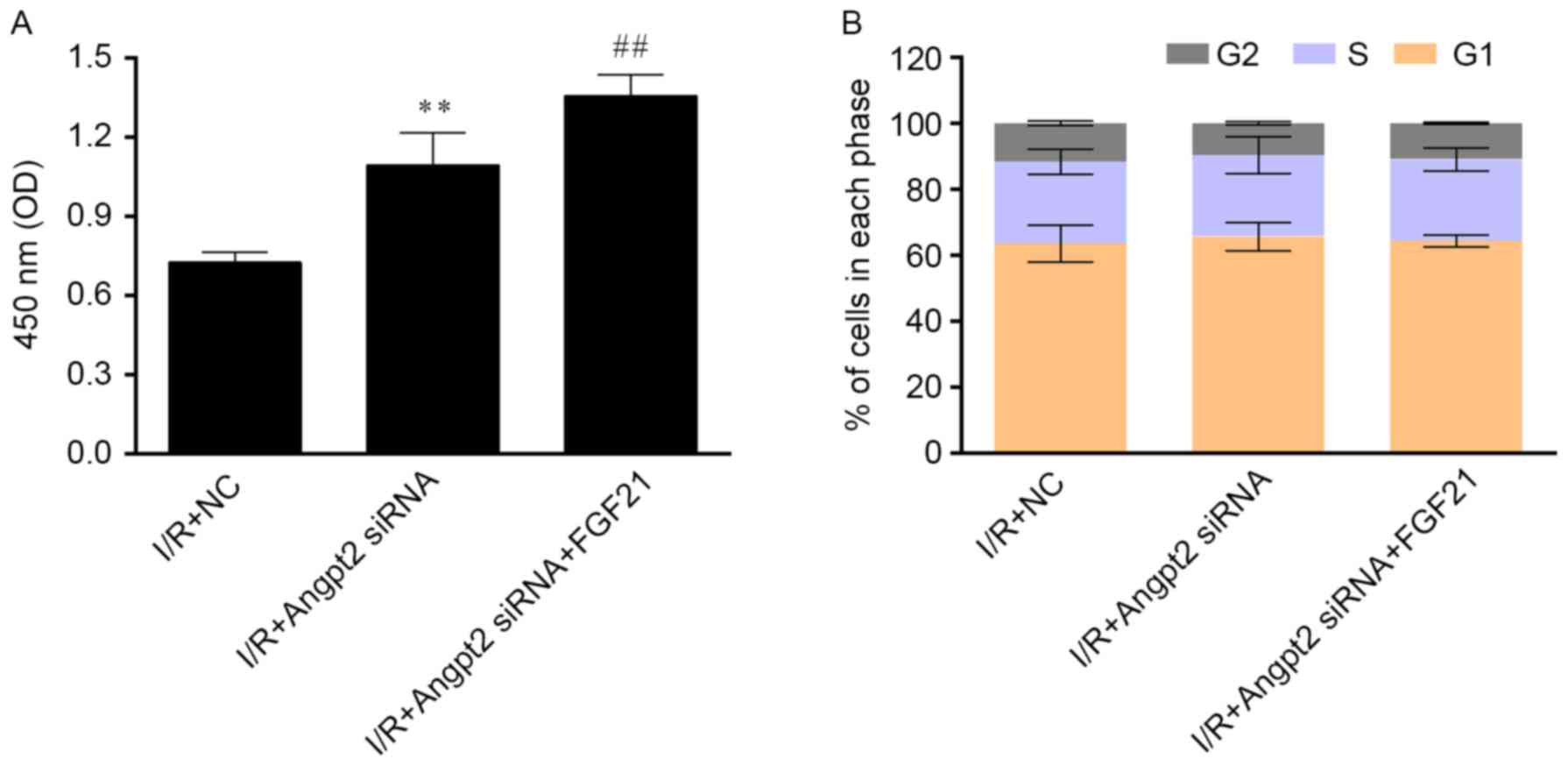
Evaluations of cell viability, cell apoptosis rate
and cell migration revealed that FGF21 administration along with
Angpt2 knockdown significantly improved cell survival compared with
Angpt2 knockdown alone (P<0.01; Fig.
7A), whereas transfection and treatment did not markedly affect
the cell cycle distribution following I/R (Fig. 7B). FGF21 exposure with Angpt2
knockdown also significantly improved the migration ability
(Fig. 8A) in comparison with Angpt2
knockdown alone (P<0.01). Furthermore, the siRNA transfection
significantly reduced cell apoptosis compared with the control
(P<0.01), and FGF21 treatment inducing significant further
reduction compared with the Angpt2 siRNA group (P<0.05; Fig. 8B).
Discussion
The development of I/R-induced injury in the heart
has been considered as a complicated pathological process, which
ranges from the cellular level to the organism level. A large
number of regulators and pathways have been reported to be involved
in the pathophysiology of I/R-induced injury, and the role of each
pathway and the exact mechanisms of I/R-induced injury remain
controversial (19).
In general, cardioprotective responses against
I/R-induced injury do not only occur in the heart itself, but also
systemic responses are observed. Several previous studies have
suggested that the liver responds to I/R-induced injury in the
heart via upregulation of cardioprotective secretory factors
(20,21), including endocrine factor FGF21.
Evidence has been provided that FGF21 protects the heart from
I/R-induced injury (22). Patel
et al reported that cardiomyocytes can also secret FGF21,
and autocrine/paracrine FGF21 can stimulate further production and
secretion of FGF21 during I/R-induced injury, thus promoting its
cardioprotective effect in the heart (23). A comprehensive proteomic analysis by
Cong et al also identified that FGF21 protects against
I/R-induced injury in cardiomyocytes through the activation of the
Akt-GSK-3β-caspase-3 pathway and modulation of the key factors for
energy supply (24).
In the present study, treatment with FGF21 markedly
increased cell survival by suppression of apoptosis. In addition,
the cell migration ability was also improved by FGF21 treatment
during simulated I/R. Cell migration contributes to the adaptive
response during I/R-induced injury (25), which may induce the regeneration of
cardiomyocytes and repair of heart injury (26). According to the data from western
blot and qPCR, FGF21 significantly reduced the expression of
caspase-3, which suggested the interruption of downstream signaling
of apoptosis during I/R-induced injury. This result provided
evidence supporting the theory that FGF21 prevents apoptosis during
I/R-induced injury via the Akt-GSK-3β-caspase-3 signaling pathway,
leading to the inhibition of apoptosis (27).
Previous studies have demonstrated that the
expression of important regulators of energy metabolism is
increased by FGF21, such as the expression of ATP-synthase
(28), pyruvate kinase (24) and GLUT1 (29). However, in contrast to those studies,
the western blot and qPCR data of the present study detected a
decreased level of GLUT1, which may result from the upregulated
Angpt2 expression following simulated I/R. GLUT1 expression can be
induced by the FGFR (tyrosine kinase)-ERK1/2-Akt signaling pathway
(11), while Angpt2 is an inhibitor
of different classes of tyrosine kinase (15). Thus, during simulated I/R injury,
increased level of Angpt2 may inhibit FGFR activation, leading to
interruption of GLUT1 expression, which consequently results in a
passive influence on the cardioprotective effect of FGF21
treatment. Therefore, the current study sought to inhibit Angpt2
expression by siRNA transfection in order to determine the role of
Angpt2 in the cardioprotective effect of FGF21.
Angpt2 suppresses the binding of Angpt1 and TEK
tyrosine kinase, inhibiting the process of angiogenesis and leading
to tissue damage and injury (30).
It has been reported that Angpt2 levels are rapidly raised in
injury of the heart (31). A number
of clinical studies have also suggested that Angpt2 may be applied
as a biomarker or predictor for several types of heart injury,
including heart failure (13) and
coronary heart disease (32). In the
present study, using Angpt2 siRNA along with FGF21 administration,
we showed an improved level of cell survival and cell migration
ability, which supports the hypothesis that inhibition of Angpt2
promoted the cardioprotective effect of FGF21. Biochemistry
analysis indicated that GLUT1 levels were significantly increased
following transfection with Angpt2 siRNA. These results provided
strong evidence supporting our hypothesis that increased level of
GLUT1 caused by Angpt2 inhibition improves the glucose
transportation and energy supply, resulting in a more efficient
cardioprotective effect of FGF21 against I/R-induce injury in
cardiomyocytes.
To the best of our knowledge, the present study is
the first to determine the factor that influences the
cardioprotective effect of FGF21. Notably, overexpression of Angpt2
in heart injury attenuated the cardioprotective effect of FGF21
treatment through inhibition of GLUT1 expression induced by the
FGFR-ERK1/2-Akt signaling pathway. In addition, selective knockdown
of Angpt2 using siRNA markedly improved the cardioprotective
efficiency of FGF21 against I/R-induced injury, suggesting a
potential combination treatment for I/R-induced injury in the
heart. Combined application of pharmacological compounds is a
classic and simple form of combination therapy, which targets
multiple mechanisms and factors contributing to the pathogenesis of
certain diseases (33), such as
heart diseases. The protective effects of pharmacological compounds
targeting different mechanisms can achieve additional or
synergistic protection against the disease (33). A classic example of combination
therapy for heart diseases is the therapeutic strategy that
includes diuretics, β-blockers, ACE inhibitor, calcium channel
blockers and other agents (34).
Recent studies have demonstrated a synergistic protection against
I/R-induced injury via several mechanisms contributing the
contractile protein degradation (35). In the current study, both Angpt2
siRNA and FGF21 were found to exert a cardioprotective effect,
since overexpression of Angpt2 is harmful to the cardiovascular
system and endocrine FGF21 has beneficial effects in heart injury.
Combination of Angpt2 siRNA and FGF21 achieves additional
cardioprotection against I/R-induced injury, which results from the
targeting of specific mechanisms by the two compounds and the
promoting effect of Angpt2 siRNA in FGF21 administration.
In conclusion, the present study provides evidence
supporting that FGF21 treatment protects cardiomyocytes from
I/R-induced injury. However, increased level of Angpt2 inhibited
GLUT1 expression, which serves a passive role in the
cardioprotective effect of FGF21 against I/R-induced injury.
Combination therapy of FGF21 and Angpt2 siRNA provided more
efficient cardioprotection through Angpt2 inhibition and induced
GLUT1 upregulation, resulting in improvement of energy supply,
increased cell migration and lower apoptosis rate. These results
may suggest a novel therapeutic strategy for I/R-induced injury in
the future.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81070227).
References
|
1
|
Ambrose JA and Singh M: Pathophysiology of
coronary artery disease leading to acute coronary syndromes.
F1000Prime Rep. 7:082015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Groot H and Rauen U:
Ischemia-reperfusion injury: Processes in pathogenetic networks: A
review. Transplant Proc. 39:pp. 481–484. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boag SE, Das R, Shmeleva EV, Bagnall A,
Egred M, Howard N, Bennaceur K, Zaman A, Keavney B and
Spyridopoulos I: T lymphocytes and fractalkine contribute to
myocardial ischemia/reperfusion injury in patients. J Clin Invest.
125:3063–3076. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zitta K, Meybohm P, Bein B, Gruenewald M,
Lauer F, Steinfath M, Cremer J, Zacharowski K and Albrecht M:
Activities of cardiac tissue matrix metalloproteinases 2 and 9 are
reduced by remote ischemic preconditioning in cardiosurgical
patients with cardiopulmonary bypass. J Transl Med. 12:942014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He K, Yan L, Pan CS, Liu YY, Cui YC, Hu
BH, Chang X, Li Q, Sun K, Mao XW, et al: ROCK-dependent ATP5D
modulation contributes to the protection of notoginsenoside NR1
against ischemia-reperfusion-induced myocardial injury. Am J
Physiol Heart Circ Physiol. 307:H1764–H1776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawicki G: Synergistic effect of
inhibitors of MMPs and ROS-dependent modifications of contractile
proteins on protection hearts subjected to oxidative stress. Curr
Pharm Des. 20:1345–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukumoto S: Actions and mode of actions of
FGF19 subfamily members. Endocr J. 55:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wente W, Efanov AM, Brenner M,
Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I,
Zitzer H and Gromada J: Fibroblast growth factor-21 improves
pancreatic beta-cell function and survival by activation of
extracellular signal-regulated kinase 1/2 and Akt signaling
pathways. Diabetes. 55:2470–2478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SQ, Roberts D, Kharitonenkov A, Zhang
B, Hanson SM, Li YC, Zhang LQ and Wu YH: Endocrine protection of
ischemic myocardium by FGF21 from the liver and adipose tissue. Sci
Rep. 3:27672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge X, Chen C, Hui X, Wang Y, Lam KS and Xu
A: Fibroblast growth factor 21 induces glucose transporter-1
expression through activation of the serum response factor/Ets-like
protein-1 in adipocytes. J Biol Chem. 286:34533–34541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Syrjälä SO, Tuuminen R, Nykänen AI,
Raissadati A, Dashkevich A, Keränen MA, Arnaudova R, Krebs R, Leow
CC, Saharinen P, et al: Angiopoietin-2 inhibition prevents
transplant ischemia-reperfusion injury and chronic rejection in rat
cardiac allografts. Am J Transplant. 14:1096–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lukasz A, Beutel G, Kümpers P, Denecke A,
Westhoff-Bleck M, Schieffer B, Bauersachs J, Kielstein JT and
Tutarel O: Angiopoietin-2 in adults with congenital heart disease
and heart failure. PLoS One. 8:e668612013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pöss J, Ukena C, Kindermann I, Ehrlich P,
Fuernau G, Ewen S, Mahfoud F, Kriechbaum S, Böhm M and Link A:
Angiopoietin-2 and outcome in patients with acute decompensated
heart failure. Clin Res Cardiol. 104:380–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scholz A, Plate KH and Reiss Y:
Angiopoietin-2: A multifaceted cytokine that functions in both
angiogenesis and inflammation. Ann NY Acad Sci. 1347:45–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winter SF, Acevedo VD, Gangula RD, Freeman
KW, Spencer DM and Greenberg NM: Conditional activation of FGFR1 in
the prostate epithelium induces angiogenesis with concomitant
differential regulation of Ang-1 and Ang-2. Oncogene. 26:4897–4907.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Z, Chen Y, Li L, Wang G, Xue H and Tang
W: Combination therapy of renin-angiotensin system inhibitors plus
calcium channel blockers versus other two-drug combinations for
hypertension: A systematic review and meta-analysis. J Hum
Hypertens. 31:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu SQ, Tefft BJ, Roberts DT, Zhang LQ,
Ren Y, Li YC, Huang Y, Zhang D, Phillips HR and Wu YH:
Cardioprotective proteins upregulated in the liver in response to
experimental myocardial ischemia. Am J Physiol Heart Circ Physiol.
303:H1446–H1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu SQ and Wu YH: Liver cell-mediated
alleviation of acute ischemic myocardial injury. Front Biosci
(Elite Ed). 2:711–724. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Planavila A, Redondo-Angulo I and
Villarroya F: FGF21 and Cardiac Physiopathology. Front Endocrinol
(Lausanne). 6:1332015.PubMed/NCBI
|
|
23
|
Patel V, Adya R, Chen J, Ramanjaneya M,
Bari MF, Bhudia SK, Hillhouse EW, Tan BK and Randeva HS: Novel
insights into the cardio-protective effects of FGF21 in lean and
obese rat hearts. PLoS One. 9:e871022014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cong WT, Ling J, Tian HS, Ling R, Wang Y,
Huang BB, Zhao T, Duan YM, Jin LT and Li XK: Proteomic study on the
protective mechanism of fibroblast growth factor 21 to
ischemia-reperfusion injury. Can J Physiol Pharmacol. 91:973–984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Li H, Wei R, Ma J, Zhao Y, Lian Z
and Liu Z: Endothelial cells regulate cardiac myocyte
reorganisation through β1-integrin signalling. Cell Physiol
Biochem. 35:1808–1820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian H, Yang Y, Li J, Huang J, Dou K and
Yang G: The role of vascular stem cells in atherogenesis and
post-angioplasty restenosis. Ageing Res Rev. 6:109–127. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanajak P, Chattipakorn SC and
Chattipakorn N: Effects of fibroblast growth factor 21 on the
heart. J Endocrinol. 227:R13–R30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji K, Zheng J, Lv J, Xu J, Ji X, Luo YB,
Li W, Zhao Y and Yan C: Skeletal muscle increases FGF21 expression
in mitochondrial disorders to compensate for energy metabolic
insufficiency by activating the mTOR-YY1-PGC1α pathway. Free Radic
Biol Med. 84:161–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moyers JS, Shiyanova TL, Mehrbod F, Dunbar
JD, Noblitt TW, Otto KA, Reifel-Miller A and Kharitonenkov A:
Molecular determinants of FGF-21 activity-synergy and cross-talk
with PPARgamma signaling. J Cell Physiol. 210:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiedler U, Krissl T, Koidl S, Weiss C,
Koblizek T, Deutsch U, Martiny-Baron G, Marmé D and Augustin HG:
Angiopoietin-1 and angiopoietin-2 share the same binding domains in
the Tie-2 receptor involving the first Ig-like loop and the
epidermal growth factor-like repeats. J Biol Chem. 278:1721–1727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lorbeer R, Baumeister SE, Dörr M, Nauck M,
Grotevendt A, Volzke H, Vasan RS, Wallaschofski H and Lieb W:
Circulating angiopoietin-2, its soluble receptor Tie-2, and
mortality in the general population. Eur J Heart Fail.
15:1327–1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Yong H, Mi L, Bai Y, Guo L, Gao W,
Cui M and Zhang Y: Changes and significance of serum angiopoietin-2
levels in patients with coronary heart disease. Biomarkers.
17:745–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piccolo MT, Menale C and Crispi S:
Combined anticancer therapies: An overview of the latest
applications. Anticancer Agents Med Chem. 15:408–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith R, McCready T and Yusuf S:
Combination therapy to prevent cardiovascular disease: Slow
progress. JAMA. 309:1595–1596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cadete VJ, Arcand SA, Lin HB and Sawicki
G: Synergistic protection of MLC 1 against cardiac
ischemia/reperfusion-induced degradation: A novel therapeutic
concept for the future. Future Med Chem. 5:389–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|