Introduction
Idiopathic pulmonary fibrosis (IPF) is a
progressive, severely debilitating disease with a high mortality
rate (1). However, there are
currently no effective treatments for IPF. The clinical features of
IPF include chronic (>6 months) exertional shortness of breath,
a dry cough, inspiratory bibasilar crackles and finger clubbing
(2,3). Although the risk factors for IPF remain
unclear, a prevailing hypothesis suggests that injuries in the
alveolar epithelium trigger pathophysiological alterations,
including increased production of transforming growth factor
(TGF)-β, which induces the differentiation of fibroblasts into
myofibroblasts and the excessive deposition of extracellular matrix
(ECM) (4). The alteration of
fibroblasts and the ECM serves a major role in the development of
fibrosis, and creates a profibrosis positive feedback loop
(5). The differentiation of
fibroblasts into myofibroblasts, which are fibroblasts that contain
α-smooth muscle actin (α-SMA) and other contractile elements, has
been identified as a key event in the development of IPF and other
profibrotic conditions (6,7).
The neurogenic locus notch homolog protein (Notch)
signaling pathway is highly conserved and serves a key role in
cellular proliferation, specification and differentiation (8). In mammals, there are four types of
Notch receptor (Notch1-4) and five Notch receptor ligands (Jagged1,
Jagged2, delta-like 1, delta-like 3 and delta-like 4). Notch
receptors are activated by ligand binding, upon which the Notch
intracellular domain (NICD) is released and translocates into the
nucleus, where it interacts with transcriptional repressors to
modulate the expression of target genes that include the
well-characterized transcription factor hairy enhance of split
(Hes).
Previous studies have reported that the Notch
signaling pathway is associated with human fibrotic diseases,
including pulmonary fibrosis (PF) (9,10).
Artesunate, the recommended first-line treatment for severe
malarias (11), was recently
demonstrated by our group to inhibit the proliferation of lung
fibroblast by promoting apoptosis and reducing collagen secretion
(12). In addition, artesunate was
determined to downregulate the expression of TGF-β1, mothers
against decapentaplegic homolog 3, heat shock protein 47, α-SMA and
collagen type I (12).
The present study aimed to evaluate the effect of
artesunate on the TGF-β1-induced differentiation of lung
fibroblasts into myofibroblast. In addition, the effect of
artesunate on bleomycin-induced pulmonary fibrosis in rats was
investigated. Furthermore, the potential underlying molecular
mechanisms of the effects of artesunate on these processes was
explored.
Materials and methods
Materials
Antibodies for cleaved Notch (NICD, cat. no.
ab52301), α-SMA (cat. no. ab5694) and Hes-1 (cat. no. ab108937)
were purchased from Abcam (Cambridge, MA, USA). Antibodies for
Jagged1 (cat. no. Sc-8303) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Antibodies for Notch1 (cat.
no. 4380) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies for β-actin (cat. no. PR0255),
horseradish peroxidase (HRP)-conjugated anti-mouse or -rabbit
immunoglobulin G antibodies (cat. no ZB-2301 and ZB-2305), and
Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit
immunoglobulin G antibody (cat. no. ZF-0311) were obtained from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China). The γ-secretase inhibitor DAPT was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Human recombinant
TGF-β1 was purchased from Peprotech, Inc. (Rocky Hill, NJ, USA).
Penicillin/streptomycin, fetal bovine serum (FBS) and Dulbecco's
modified Eagle's medium (DMEM) were obtained from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bleomycin
hydrochloride for injection was purchased from Nippon Kayaku Co.,
Ltd. (Tokyo, Japan). Artesunate was purchased from Guilin
Pharmaceutical (Shanghai) Co., Ltd. (Guilin, China). The DAB kit
(cat. no. ZLI-9017) was obtained from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China) according to the
manufacturer's protocol. Other reagents were purchased from
Sigma-Aldrich (Merck KGaA).
Lung fibroblast culture
Lung fibroblasts were isolated from the rats
described below according to a previous method (13). The cells were cultured (at 37°C, 95%
humidity and 5% CO2) in DMEM supplemented with 10% FBS.
The identity of fibroblast cells was verified at passage four by
testing the expression of vimentin (1:200 dilution; cat. no. 5741;
CST, Inc., Danvers, MA, USA) by immunofluorescence staining. Cells
at passage 5 were used for further experiments.
The primary fibroblasts were synchronized by
incubation (at 37°C in 5% CO2) in serum-free DMEM for 24
h and then randomly divided into five groups as follows: The
control group, incubated in serum-free DMEM; the TGF-β1 group,
incubated in DMEM containing 5 ng/ml TGF-β1; the Notch inhibitor
(DAPT) + TGF-β1 group, incubated in DMEM containing 5 ng/ml TGF-β1
and 10 µΜ DAPT; the artesunate + TGF-β1 group, incubated in DMEM
containing 5 ng/ml TGF-β1 and 8 µg/ml artesunate; and the
artesunate control group, incubated in DMEM containing 8 µg/ml
artesunate at 37°C for 24 h.
Animal experiment protocols
Male Sprague Dawley rats (n=24) weighing 180–250 g
at 8 weeks old were obtained from the Center for Experimental
Animals at Guilin Medical University (Guilin, China). The rats were
housed in specific pathogen-free conditions at 20–24°C and humidity
30–50% with a 12-h light/dark cycle and ad libitum access
food and water. The animal protocol was reviewed and approved by
the Institutional Animal Care and Use Committee of the Affiliated
Hospital of Guilin Medical University (Guilin, China).
For in vivo study, the rats were randomly
divided into the following four groups: The control group (n=6),
which received intratracheal administration of 0.9% NaCl solution
on day 1, followed by daily intraperitoneal injections of 0.9% NaCl
solution (1 ml) for 27 days; the bleomycin group (n=6), which
received intratracheal administration of bleomycin (5 mg/kg) at day
1, followed by daily intraperitoneal injections of 0.9% NaCl
solution (1 ml) for 27 days; the artesunate group (n=6), which
received intratracheal administration of 0.9% NaCl solution at day
1, followed by daily intraperitoneal injections of artesunate (100
mg/kg) for 27 days; and the bleomycin + artesunate group (n=6),
which received intratracheal administration of bleomycin (5 mg/kg)
at day 1, followed by daily intraperitoneal injection of artesunate
(100 mg/kg) for 27 days. All animals were euthanized by
pentobarbital overdose (100 mg/kg) at the end of treatment period,
and lung tissues were quickly removed and processed for further
analysis.
Masson's trichrome staining
Masson's trichrome staining was performed to observe
lung fibroblast collagen secretion. Lung fibroblasts
(0.3×106/well) were plated in 6-well plates with or
without TGF-β1 (5 ng/ml) at 37°C in 5% CO2) for 24 h,
and the cultured cells were washed 3 times for 1 min each with
ice-cold PBS and fixed at 4°C with 4% paraformaldehyde for 30 min.
Then lung fibroblasts were stained with Masson trichrome staining
kit manual at 20–24°C for 5 min and subsequently examined with a
light microscope (Olympus Corporation, Beijing, China).
Immunofluorescence staining
Lung fibroblasts (7,000 cells per well) were plated
on 6-well chamber slides with or without TGF-β1 (5 ng/ml) at 37°C
for 24 h, then the culture medium was then aspirated, and the
slides were washed with PBS and fixed with 4% paraformaldehyde at
room temperature for 20 min. Cells were permeabilized with 0.05%
Triton X-100 at 4°C for 10 min and blocked with 3% bovine serum
albumin (BSA; cat. no. OR0015; Leagene Co., Ltd. Beijing, China)
for at 4°C 1 h. The slides were incubated at 4°C overnight with
primary antibodies directed against α-SMA (dilution, 1:100 in 3%
BSA) or against vimentin (1:200 dilution; cat. no. 5741; CST,
Inc.). Subsequently, the slides were washed with PBS and incubated
with secondary anti-rabbit Texas green-conjugated antibodies at 4°C
(dilution, 1:200 in 3% BSA). Slides were then washed in PBS for 20
min. Two drops (10–15 µl) of ProLong® Gold Antifade
Mountant (Thermo Fisher Scientific, Inc.) was added to each well
prior to the addition of coverslips. The slides were then examined
by fluorescence microscopy.
Western blot analysis
The lung tissue was homogenized in
radioimmunoprecipitation assay (RIPA) buffer with a protease
inhibitor cocktail (cat. no. 04693159001; Roche Diagnostics, Basel,
Switzerland) containing 1 mmol/l phenylmethanesulfonyl fluoride
(PMSF) using a tissue grinder. Cultured cells were lysed on ice in
ice-cold RIPA buffer with a protease inhibitor cocktail containing
1 mmol/l PMSF for 20 min. Proteins from lung tissue and fibroblast
were prepared as previously described (12,13). A
total of 20 µg protein lane was loaded into each lane and separated
via 10% SDS-PAGE gel and then transferred to a polyvinylidene
difluoride membrane, which was blocked with 5% non-fat milk in
Tris-buffered saline (TBS) at room temperature for 1.5 h. The
membranes were then incubated at 4°C overnight with cleaved Notch
(NICD 1:1,000 dilution; cat. no. ab52301), α-SMA (1:1,000 dilution;
cat. no. ab5694), Hes-1 (1:800 dilution; cat. no. ab108937),
Jagged1 (1:500 dilution; cat. no. Sc-8303) and Notch1 (1:400
dilution; cat. no. 4380), followed by three washes with TBS
containing Tween-20 (5 min/wash). The membrane was subsequently
incubated with secondary antibodies, horseradish
peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G
antibodies (1:5,000 dilution; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), cat. no ZB-2301 and ZB-2305,
respectively) at 20–24°C for 1.5 h. Protein bands were detected
using Enhanced Chemiluminescent reagent and imaged with a ChemiDoc
MP Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Immunohistochemical staining
Tissues were fixed with formalin at 20–24°C for 24 h
and embedded with paraffin, then sliced into 5 µm thick sections,
which were deparaffinized in xylene followed by rehydration in a
graded series of alcohols. Antigen retrieval was performed through
pressure cooking (at 121°C) in citrate buffer solution for 3 min.
Slides were rinsed with PBS and then incubated in 3% hydrogen
peroxide solution for at 20–24°C for 20 min. After rinsing with PBS
three times, the slides were incubated with 10% fetal bovine serum
(cat. no. 10437028; Gibco; Thermo Fisher Scientific, Inc.) in PBS
for 30 min to block nonspecific binding. The slides were then
incubated with primary antibodies directed against Jagged1 (1:400),
NICD (1:200), Hes-1 (1:200), α-SMA (1:200) or Type-IV collagen
(1:400 dilution; cat. no. ab6586; Abcam) at 4°C overnight, followed
by three washes in PBS (5 min/wash). Subsequently, the slides were
incubated with biotinylated goat anti-rabbit polyclonal secondary
antibody (cat. no. XIT-9901; Maixin-Bio, Fuzhou, China) at room
temperature for 30 min, followed by three washes in PBS (5
min/wash). The slides were then stained with the DAB kit at 20–24°C
for 5–10 min according to manufacturer's instruction. Finally, the
slides were dehydrated, cleared with xylene and mounted with
neutral gum. Images of tissue sections were captured using a BX53
digital light microscope (Olympus Corporation, Tokyo, Japan).
ECM-deposition assay
Lung fibroblasts (0.3×106/well in 6-well
plate) were stimulated by incubation with TGF-β1 (5 ng/ml) for 24 h
at 37°C with or without artesunate (8 µg/ml) or DAPT (10 µmol/l),
then in serum-free DMEM at 37°C for 48 h. Collagen secretion and
deposition (collagen type I–V) were determined using the Sirius Red
Collagen Detection kit (cat. no. 9062; Chonrex, Inc., Redmond, WA,
USA) according to the manufacturer's protocol.
Statistical analysis
Statistical tests were performed using SPSS software
(version 18.0; SPSS, Inc., Chicago, IL, USA). Results are presented
as the mean ± standard error. The statistical significance of
differences between groups was determined using one-way analysis of
the variance with a post hoc Tukey's range test. P<0.05 was
considered to indicate a statistically significant difference.
Results
TGF-β1 induces the differentiation of
rat primary lung fibroblasts
To assess whether TGF-β1 serves a role during the
differentiation of primary lung fibroblasts into myofibroblasts,
the expression of α-SMA, a myofibroblast marker and all type of
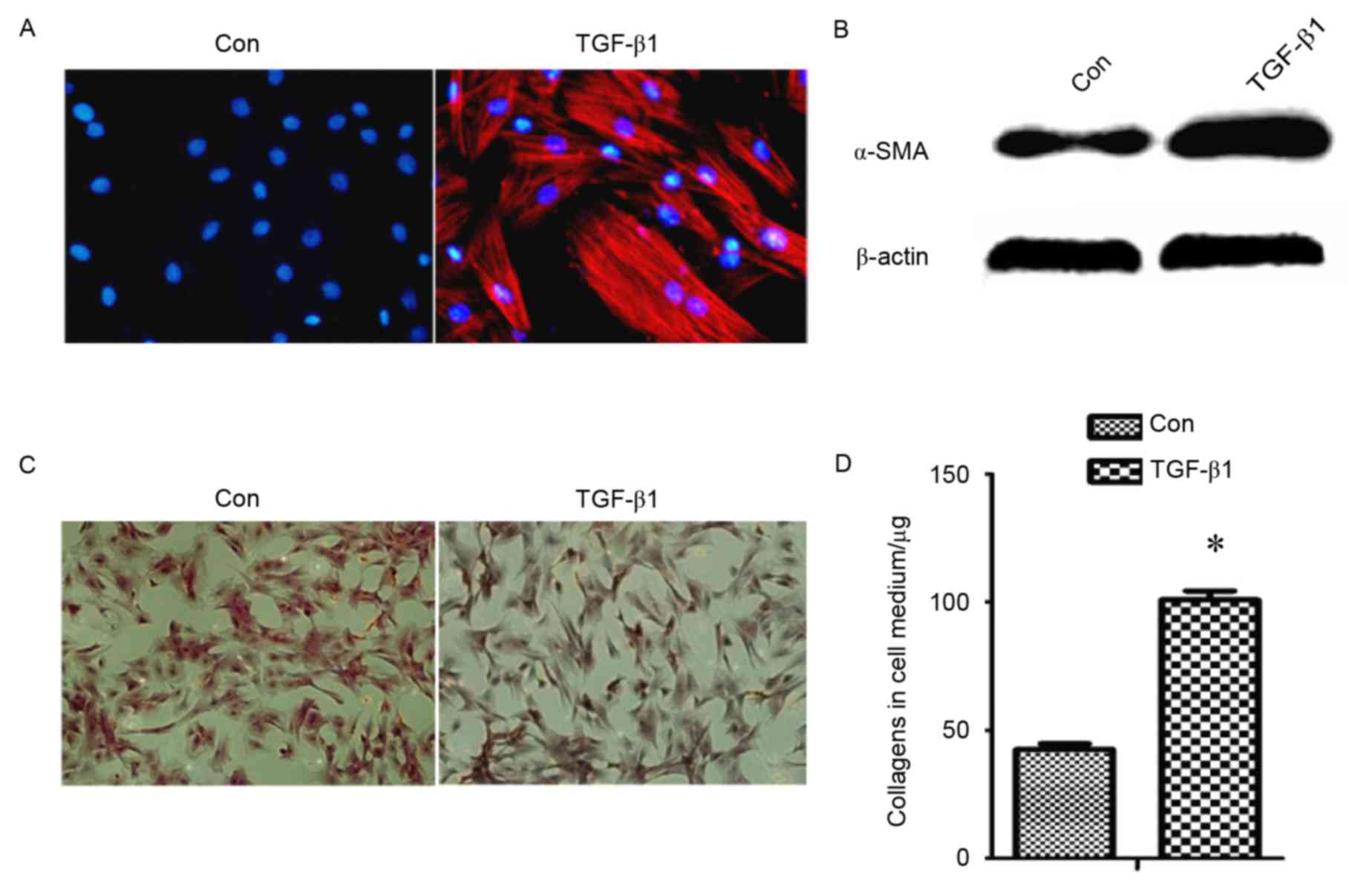
collagen, was detected (Fig. 1).
This revealed that protein levels of α-SMA (Fig. 1A and B) and collagens (Fig. 1C and D) were increased in
TGF-β1-treated fibroblasts compared with the control group,
indicating that TGF-β1 induces the differentiation of fibroblast
into myofibroblasts.
TGF-β1 activates the Notch signaling
pathway in primary lung fibroblasts
The Notch signaling pathway is an important
signaling pathway in PF (8). To
investigate the role of the Notch signaling pathway in the
TGF-β1-induced differentiation of fibroblasts into myofibroblasts,
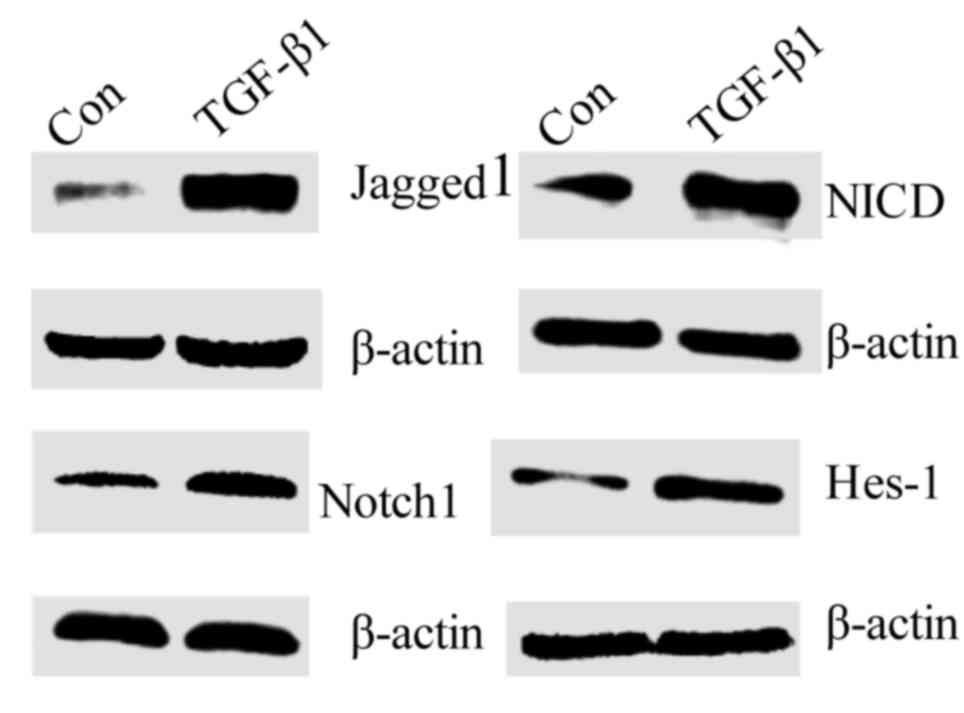
the protein expression of Jagged1, Notch1, NICD and Hes-1 was
measured. This revealed that all were increased in TGF-β1-treated
fibroblasts compared with the control group (Fig. 2). Furthermore, DAPT, a γ-secretase
inhibitor and inhibitor of the Notch signaling pathway,
significantly suppressed the TGF-β1-induced overexpression of α-SMA
(P<0.01; Fig. 3). These findings
indicate that the Notch signaling pathway is associated with the
TGF-β1-induced differentiation of fibroblasts into
myofibroblasts.
Artesunate inhibits the
differentiation of primary lung fibroblasts
Based on our groups previous finding that artesunate
exerts an antiprofibrotic effect in rats with bleomycin-induced PF
(12), and the finding of the
current study that the expression of α-SMA and collagens was
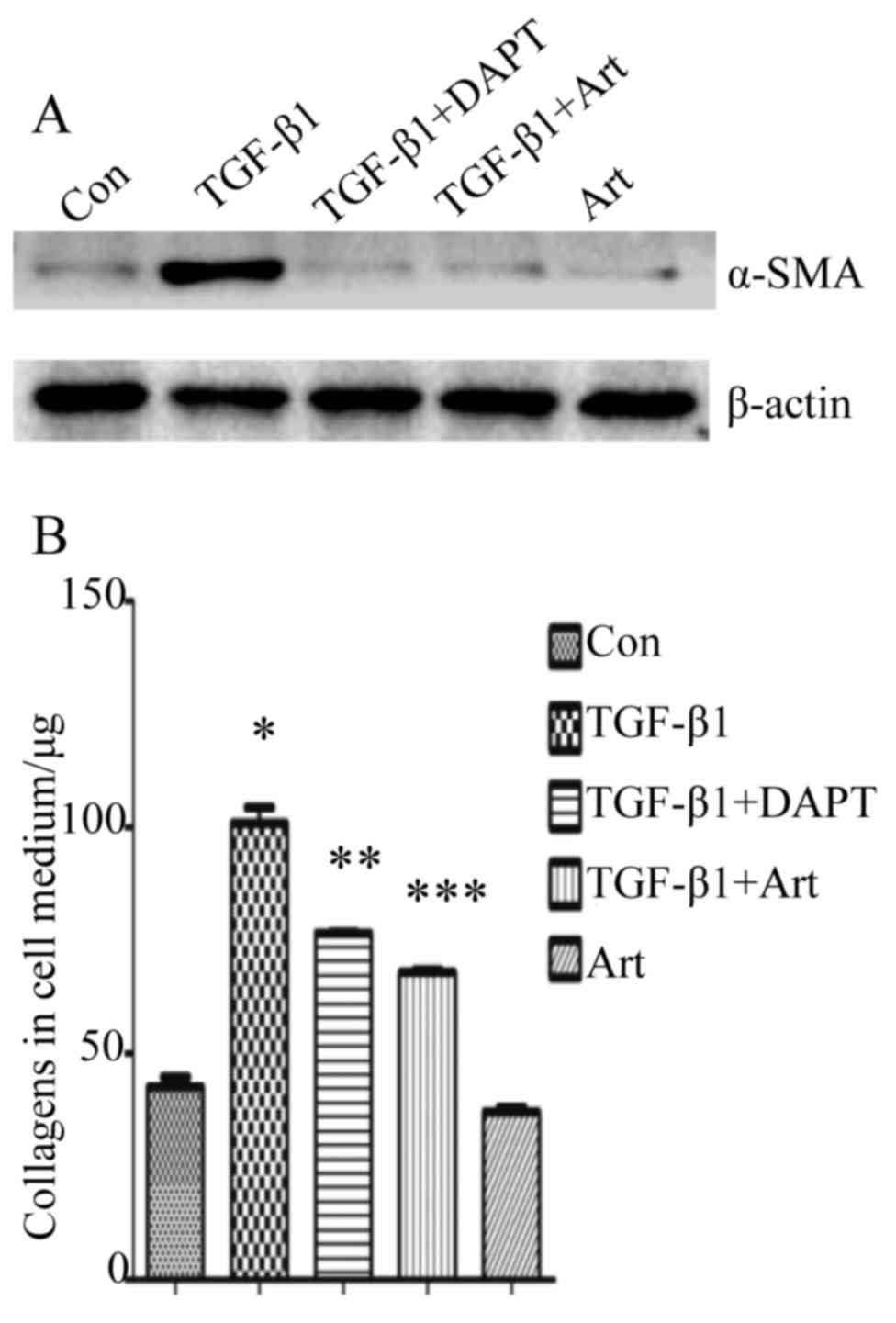
upregulated in TGF-β1-treated fibroblasts (Fig. 1), it was hypothesized that artesunate
may serve a role in the differentiation of lung fibroblasts. To
test this hypothesis, the effect of artesunate on the expression of
α-SMA and collagens in TGF-β1-treated lung fibroblasts was
examined. The protein expression of α-SMA (Fig. 3A) and the amount of collagens
secreted in culture medium (Fig. 3B)
were significantly decreased after treatment with artesunate. In
addition, the effect of artesunate was only slightly less compared
with that of DAPT (Fig. 3). These
findings suggest that artesunate is able to inhibit the
differentiation of fibroblasts into myofibroblasts.
Artesunate inhibits the Notch
signaling pathway in primary lung fibroblasts
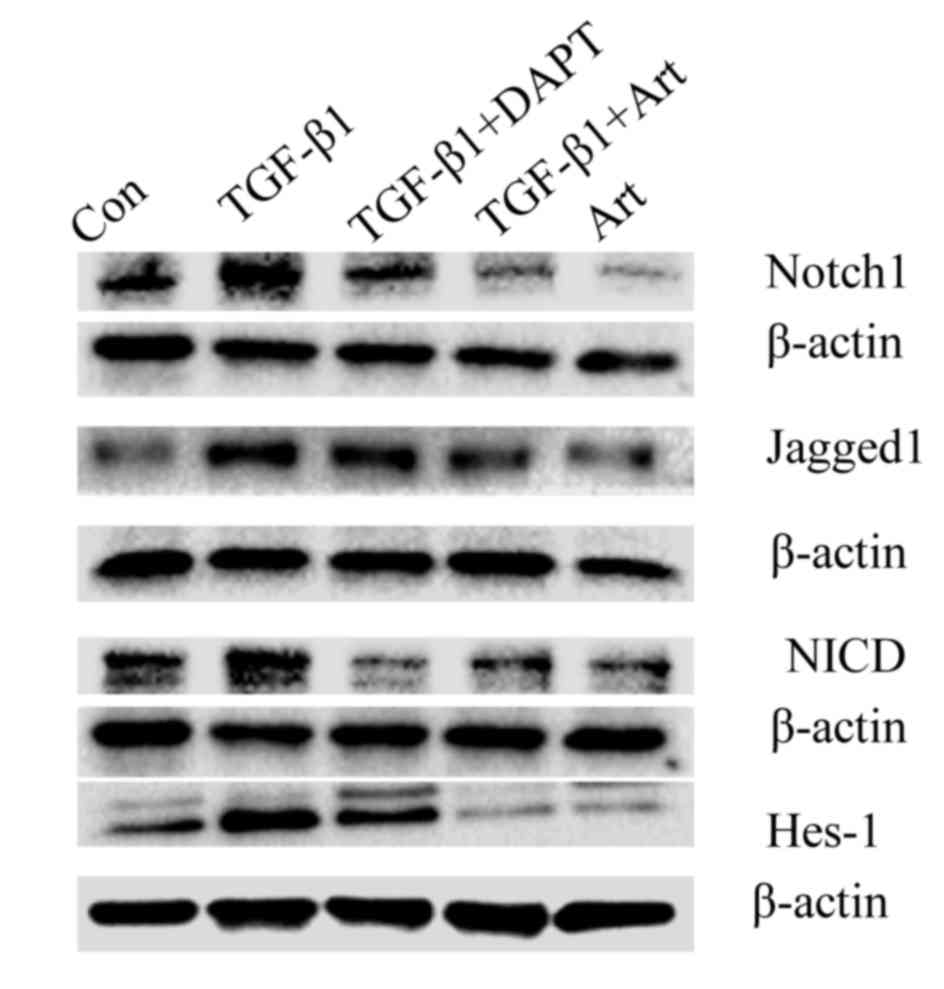
The aforementioned experiments indicated that the
Notch signaling pathway serves a role in the TGF-β1-induced
differentiation of fibroblasts to myofibroblasts, and that
artesunate is able to inhibit this. To evaluate whether the
inhibitory effect of artesunate on this differentiation is via the
Notch signaling pathway, the protein levels of Notch1, Jagged1,
NICD and Hes-1 in lung fibroblasts treated with artesunate was
measured. The expression of these proteins was markedly decreased
in lung fibroblasts following treatment with Art or DAPT compared
with the control group (Fig. 4),
suggesting that artesunate inhibits the Notch signaling
pathway.
Artesunate suppresses the expression
of collagens, α-SMA and certain components of the Notch signaling
pathway in the lung tissues of rats with bleomycin-induced PF
Bleomycin induces inflammation and collagen
deposition in the lungs of rats, and a previous study by our
previously groups demonstrated that artesunate can ameliorate these
pathological alterations in cultured frbroblasts (12). In the present study, an in
vivo experiement was performed to confirm these findings.
Immunohistochemistry (Fig. 5A) and
western blotting (Fig. 5B) were
employed to detect the expression of collagen, α-SMA and certain
key components of the Notch signaling pathway in the lung tissue
from rats with bleomycin-induced PF treated with artesunate. The
results demonstrated that the protein levels of collagen, α-SMA,
Jagged1, Notch, NICD and Hes-1 were markedly increased following
exposure to bleomycin, indicating that the Notch signaling pathway
is activated by bleomycin. However, the protein levels of collagen,
α-SMA, Jagged1, Notch1, NICD and Hes-1 were decreased in the lung
tissues from rats treated with artesunate, or bleomycin and
artesunate, suggesting that artesunate inhibits the Notch signaling
pathway.
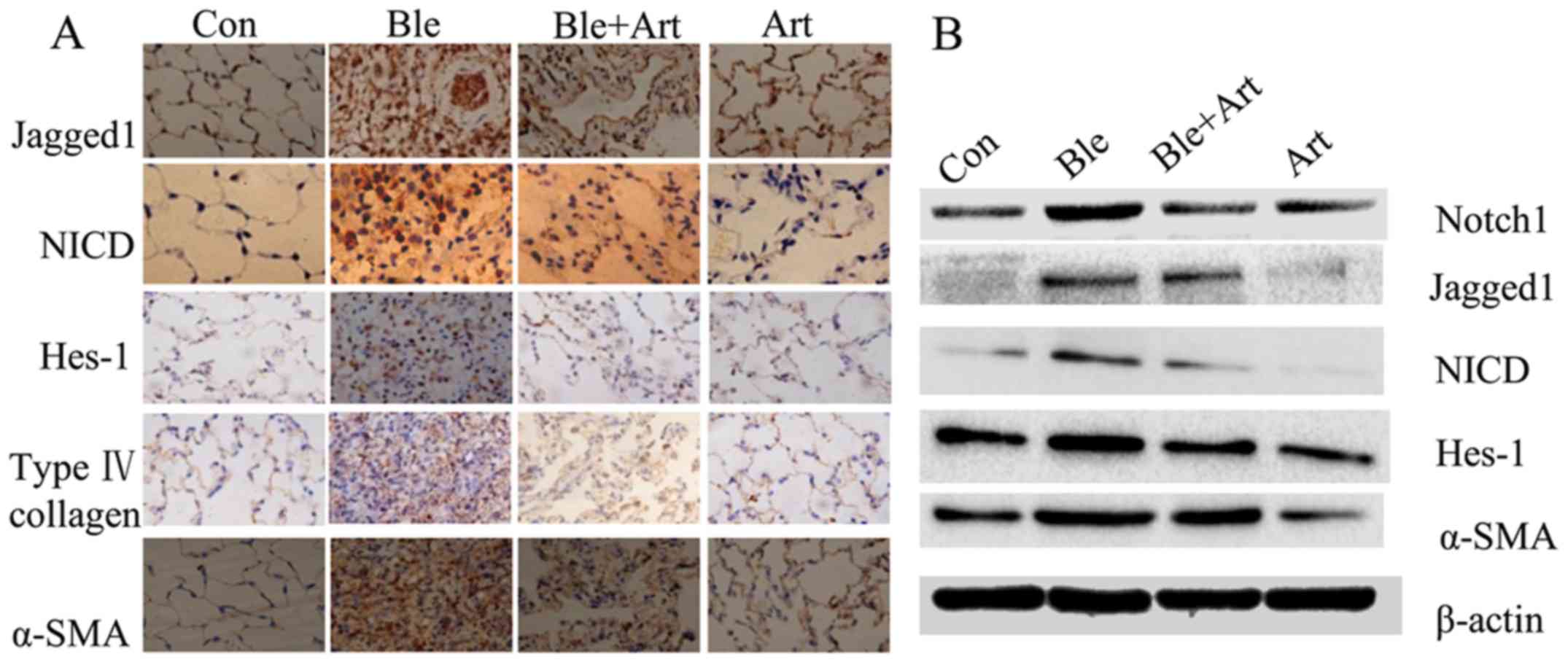 | Figure 5.Artesunate inhibits the expression of
collagen, α-SMA and components of the Notch signaling pathway in
the lung tissues of rats with bleomycin-induced pulmonary fibrosis.
Representative (A) immunohistochemical images (magnification ×40)
and (B) western blotting results for Jagged1, NICD, Hes-1, collagen
and α-SMA. Con, control group; Ble, bleomycin; Art, artesunate;
α-SMA, α smooth muscle actin; Notch1, neurogenic locus notch
homolog protein 2; NICD, Notch intracellular domain; Hes-1, hairy
enhance of split 1. |
Discussion
The results of the present study indicate that
artesunate inhibits the TGF-β1-induced expression of collagen and
α-SMA in lung fibroblasts, and that artesunate reduces
bleomycin-induced PF in rats. In addition, artesunate was
identified to downregulate key components of the Notch signaling
pathway in vitro and in vivo, including Jagged1,
Notch1, NICD and Hes-1.
PF is a chronic lung disorder characterized by the
dysregulated recruitment, proliferation and differentiation of
fibroblasts, excessive deposition of ECM and abnormal lung
remodeling (4). Fibroblasts serve an
important role in the pathogenesis of PF, and several factors
influence their proliferation and synthesis of ECM (14). Myofibroblasts, a marker of fibrotic
diseases, secrete ECM, collagens and a-SMA, which leads to the loss
of alveolar function (4,14). Numerous profibrotic cytokines,
including TGF-β1, platelet-derived growth factor and tumor necrosis
factor α, serve roles in the differentiation of fibroblasts into
myofibroblasts. The present study demonstrated that TGF-β1 could
induce the differentiation of fibroblasts into myofibroblasts, in
addition to activating the Notch signaling pathway.
There are few reports on the role of the Notch
signaling pathway in the differentiation of lung fibroblasts into
myofibroblasts. The Notch signaling pathway is a highly conserved
pathway, which is essential to normal embryonic development,
cellular proliferation, specification and differentiation, and is
associated with fibrotic disease (8). The findings of the present study aid in
the better understanding of the pathophysiology of lung fibrosis,
in addition to suggesting that the Notch signaling pathway is a
potential therapeutic target for this disease. Since overexpression
of Hes1 enhances the promoter activities of α-SMA and collagen type
1 α2 (15), therefore TGF-β1 may
function, at least partially, via the Notch signaling pathway to
upregulate Hes1 expression and promote α-SMA expression.
The results of the present study are consistent with
previous reports that Notch deficiency has a significant inhibitory
effect on the response to bleomycin-induced PF (10,16),
suggesting that cellular signaling pathway crosstalk serves an
essential role in the pathogenesis of PF. Our group recently
reported that artesunate, a drug used for the treatment of severe
malaria in adults and children worldwide, may inhibit the
development of PF (12,17). Previous experiments by our group
demonstrated artesunate can induce the apoptosis of fibroblasts,
inhibit TGF-β1-induced epithelial-to-mesenchymal transition and
downregulate the expression of TGF-β1 in an animal model of PF
(12,18). The present study provided additional
evidence revealing the potential underlying molecular mechanism of
the antifibrotic effect of artesunate. However, further experiments
are required to test whether artesunate functions via the same
mechanism in other types of pulmonary cells.
In conclusion, the current study demonstrated that
artesunate effectively inhibits the Notch signaling pathway and the
TGF-β1-induced differentiation of primary lung fibroblasts into
myofibroblasts in vitro, and ameliorates PF in vivo.
The results of the present study indicate that the Notch signaling
pathway serves a role in the differentiation of fibroblasts into
myofibroblasts, and may be a potential novel therapeutic target for
PF.
Acknowledgements
The present study was supported the Natural Science
Foundation of Guangxi (grant nos. 2014GXNSFAA118151 and
2015GXNSFAA139178), the National Natural Science Foundation of
China (grant no. 81360010), and the Guangxi Health and Family
Planning Commission (grant no. S2015-34). G. H. was supported by
the Hundred Talents Program of Guangxi.
References
|
1
|
Puglisi S, Torrisi SE, Giuliano R,
Vindigni V and Vancheri C: What we know about the pathogenesis of
idiopathic pulmonary fibrosis. Semin Respir Crit Care Med.
37:358–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HJ, Perlman D and Tomic R: Natural
history of idiopathic pulmonary fibrosis. Respir Med. 109:661–670.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker MW, Rossi D, Peterson M, Smith K,
Sikström K, White ES, Connett JE, Henke CA, Larsson O and Bitterman
PB: Fibrotic extracellular matrix activates a profibrotic positive
feedback loop. J Clin Invest. 124:1622–1635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiskirchen R and Tacke F: Liver fibrosis:
From pathogenesis to novel therapies. Dig Dis. 34:410–422. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardo A, Cabrera S, Maldonado M and Selman
M: Role of matrix metalloproteinases in the pathogenesis of
idiopathic pulmonary fibrosis. Respir Res. 17:232016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Liao S, Zhang Z, Wang B and Wan L:
Astragalus injection attenuates bleomycin-induced pulmonary
fibrosis via down-regulating Jagged1/Notch1 in lungs. J Pharm
Pharmacol. 68:389–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu T, Hu B, Choi YY, Chung M, Ullenbruch
M, Yu H, Lowe JB and Phan SH: Notch1 signaling in FIZZ1 induction
of myofibroblast differentiation. Am J Pathol. 174:1745–1755. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reyburn H: New WHO guidelines for the
treatment of malaria. BMJ. 340:c26372010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Xuan X, Yao W, Huang G and Jin J:
Anti-profibrotic effects of artesunate on bleomycin-induced
pulmonary fibrosis in Sprague Dawley rats. Mol Med Rep.
12:1291–1297. 2015.PubMed/NCBI
|
|
13
|
Wang Y, Huang G, Mo B and Wang C:
Artesunate modulates expression of matrix metalloproteinases and
their inhibitors as well as collagen-IV to attenuate pulmonary
fibrosis in rats. Genet Mol Res. 15:2016.
|
|
14
|
Hardie WD, Glasser SW and Hagood JS:
Emerging concepts in the pathogenesis of lung fibrosis. Am J
Pathol. 175:3–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Zhang YQ, Ai WB, Hu QT, Zhang QJ,
Wan LY, Wang XL, Liu CB and Wu JF: Hes1, an important gene for
activation of hepatic stellate cells, is regulated by Notch1 and
TGF-β/BMP signaling. World J Gastroenterol. 21:878–887. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu B, Wu Z, Bai D, Liu T, Ullenbruch MR
and Phan SH: Mesenchymal deficiency of Notch1 attenuates
bleomycin-induced pulmonary fibrosis. Am J Pathol. 185:3066–3075.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Liu W, Fang B, Gao S and Yan J:
Artesunate ameliorates hepatic fibrosis induced by bovine serum
albumin in rats through regulating matrix metalloproteinases. Eur J
Pharmacol. 744:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang CM, Chen J, Jiang M, Xuan XP and Li
HX: Relationship between artesunate influence on the process of
TGF-beta1 induced alveolar epithelial cells transform into
mesenchymal cells and on idiopathic pulmonary fibrosis. Yao Xue Xue
Bao. 49:142–147. 2014.(In Chinese). PubMed/NCBI
|