Introduction
Intervertebral disc degeneration (IDD) is the
pathological basis of intervertebral disc protrusion, unstable
spine, spinal canal stenosis, spinal cord, nerve root compression,
and the major cause leading to low back pain (1). The reduction of type II collagen (Col
II) and aggrecan contents are typical pathological changes of IDD
and matrix metalloproteinase (MMP) is the most significant
proteinase that can degrade the extracellular matrix (ECM)
(2). A disintegrin-like and
metalloproteinase with thrombospondin type I motifs (ADAMTS) also
exerts important effects in degrading proteoglycans (3).
Previous findings showed that inflammatory reaction
plays a significant role in the occurrence and development of IDD
(4). The JAK2/STAT3 signaling
pathway is key in central nervous system inflammation and immune
response, and exerts an important role in synaptic plasticity,
neurodegeneration, and memory formation (5). STAT3 is activated by the IL-6 cytokine
and is regarded as a major factor mediating IL-6 function (6). Differential microRNA (miR) chip
screening in degenerative nucleus pulposus indicates that the
occurrence of IDD may be regulated by an abnormally high expression
of miR-146a (7).
Based on this, we further analyzed the mechanism of
miR-146a-mediated interleukin-6/signal transducer and activator of
transcription 3 (IL-6/STAT3) activation in IDD. This study has the
potential to identify a new target to improve our understanding of
IDD and for therapeutic intervention.
Materials and methods
Patients and tissue collection
Five patients diagnosed with lumbar intervertebral
disc herniation were selected as the experimental group and another
five patients with lumbar burst fracture were enrolled as the
control group. The experimental group had three males and two
females, aged 50–70 years, with an average age of 57.5±8.9 years.
According to the position of disc damage, two cases affected lumbar
spinal segments L2-3, two affected L4-5 and one affected L5-S1. The
control group had three males and two females, aged 52–69 years,
with an average age of 56.6±7.5 years. The control group included
two cases involving L3-4 and three cases affected L4-5.
Written informed consent was obtained
from patients
The study was approved by the Ethics Committee of
Shanghai General Hospital of Nanjing Medical University.
Cell culture
The nucleus pulposus tissue of the lesion was
removed by surgery, and cell culture and identification were
performed. The tissue was digested with parenzyme and centrifuged
at 2,000 × g for 5 min. Subsequently, 0.2% Col II was added and
mixed uniformly for digestion within 4 h. Dulbeccos modified Eagles
medium Nutrient Mixture F-12 (DMEM/F12) complete culture solution
was added to stop the digestion. After filtration, the samples were
centrifuged at 1,000 × g for 5 min and DMEM/F12 complete culture
solution was added. The cultures were re-suspended at a density of
1×105 cells/ml and cultured at 37°C, and 5%
CO2. The cultures were sub-cultured after the solution
was changed the following day and cell attachment, growth, and
morphological changes were observed under an inverted microscope
(BX-42; Olympus, Tokyo, Japan). Second-generation cells were used
for immunocytochemical staining to identify Col II expression in
order to demonstrate the identity of the cultures as nucleus
pulposus cells.
Research methods
Cell transfection was performed according to ribo
FECTTMCP (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
transfection kit instructions. miR-146a empty vector, mimic and
inhibitor were cultured with the two groups of cells for 24 h and
transfection efficiency was detected. The levels of IL-6, Col II,
and aggrecan were detected by ELISA, and the expression levels of
STAT3, MMP-3, and ADAMTS were detected by western blotting.
Cell transfection
Cells were transferred onto 24-well plates at a
density of 5×105 cells/ml. A total of 440 µl DMEM/F12
(1:1) medium was added into each well and the transfection was
performed when the cell volume reached 85%. Then, 5 µl 40 nM
miR-146a empty vector, miR-146a mimic, miR-146a mimic negative
control, miR-146a inhibitor and miR-146a inhibitor negative control
were added into 50 µl FECTTMCP buffer solution, mixed well, and
incubated at room temperature for 5 min. FECTTMCP transfection
agent (5 µl) was added, mixed well, and incubated at room
temperature for 15 min. The 24-well plate was incubated at 37°C, 5%
CO2 for 24 h. The cell transfection efficacy was
detected by reverse transcription quantitative PCR.
ELISA method
IL-6, Col II and aggrecan agents were purchased from
Beyotime Institute of Biotechnology (Jiangsu, China), and the cell
extract was centrifuged at 3,000 × g for 20 min and detected by
microplate reader. The average value was obtained in
triplicate.
Western blotting
Radio-immunoprecipitation assay (RIPA) lysis buffer
was added to the cells and the total protein was extracted.
Coomassie brilliant blue was used for coarse quantitation and
β-actin antibody was used for standardization. Total protein (30
µg) was used for 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) separation. The proteins were
transferred onto a polyvinylidene fluoride (PVDF) membrane,
incubated overnight with mouse monoclonal STAT3 antibody (dilution,
1:500; cat. no. AMAB90777), mouse monoclonal MMP-3 antibody
(dilution, 1:500; cat. no. SAB530042) and mouse monoclonal ADAMTS
antibody (dilution, 1:500; cat. no. WH0170691M1). Rabbit
anti-mouse-HRP polyclonal secondary antibody (dilution, 1:2,000;
cat. no. A9044MSDS) was incubated at room temperature for 4 h. All
the antibodies were purchased from Sigma (St. Louis, MO, USA).
Phosphate-buffered saline (PBS) was used for washing and
electrochemiluminescence (ECL) was used for signal development. The
results were scanned and saved. Lab Works 4.5 gel imaging software
(Invitrogen, Carlsbad, CA, USA) was used for semi-quantitative
analysis.
Statistical methods
SPSS20.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. Measurement data were expressed as
mean ± standard deviation. Independent sample t-test was used for
the intergroup comparison and one-way analysis of variance (ANOVA)
was used for the comparison among multiple groups. Least
significant difference (LSD) test was used for the paired
comparisons and the paired t-test was used for a comparison before
and after intervention in each group. Enumeration data were
expressed as case or percentage, and the Chi-square test was used
for the intergroup comparison. P<0.05 indicated that the
difference was statistically significant.
Results
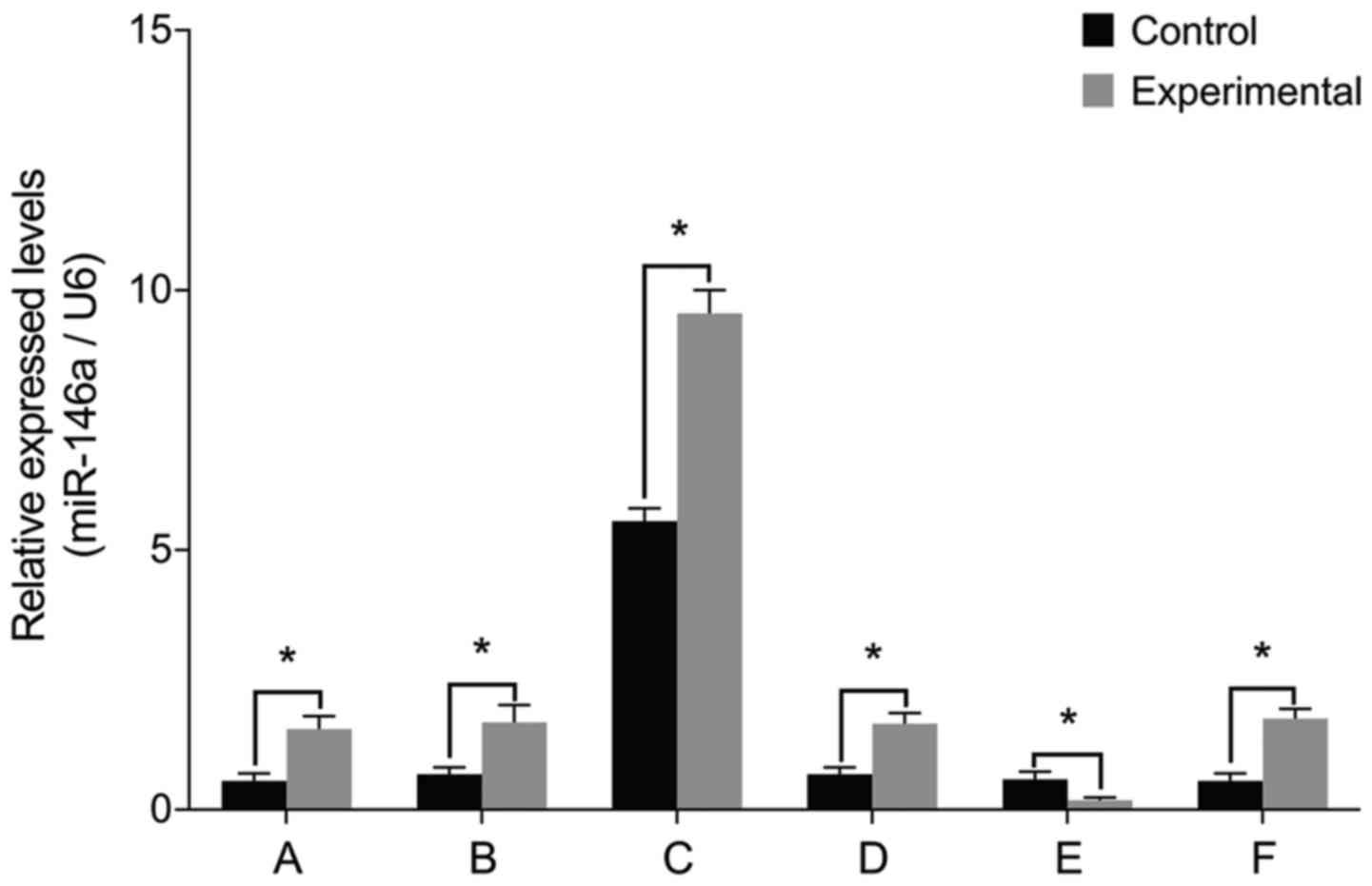
miR-146a levels
To examine the role of miR-146a in the pathology of
intervertebral discs, we cultured nucleus pulposus cells from
patients with IDD and then manipulated miR-146a activity in these
cells. The levels of miR-146a mRNA were significantly higher in the
experimental group than those in the control group prior to
transfection (P<0.05) (Fig. 1).
Cell transfections confirmed that there were no differences between
miR-146a mRNA levels before and after treatment with miR-146a empty
vector, miR-146a mimic negative control and miR-146a inhibitor
negative control (P>0.05). miR-146a mRNA levels increased
15-fold after transfection with miR-146a mimic and decreased by 66%
after transfection with miR-146a inhibitor.
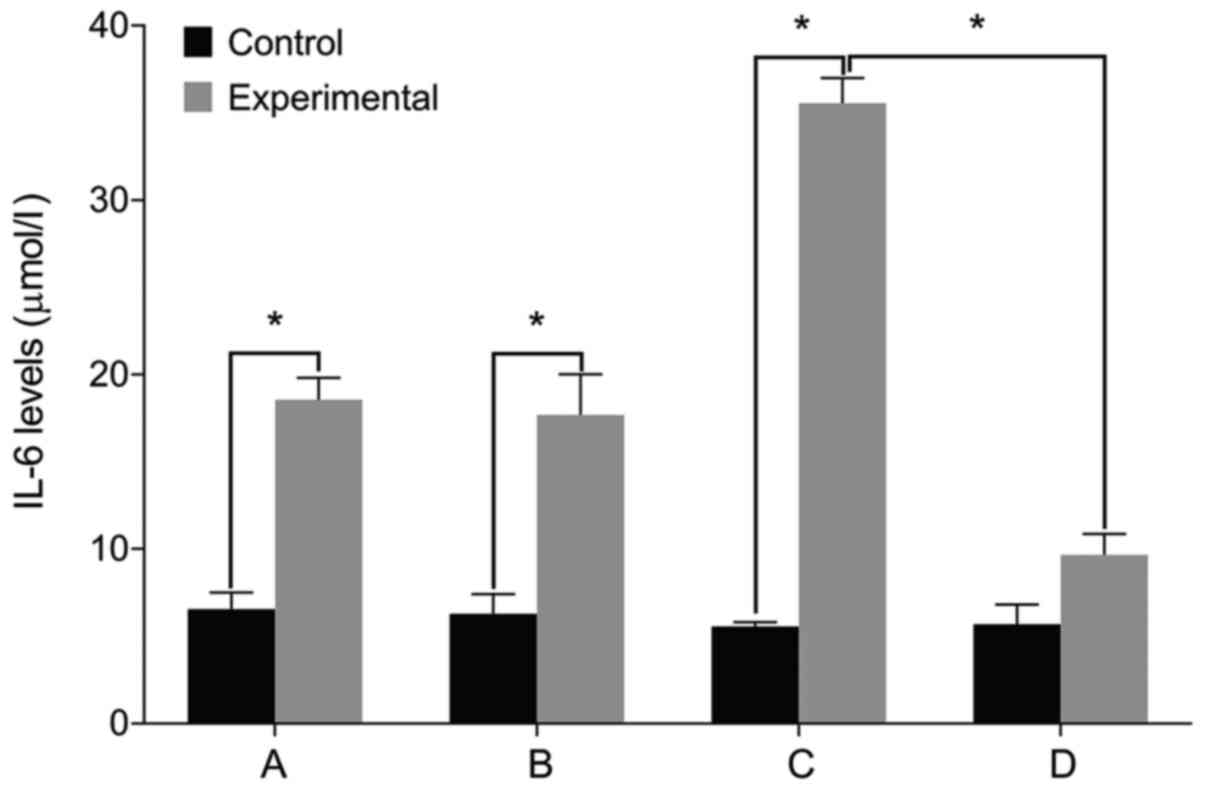
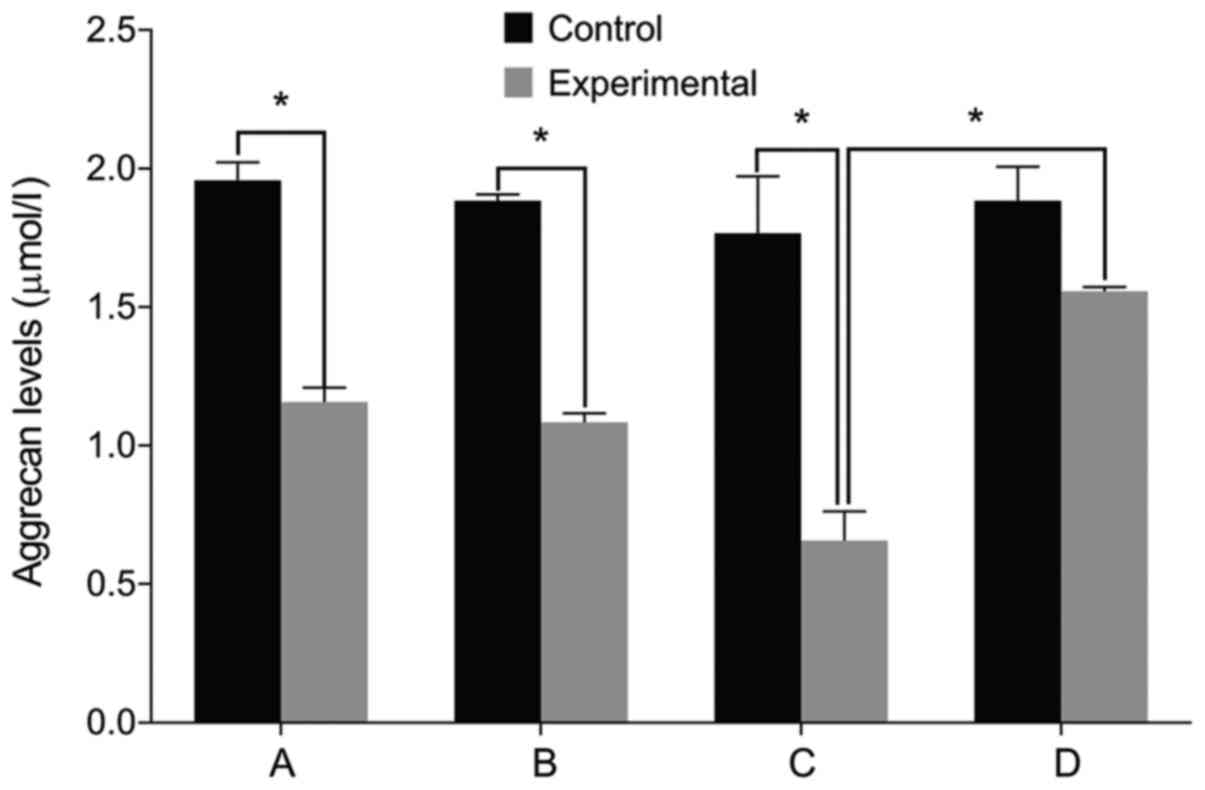
IL-6, Col II and aggrecan levels
We then examined the expression of relevant markers
in the cultured cells. We found no differences in the levels of
IL-6, Col II and aggrecan before and after treatment in the control
group (P>0.05). The level of IL-6 was significantly higher in
the experimental group than that in the control group before
treatment. The levels of Col II and aggrecan were lower in the
experimental group compared with the control group (P<0.05). The
level of IL-6 increased in the experimental group after treatment
with miR-146a mimic when compared with the miR-146a empty vector.
The levels of Col II and aggrecan levels decreased after treatment
with miR-146a mimic (P<0.05). By contrast, the cells treated by
miR-146a inhibitor had the opposite result (P<0.05) (Figs. 2–4).
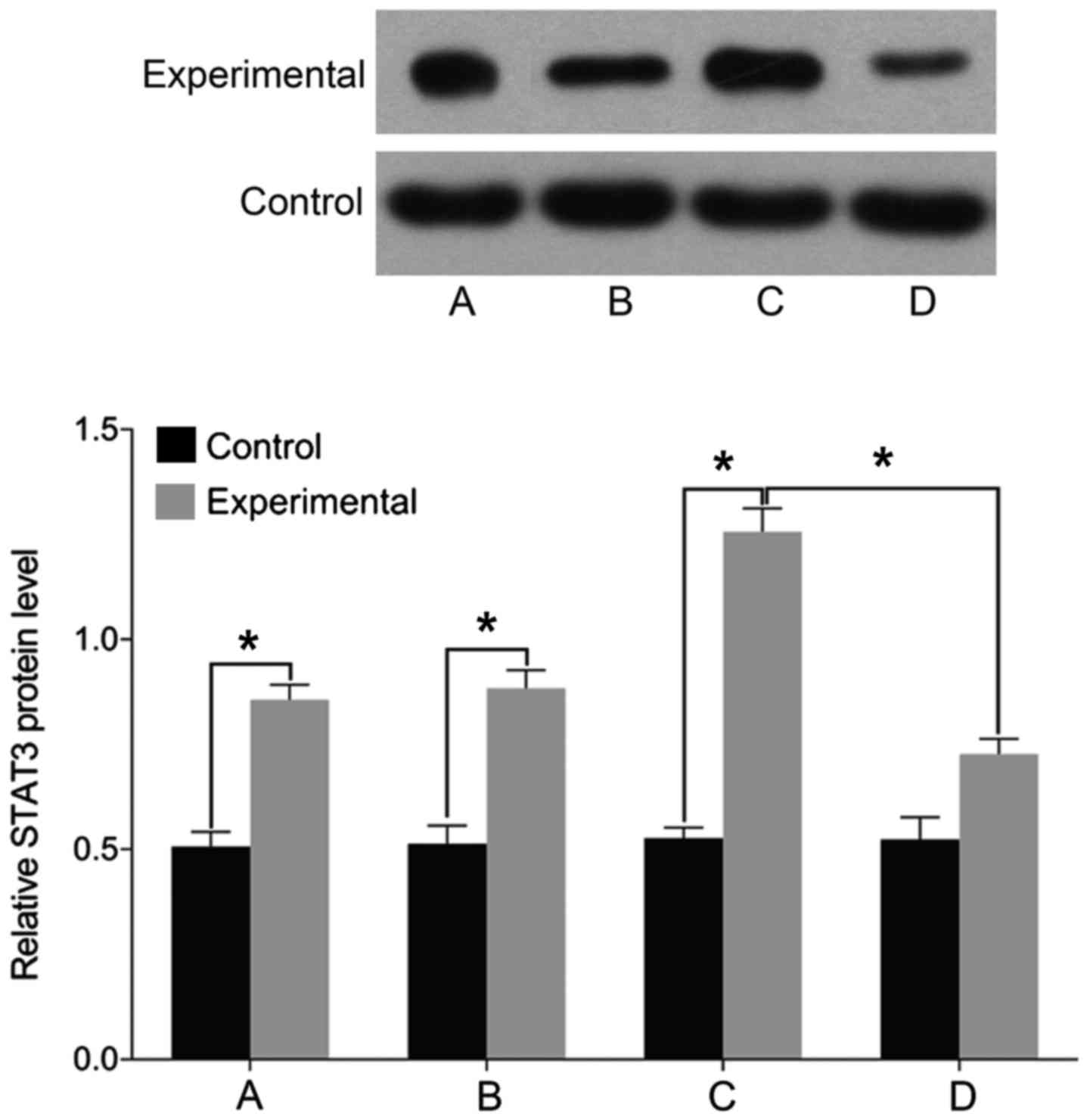
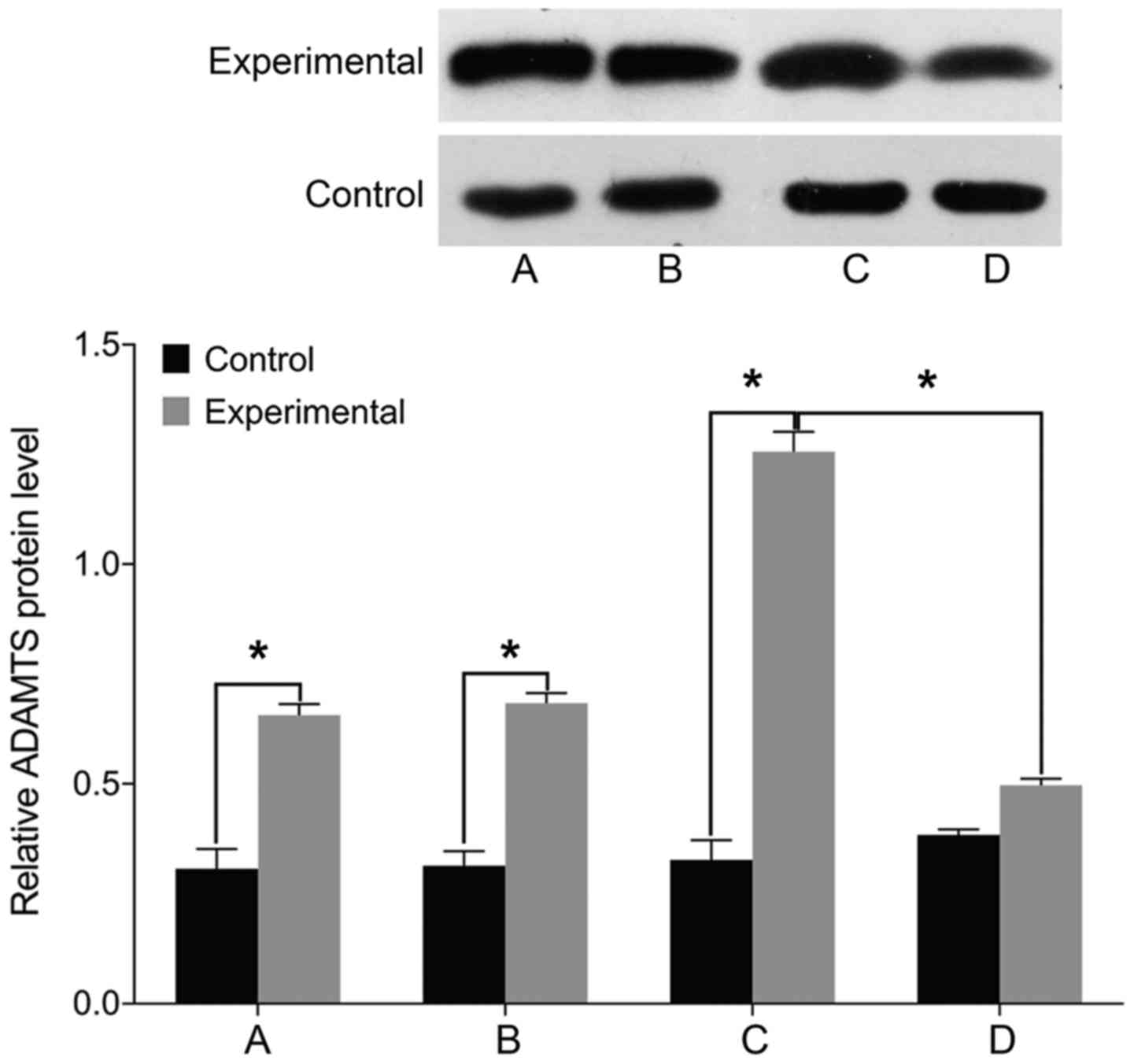
STAT3, MMP-3 and ADAMTS levels
Finally, we examined the expression of markers
associated with intervertebral disc pathology. We found no
differences in the levels of STAT3, MMP-3, and ADAMTS before and
after treatment in the control group (P>0.05). The levels of
cell STAT3, MMP-3, and ADAMTS were significantly higher in the
experimental group than those in the control group before treatment
(P<0.05). In the experimental group, the levels of STAT3, MMP-3,
and ADAMTS were elevated following treatment with miR-146a mimic
and decreased after treatment with miR-146a inhibitor (P<0.05)
(Figs. 5–7).
Discussion
Results of the present study revealed that treatment
of control cells with miR-146a had no effect on the levels of the
pathogenic markers IL-6, Col II, aggrecan, STAT3, MMP-3 and ADAMTS,
suggesting that miR-146a is not the initiating factor in the
degeneration of nucleus pulposus. The levels of miR-146a, IL-6,
STAT3, MMP-3 and ADAMTS were significantly higher in the
experimental group, whereas Col II and aggrecan were lower in the
experimental group. The abnormally high expression of miR-146a was
positively correlated with the Pfirrmann classification of
degeneration (8). The study of Gu
et al indicated that miR-146a expression is associated with
intervertebral disc IL-1-mediated inflammatory reaction (9).
Cell transfection experiments suggested that
IL-6/STAT3 signaling pathway can be activated by a high expression
of miR-146a to promote lumbar intervertebral disc degeneration.
Bioinformatics analysis revealed that IL-6/STAT3 may be a direct
target of miR-146a (10). An IDD rat
model demonstrated that the inflammatory reaction with elevated
IL-6 and TNF-α and prolonged duration was highly significant
(11). Recombinant IL-6 injected in
the rat lumbar dorsal root ganglion can lead to hyperalgesia, but
the trigger pain can be significantly reduced by IL-6 inhibitors.
The mechanical hyperalgesia can be alleviated by silencing the
IL-6 gene (12).
Additionally, anti-inflammatory cytokines such as IL-10 show a
significant analgesic effect (13).
Burke et al also indicated that the high level of
inflammatory molecules secreted by intervertebral disc tissue is an
important pathogenic factor leading to low back pain (14). JAKs can be activated through IL-6
binding with its receptor, activating STAT3 phosphorylation, which
enables its nuclear translocation where it combines with the
promoters of its target genes. Inflammation is an important factor
that can promote tumor formation and malignant lesions (15). Efficient blocking of IL-6/STAT3
signaling pathway can be achieved with multiple small molecule
inhibitors, such as STA-21 and STX-0119, which may be beneficial in
the treatment of IDD (16).
Apoptosis and the increase of proteases are major reasons leading
to the reduction of ECM component in the intervertebral disc. MMPs
and ADAMTS are Zn2+-dependent proteases with broad
substrate specificity that can be activated by multiple
inflammatory factors, including as IL-6 and TNF-α (17,18).
In summary, this study revealed that IDD is
associated with high levels of miR-146a, inflammatory reaction due
to IL-6 and activation of the STAT3 signaling pathway, thereby
affecting the expression of Col II and aggrecan in the nucleus
pulposus tissue. Future studies are to investigate whether IDD can
be reversed by the single or combined effects of targeting
miR-146a, IL-6, or STAT3, which will provide an important reference
for the clinical intervention in IDD.
References
|
1
|
Ma T, Guo CJ, Zhao X, Wu L, Sun SX and Jin
QH: The effect of curcumin on NF-κB expression in rat with lumbar
intervertebral disc degeneration. Eur Rev Med Pharmacol Sci.
19:1305–1314. 2015.PubMed/NCBI
|
|
2
|
Wang X, Wang H, Yang H, Li J, Cai Q,
Shapiro IM and Risbud MV: Tumor necrosis factor-α- and
interleukin-1β-dependent matrix metalloproteinase-3 expression in
nucleus pulposus cells requires cooperative signaling via syndecan
4 and mitogen-activated protein kinase-NF-κB axis: Implications in
inflammatory disc disease. Am J Pathol. 184:2560–2572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Z, Yin Z, Liu C, Liang H, Jiang M and
Tian J: IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation
through NF-κB in human intervertebral disc. J Orthop Surg.
10:1592015. View Article : Google Scholar
|
|
4
|
Liu C, Fei HD, Sun ZY and Tian JW:
Bioinformatic analysis of the microarray gene expression profile in
degenerative intervertebral disc cells exposed to TNF-α. Eur Rev
Med Pharmacol Sci. 19:3332–3339. 2015.PubMed/NCBI
|
|
5
|
Zhang SY, Zhao QF, Fang NN and Yu JG:
Betulin inhibits pro-inflammatory cytokines expression through
activation STAT3 signaling pathway in human cardiac cells. Eur Rev
Med Pharmacol Sci. 19:455–460. 2015.PubMed/NCBI
|
|
6
|
Lu X, Luo F, Liu Y, Zhang A, Li J, Wang B,
Xu W, Shi L, Liu X, Lu L, et al: The IL-6/STAT3 pathway via miR-21
is involved in the neoplastic and metastatic properties of
arsenite-transformed human keratinocytes. Toxicol Lett.
237:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye EA and Steinle JJ: miR-146a attenuates
inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect
primary human retinal microvascular endothelial cells grown in high
glucose. Mediators Inflamm. 2016:39584532016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao B, Yu Q, Li H, Guo X and He X:
Characterization of microRNA expression profiles in patients with
intervertebral disc degeneration. Int J Mol Med. 33:43–50.
2014.PubMed/NCBI
|
|
9
|
Gu SX, Li X, Hamilton JL, Chee A, Kc R,
Chen D, An HS, Kim JS, Oh CD, Ma YZ, et al: MicroRNA-146a reduces
IL-1 dependent inflammatory responses in the intervertebral disc.
Gene. 555:80–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu P, Feng B, Wang G, Ning B and Jia T:
Microarray based analysis of gene regulation by microRNA in
intervertebral disc degeneration. Mol Med Rep. 12:4925–4930.
2015.PubMed/NCBI
|
|
11
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng X, Zhao F, Kang B and Zhang X:
Elevated interleukin-6 expression levels are associated with
intervertebral disc degeneration. Exp Ther Med. 11:1425–1432.
2016.PubMed/NCBI
|
|
13
|
Molinos M, Almeida CR, Caldeira J, Cunha
C, Gonçalves RM and Barbosa MA: Inflammation in intervertebral disc
degeneration and regeneration. J R Soc Interface. 12:201411912015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burke JG, Watson RWG, McCormack D, Dowling
FE, Walsh MG and Fitzpatrick JM: Intervertebral discs which cause
low back pain secrete high levels of proinflammatory mediators. J
Bone Joint Surg Br. 84:196–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson ZI, Schoepflin ZR, Choi H, Shapiro
IM and Risbud MV: Disc in flames: Roles of TNF-α and IL-1β in
intervertebral disc degeneration. Eur Cell Mater. 30:104–116;
discussion 116–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miao D and Zhang L: Leptin modulates the
expression of catabolic genes in rat nucleus pulposus cells through
the mitogen-activated protein kinase and Janus kinase 2/signal
transducer and activator of transcription 3 pathways. Mol Med Rep.
12:1761–1768. 2015.PubMed/NCBI
|
|
17
|
Wang XH, Zhu L, Hong X, Wang YT, Wang F,
Bao JP, Xie XH, Liu L and Wu XT: Resveratrol attenuated
TNF-α-induced MMP-3 expression in human nucleus pulposus cells by
activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med
(Maywood). 241:848–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF-κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|