Introduction
Developments in modern emergency treatments and
life-support systems have substantially improved the treatment of
severe trauma or cardiac accidents for millions of patients.
Traumatic brain injury-related mortality has reduced from 17% in
2000 to 11% in 2010, as reported by Siman-Tov, et al
(1). However, 10–15% of patients who
sustain severe acquired brain injury enter a condition termed a
disorder of consciousness (DOC), which broadly includes the
syndromes of coma, vegetative state [VS; also known as unresponsive
wakefulness syndrome (UWS)] and minimally conscious state (MCS)
(2,3). Over the past century, there have been
few methods that reliably assess the consciousness of DOC patients,
as methods of traditional behavioral assessment may be easily
limited. In DOC patients, responses to command may be only minimal
or inconsistently present and can be very difficult to identify
clinically. This may be a primary factor contributing to a ~40%
misdiagnosis rate for VS (4).
However, recent advances in neuroimaging, such as
functional magnetic resonance imaging (fMRI) and brain computer
interface (BCI) have improved the identification of residual
cognition in DOC patients (5). These
recently developed techniques have provided novel insights into the
brain function of DOC patients and may partly complement the
clinical behavioral assessment. They may also be employed to
examine the effects of certain therapeutic interventions, including
pharmacological agents, brain stimulations and music training
(5,6). In the current article, advances in the
clinical assessment of DOC patients are reviewed, in addition to
current concerns and future perspectives in the treatment of
DOC.
Controversy over the concept of
consciousness
Due to improvements in emergency therapy,
particularly in life-support systems, the mortality rates of
patients with severe head trauma or cardiac arrest have been
markedly decreased (1). However,
10–15% patients who survive the acute coma stage enter into a
chronic DOC (2,3). Therefore, the treatment and
rehabilitation of patients with DOC is a major challenge in
clinical neuroscience. As a pre-requisite for effective treatment,
an objective assessment of the conscious state of DOC patients is
necessary, which has been a focus of research from both clinical
and neuroscience perspectives (7).
A plausible definition of consciousness limits the
in-depth investigation of DOC that may otherwise provide insight
into the different states of consciousness. Several definitions of
consciousness have so far been proposed (8). A commonly accepted viewpoint is that
consciousness is the brain's ability to form cognition of the
world, including sensations and perceptions of oneself and the
environment. The majority of researchers believe that consciousness
can be separated into a minimum of two components; wakefulness and
awareness. The former refers to the level of consciousness and the
latter the content of consciousness. The majority of patients with
traumatic brain injuries transit through the following states of
consciousness during recovery: Coma, VS (UWS), MCS and emergence
from MCS. This recovery path is consistent with the two aspects of
consciousness, as first the level of consciousness is recovered,
which is then succeeded by re-building of its contents (9,10). This
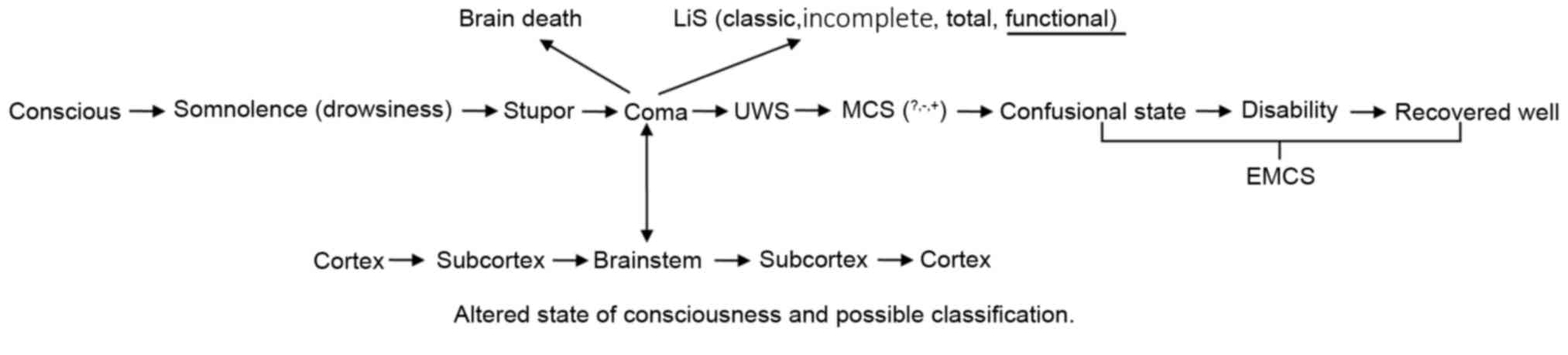
is indicated by the recovery of DOC patients, of which there are
two contradicting nomenclatures (Fig.
1). One is functional locked-in syndrome (fLiS), the other is
functional MCS (fMCS). Locked-in syndrome (LiS) is caused by an
insult to the ventral pons, most commonly an infarct, haemorrhage
or trauma. The typical signs of LiS are quadriplegia and anarthria
with preservation of consciousness and vertical eye movement,
facilitating non-verbal communication. Bauer et al (11) reviewed the history of LiS and divided
it into three types: classical LiS (total immobility with the
exception of vertical eye movements and blinking), incomplete LiS
(If any other movements are present) and total LiS (total
immobility, including all eye movements, combined with signs of
undisturbed cortical function in the EEG). The neuroanatomical
basis of LiS was also analyzed and it was indicated that a
de-efferented state with preserved consciousness appears to be
possible with lesions in both cerebral peduncles (11). This state may preserve all or partial
consciousness, detected using modern neuroimaging technologies,
which demonstrate functional communication (active paradigm) and
between-network anti-correlation (passive paradigm) (12). The present study therefore
hypothesized that fLiS should be kept as a separate nomenclature
for this state and as one member of the LiS sequence. To be
consistent with the classification of other MCS (MCS- and MCS+),
Gosseries et al (13)
proposed MCS* as an indicator of VS with covert awareness. However,
para-clinical tests indicate no functional communication and no
between-network anti-correlation in patients with fMCS (12).
Consciousness, however, may not simply consist of
two dimensions (14). Laureys and
Schiff (15) proposed a revised
recovery path of DOC, which due to its complexity is difficult to
accurately replicate. Monti (16)
thus proposed that alternative recovery behavior belonged to a
third dimension of consciousness; however, not all types of DOC can
be accurately described by the improved model. Therefore, other
dimensions are considered to support consciousness, including those
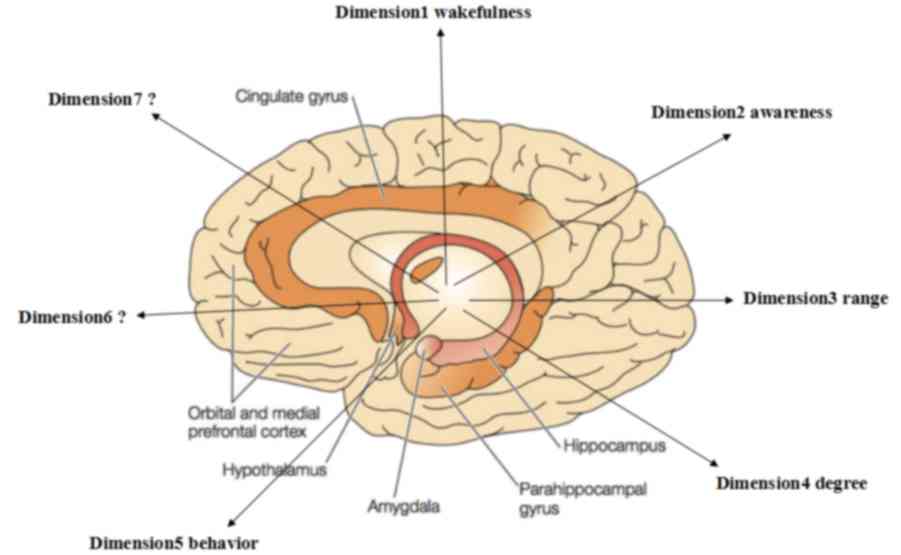
depicted in Fig. 2 (9,10). With
the exception of the familiar concepts such as level of
consciousness (wakefulness) and content of consciousness
(awareness), behavior, degree and range were also proposed as
dimensions of consciousness (9,14). It is
therefore possible that more dimensions may exist (Fig. 2).
Reliability of novel diagnostic
technologies
Given the uncertainty surrounding the definition of
consciousness, difficulties remain in the diagnosis and assessment
of DOC. Behavioral assessment is considered to be a golden standard
method for evaluating the awareness of patients with DOC (7). Various other methods, including skin
conductance response (SCR), diameter of the pupils, breathing
control and mini movement micro-switch have also been proposed to
advance the diagnosis and assessment of DOC (17–20).
According to the Coma Recovery Scale-Revised (CRS-R), any early
signs, including visual fixation, visual pursuit and pain location,
may aid to determine whether a patient has transited from VS to MCS
(4). However, the rate of clinical
misdiagnosis based on the CRS-R remains high, due to subjectivity
of the evaluator and the physical status of the patient (21).
With the development of positron emission tomography
(PET), fMRI and electroencephalographic (EEG) recording of event
related potentials (ERP), detection and analysis of brain activity
signals are now possible in clinical practice (22). In turn, these techniques may enable
the detection of residual cognitive brain functions in DOC
patients. Previous results have demonstrated that 17–19% of
patients may be distinguished from VS/UWS by the detection of brain
activity changes with fMRI or EEG (23). In turn, this patient ratio was
increased up to 33% with the use of Sitt's automatic classification
of the state of consciousness (24).
Therefore, previous evaluations based on behavior may have
underestimated the brain function of DOC patients.
Brain activity may become a target for evaluation of
consciousness, as neuroimaging techniques are able to detect
cognitive function by decoding conscious responses based on brain
activity (25). Advances in computer
science have also made a form of communication with DOC patients
possible, by modulating the brain activity or through the control
of electrical brain activity (26).
With the development of these novel imaging methods, the current
guideline or consensus on behavior-based diagnosis and evaluation
should be revised in order to progress and allow these newly
developed imaging techniques to be considered in discussing the
diagnoses, treatment and prognosis of DOC. However, cognitive
function detected by current technologies is still unable to
reliably prove the consciousness of a DOC patient. Brain activity
alone cannot prove patient consciousness, though it has been
hypothesized that consciousness is associated with brain activity
(27). Importantly, a fundamental
distinction between consciousness and unconsciousness is yet to be
determined. This should be defined based on a more feasible,
sensitive and accurate diagnostic criteria, built on a combination
of the available behavioral, brain imaging and electrophysiological
measures.
Prognosis of DOC patients
Prognosis is also a concern for DOC patients.
Classical prognostic indicators include age, etiology, coma period,
CRS-R or Disability Rating Scale (DRS), S100 protein and
neuron-specific enolase (NSE) expression and N20 neuron potential.
CRS-R or DRS scores are associated with patient prognosis and have
a marked predictive power with respect to the time until commands
are followed (28).
According to the biomarkers, NSE and S-100B are
released following injury to neurons and glial cells respectively
and are likely to be associated with the extent of anoxic-ischemic
neurological injury following cardiac arrest and therefore, with
the severity of neurological outcome (29,30).
Median nerve somatosensory evoked potentials (SEPs; primarily N20)
also provide useful prognostic information regarding the outcome
following coma and are becoming increasingly used (31,32).
The development of PET, fMRI, EEG and BCI has made
it possible to predict the prognosis of DOC patients. For instance,
Vogel et al (33) tested
active signals within regions of interest (ROIs) of 22 DOC patients
using a mental imagery fMRI paradigm. It was suggested that VS
patients with ROIs that were activated significantly had the
potential to recover from DOC to MCS at minimum, while those with
inactivated ROIs were likely to remain in VS. In addition, Luyt
et al (34) analyzed
Diffusion Tensor Imaging results of 57 post-cardiopulmonary
resuscitation patients two weeks after entering a coma, revealing
that the specificity and sensibility of the Fractional Anisotropy
index as a one-year prognostic indicator were 100 and 94%,
respectively.
Di et al (35)
also systematically reviewed previous studies on the use of PET and
fMRI in DOC patients, and based on results, classified the
neuroimaging activation into three patterns: Absence of cortical
activation, typical low-level primary cortical activation and
higher level associative cortical activation. The latter high level
activation mode was demonstrated to predict recovery from VS with
93% specificity and 69% sensitivity.
EEG is also a simple and effective method of
detecting brain function in DOC patients, and Lehembre et al
(36) suggested that quantitative
EEG may be a useful way of distinguishing MCS from VS/UWS. In
addition, Qin et al (37) and
Cavinato et al (38)
evaluated the prognostic values of cognitive event-related
potential (ERP). It was observed that MMN and P300, evoked by the
subject's own name, exhibited potential prognostic values in
predicting recovery of consciousness and therefore, they may serve
as good prognostic markers. However, through the use of active task
paradigms, it may be possible to determine whether a patient has an
appropriate level of residual brain function to process
stimulation, and thus if they are likely to regain consciousness
(39). However, current technology
remains unable to accurately determine the prognosis of DOC
patients. Future multi-center studies are warranted to determine
the efficacy of novel neuroimaging technologies as prognostic
indicators in DOC.
Communication with DOC patients
Communication with DOC patients is now possible due
to the aforementioned imaging technologies, particularly with fMRI.
Owen et al (40) were among
the first to establish successful communication with DOC patients
using real-time fMRI combined with mental imaging stimuli,
demonstrating the possibility of binary communication with DOC
patients through use of their residual brain functions. Although
these results are controversial due to the voluntary activation of
task-specific brain areas in response to passive exposure to
stimuli associated with a specific action can be with or without
conscious awareness, they are considered to be a key paradigm in
DOC research (41).
However, there are limitations to the application of
fMRI in the management of DOC, including high cost, immobility,
inconvenience of operation and a complicated operational procedure
(42). Other non-invasive methods
are therefore employed for communication with DOC patients,
including EEG and functional near-infrared spectroscopy (fNISS).
Naci et al (43) compared the
efficacy of fMRI, FNISS and EEG with BCI and observed that EEG may
be a suitable method of communication. Although EEG signals are
affected by involuntary muscle or eye movements and has limited
spatial resolution, particularly within deep brain structures, EEG
has a number of advantages over other methods such as fMRI and PET,
including low cost, noninvasiveness, a relatively simple
operational procedure, high temporal resolution and further BCI
applications (42). Thus, EEG may be
an ideal communicative apparatus.
BCI based around EEG is used to evaluate the
different components identified by EEG, including P3 potential,
sensorimotor rhythm, also known as mu-rhythm, and slow cortical
potentials (44). Münßinger et
al (45) assessed two different
versions of the P300-Brain Painting application: A colored matrix
in 3 ALS-patients and 10 healthy participants, and a black and
white matrix assessed by 10 healthy participants. The ALS-patients
were able to use the application with the same accuracy as healthy
subjects and greatly enjoyed P300-Brain Painting. Coyle et
al (46) also used image-based
BCI to examine the effect of real-time feedback in an MCS patient.
It was revealed that, with no feedback, two motor imagery (MI; hand
grasp vs. toe movement) could be classified with ~82% accuracy with
only three EEG channels. When providing real-time feedback with two
games where the participant was instructed to move a ball and a
spaceship respectively, to reach a target by performing the same MI
tasks, 77.5% ball and 80% spaceship control were achieved. This
means real-time feedback may be used in the detection of awareness
and as a means of communication.
The aforementioned studies indicate the preliminary
applications of BCI in DOC patients. As nearly 20% of the accuracy
obtained by potential users does not reach criterion level, BCI
control is not currently accurate enough for clinical use (47). Despite the requirement for further
adaptation of BCI to patients with disorders of consciousness, this
assisted communication technology does, however, provide insight
into the cognitive state of DOC patients. With future improvements
in the design of BCI paradigms, it may be possible to elucidate the
cognitive function of DOC patients, particularly regarding their
decision-making processes. Verbal communication with DOC patients
may also become possible with improved BCI techniques (48,49).
Future therapies for DOC patients
Although there have been advances in research
regarding the treatment of DOC, there remain few effective
therapies in clinical use. In clinical trials, several drugs have
been documented to improve motor ability and cognitive functions,
including Bromocrirtine, Levodopa, Baclofen, Chalybeate, Zolpidem,
Apomorphine, Ritalin, Meclofenoxate and Amantadine (50). However, no drugs have been further
approved thus far. Therefore basic life support, including
sufficient hydration and nutrition, remain the recommended
therapeutic strategies for patients with DOC in China (51).
An alternative therapeutic method is neural
stimulation, including median nerve electrical stimulation,
cervical spinal cord stimulation to the dorsal column and deep
brain stimulation to the central thalamic nuclei (52). These methods have been demonstrated
to alleviate the restrictions in functional communication, motor
performance, feeding and object naming experienced by DOC patients,
particularly in MCS patients with traumatic brain injuries
(53). In addition, the
administration of transcranial direct current stimulation in DOC
has exhibited promising rehabilitative effects, particularly within
the left dorsolateral prefrontal cortex in MCS patients following
severe brain damage (54).
The significance of the aforementioned studies
remains to be elucidated by large sample clinical trials, as their
applications are currently questioned (55). Effective therapies for DOC are
expected to emerge with improved understanding of brain function,
which in turn warrants further study. In the future, a combination
therapy involving medicine, physical stimuli and genetic/cell
therapy may be a viable therapeutic option in the treatment of
DOC.
Ethical, legal and social issues
Ethical, legal and social issues have arisen due to
the lack of effective therapies for VS/UWS. A principal controversy
is the decision to end life support for VS/UWS patients,
particularly those deemed to be permanent VS patients (remaining in
an unconsciousness state for one year following traumatic injury or
three months following non-traumatic injury) (56). Whether any one individual should be
responsible for the survival or mortality of DOC patients is also
questioned by clinicians and lawyers. Patient caregivers
additionally present ethical and social issues, due to concerns
over their physical and psychological state, attitude toward
patients and economical or social pressures. Views of the patient
are also difficult to obtain, and even with novel technologies, the
only communications currently possible are binary ‘yes or no’
choices (57–60).
Furthermore, as the cognitive functions of DOC
patients are severely compromised, and their expressions may be
inaccurate. Indeed, it has been suggested that concerns regarding
the ethical treatment and social needs of patients should be
expressed by relatives and guardians, despite the development of
novel technologies (61).
The quality of life of DOC patients is also
questioned, although a previous study in patients with locked-in
syndrome (LIS) contradicts traditional views that DOC patients have
a low quality of life (62).
Specifically, the study by Bruno et al (62) documented that 72% of LIS patients
stated their lives were meaningful despite their living in social
isolation or being severely disabled, while only 28% of patients
declared that they were unhappy. It has also been reported that
VS/UWS patients may experience pain following electrical
stimulation of the median nerve (63), indicating that VS/UWS patients may
benefit from additional therapy, such as analgesia. Thus, patient
quality of life as well as survival should be considered in the
long-term treatment of DOC. Optimizing the aforementioned
technologies in the diagnosis and treatment of DOC may be useful in
addressing these ethical and social issues.
Current limitations and future
perspectives
Although there have been advances in experimental
research on DOC, their detailed underlying mechanisms remain to be
elucidated, and existing classification systems are unable to
describe all clinical sub-types. In addition, the efficacy of novel
neuroimaging technologies regarding the diagnosis, prognostic
assessment and treatment of DOC require verification from
large-sample clinical trials. The ethical, legal and social issues
associated with DOC should also be addressed alongside the
development of clinical methods.
Clinical-based studies may aid to determine the
efficacy of neuroimaging methods. Specifically, studies that
combine assessments of behavior and brain activity using BCI are
warranted, which first requires improvements in current BCI
technology. Increased understanding of residual cognitive functions
may also aid the diagnosis, treatment and prognostic evaluation of
DOC patients. The ethical and social issues concerning the quality
of life, survival and treatment of DOC patients should also be
considered rather than solely their physical condition.
In China, ethical and clinical guidelines, similar
to those formulated by Coleman and Dolce (64), should be formulated for Chinese DOC
patients by multi-disciplinary DOC research groups. Regular
consultations across departments, including neurology, neurosurgery
and neurological rehabilitation and intensive care units, may aid
to better conserve brain function during the early stages of brain
injury, potentially reducing the risk of DOC. In addition, Chinese
epidemiological surveys are warranted to obtain data that may aid
in decision-making by the government: For example, the incidence
and prevalence of DOCs, the average cost of a chronic DOC patient
in one year and the caregivers' burden (65), which are all worthy of consideration
when discussing public health expenditure. In addition,
internet-based DOC associations, comprised of doctors, patients,
relatives and social workers, may facilitate information exchange,
education and communication between clinicians, relatives and
caregivers.
Conclusion
Our group is the first clinical DOC research group
in China and has established a coma recovery unit in the Institute
of Neuroscience, which has thus treated 60–80 DOC patients
annually. Multi-disciplinary studies including functional
neuroimaging and electrophysiological recording, namely PET, fMRI
and BCI, have also been performed in our laboratory (66,67). In
addition, our group has established cooperation among research
institutes and non-governmental organizations to promote the
welfare of DOC patients, with an ultimate goal of establishing the
first DOC society in China. For DOC patients, therapy alone is
unable to resolve all the issues presented by altered conscious
states, even after the emergence of novel technologies. Thus,
provision of humanistic care for DOC patients and their relatives
is currently the primary goal in the treatment of DOC.
Acknowledgements
The current study was supported by the National
Natural Science Foundation, China (grant nos. 81271548 and
81371535), the Guangdong Province Project for Science and
Technology (grant no 2012A030400025) and the Guangzhou Municipal
Project for Science and Technology (grant no. 201508020253).
References
|
1
|
Siman-Tov M, Radomislensky I and K; ITG
Peleg: Reduction in trauma mortality in Israel during the last
decade (2000–2010): The impact of changes in the trauma system.
Injury. 44:1448–1452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Sun X and Liu H: Clinical analysis
and misdiagnosis of cerebral venous thrombosis. Exp Ther Med.
4:923–927. 2012.PubMed/NCBI
|
|
3
|
Levin HS, Saydjari C, Eisenberg HM,
Foulkes M, Marshall LF, Ruff RM, Jane JA and Marmarou A: Vegetative
state after closed-head injury. A traumatic coma data bank report.
Arch Neurol. 48:580–585. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schnakers C, Vanhaudenhuyse A, Giacino J,
Ventura M, Boly M, Majerus S, Moonen G and Laureys S: Diagnostic
accuracy of the vegetative and minimally conscious state: Clinical
consensus versus standardized neurobehavioral assessment. BMC
Neurol. 9:352009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gosseries O, Pistoia F, Charland-Verville
V, Carolei A, Sacco S and Laureys S: The role of neuroimaging
techniques in establishing diagnosis, prognosis and therapy in
disorders of consciousness. Open Neuroimag J. 10:52–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zatorre RJ: Predispositions and plasticity
in music and speech learning: Neural correlates and implications.
Science. 342:585–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owen AM and Coleman MR: Detecting
awareness in the vegetative state. Ann N Y Acad Sci. 1129:130–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown JW: The science of consciousness:
Psychological, neuropsychological and clinical reviews. J Nerv Ment
Dis. 186:621998. View Article : Google Scholar
|
|
9
|
Zeman A: Consciousness. Brain.
124:1263–1289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayne T, Hohwy J and Owen AM: Are there
levels of consciousness? Trends Cogn Sci. 20:405–413. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer G, Gerstenbrand F and Rumpl E:
Varieties of the locked-in syndrome. J Neurol. 221:77–91. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Perri C, Thibaut A, Heine L, Annen J
and Laureys S: Towards new methods of diagnosis in disorders of
consciousness - Authors' reply. Lancet Neurol. 15:1115–1116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gosseries O, Zasler ND and Laureys S:
Recent advances in disorders of consciousness: Focus on the
diagnosis. Brain Inj. 28:1141–1150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fazekas P and Overgaard M:
Multidimensional models of degrees and levels of consciousness.
Trends Cogn Sci. 20:715–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laureys S and Schiff ND: Coma and
consciousness: Paradigms (re)framed by neuroimaging. Neuroimage.
61:478–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monti MM: Cognition in the vegetative
state. Annu Rev Clin Psychol. 8:431–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scott RB, Minati L, Dienes Z, Critchley HD
and Seth AK: Detecting conscious awareness from involuntary
autonomic responses. Conscious Cogn. 20:936–942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stoll J, Chatelle C, Carter O, Koch C,
Laureys S and Einhäuser W: Pupil responses allow communication in
locked-in syndrome patients. Curr Biol. 23:R647–R648. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charland-Verville V, Lesenfants D, Sela L,
Noirhomme Q, Ziegler E, Chatelle C, Plotkin A, Sobel N and Laureys
S: Detection of response to command using voluntary control of
breathing in disorders of consciousness. Front Hum Neurosci.
8:10202014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lancioni GE, Singh NN, O'Reilly MF,
Sigafoos J, Amenduni MT, Navarro J, Buonocunto F, Scarabino T and
Belardinelli MO: Microswitch technology and contingent stimulation
to promote adaptive engagement in persons with minimally conscious
state: A case evaluation. Cogn Process. 13:133–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Majerus S, Gill-Thwaites H, Andrews K and
Laureys S: Behavioral evaluation of consciousness in severe brain
damage. Prog Brain Res. 150:397–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruse D and Owen AM: Consciousness
revealed: New insights into the vegetative and minimally conscious
states. Curr Opin Neurol. 23:656–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cruse D, Chennu S, Chatelle C,
Bekinschtein TA, Fernández-Espejo D, Pickard JD, Laureys S and Owen
AM: Bedside detection of awareness in the vegetative state: A
cohort study. Lancet. 378:2088–2094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sitt JD, King JR, El Karoui I, Rohaut B,
Faugeras F, Gramfort A, Cohen L, Sigman M, Dehaene S and Naccache
L: Large scale screening of neural signatures of consciousness in
patients in a vegetative or minimally conscious state. Brain.
137:2258–2270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Owen AM: Detecting consciousness: A unique
role for neuroimaging. Annu Rev Psychol. 64:109–133. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lule D, Noirhomme Q, Kleih SC, Chatelle C,
Halder S, Demertzi A, Bruno MA, Gosseries O, Vanhaudenhuyse A,
Schnakers C, et al: Probing command following in patients with
disorders of consciousness using a brain-computer interface. Clin
Neurophysiol. 124:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu T, Lang S, Vogel D, Markl A, Müller F
and Kotchoubey B: Patients with unresponsive wakefulness syndrome
respond to the pain cries of other people. Neurology. 80:345–352.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Whyte J, Katz D, Long D, DiPasquale MC,
Polansky M, Kalmar K, Giacino J, Childs N, Mercer W, Novak P, et
al: Predictors of outcome in prolonged posttraumatic disorders of
consciousness and assessment of medication effects: A multicenter
study. Arch Phys Med Rehabil. 86:453–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosén H, Sunnerhagen KS, Herlitz J,
Blomstrand C and Rosengren L: Serum levels of the brain-derived
proteins S-100 and NSE predict long-term outcome after cardiac
arrest. Resuscitation. 49:183–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandroni C, Cariou A, Cavallaro F,
Cronberg T, Friberg H, Hoedemaekers C, Horn J, Nolan JP, Rossetti
AO and Soar J: Prognostication in comatose survivors of cardiac
arrest: An advisory statement from the European resuscitation
council and the European society of intensive care medicine.
Resuscitation. 85:1779–1789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee YC, Phan TG, Jolley DJ, Castley HC,
Ingram DA and Reutens DC: Accuracy of clinical signs, SEP, and EEG
in predicting outcome of hypoxic coma: A meta-analysis. Neurology.
74:572–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robinson LR, Micklesen PJ, Tirschwell DL
and Lew HL: Predictive value of somatosensory evoked potentials for
awakening from coma. Crit Care Med. 31:960–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vogel D, Markl A, Yu T, Kotchoubey B, Lang
S and Müller F: Can mental imagery functional magnetic resonance
imaging predict recovery in patients with disorders of
consciousness? Arch Phys Med Rehabil. 94:1891–1898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luyt CE, Galanaud D, Perlbarg V,
Vanhaudenhuyse A, Stevens RD, Gupta R, Besancenot H, Krainik A,
Audibert G, Combes A, et al: Diffusion tensor imaging to predict
long-term outcome after cardiac arrest: A bicentric pilot study.
Anesthesiolog. 117:1311–1321. 2012. View Article : Google Scholar
|
|
35
|
Di H, Boly M, Weng X, Ledoux D and Laureys
S: Neuroimaging activation studies in the vegetative state:
Predictors of recovery? Clin Med (Lond). 8:502–507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lehembre R, Gosseries O, Lugo Z, Jedidi Z,
Chatelle C, Sadzot B, Laureys S and Noirhomme Q:
Electrophysiological investigations of brain function in coma,
vegetative and minimally conscious patients. Arch Ital Biol.
150:122–139. 2012.PubMed/NCBI
|
|
37
|
Qin P, Di H, Yan X, Yu S, Yu D, Laureys S
and Weng X: Mismatch negativity to the patient's own name in
chronic disorders of consciousness. Neurosci Lett. 448:24–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cavinato M, Freo U, Ori C, Zorzi M, Tonin
P, Piccione F and Merico A: Post-acute P300 predicts recovery of
consciousness from traumatic vegetative state. Brain Inj.
23:973–980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schnakers C, Giacino JT, Løvstad M, Habbal
D, Boly M, Di H, Majerus S and Laureys S: Preserved covert
cognition in noncommunicative patients with severe brain injury?
Neurorehabil Neural Repair. 29:308–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Owen AM, Coleman MR, Boly M, Davis MH,
Laureys S and Pickard JD: Detecting awareness in the vegetative
state. Science. 313:14022006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nachev P and Husain M: Comment on
‘Detecting awareness in the vegetative state’. Science.
315:12212007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Di Perri C, Heine L, Amico E, Soddu A,
Laureys S and Demertzi A: Technology-based assessment in patients
with disorders of consciousness. Ann Ist Super Sanita. 50:209–220.
2014.PubMed/NCBI
|
|
43
|
Naci L, Monti MM, Cruse D, Kübler A,
Sorger B, Goebel R, Kotchoubey B and Owen AM: Brain-computer
interfaces for communication with nonresponsive patients. Ann
Neurol. 72:312–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Donchin E, Spencer KM and Wijesinghe R:
The mental prosthesis: Assessing the speed of a P300-based
brain-computer interface. IEEE Trans. Rehabil Eng. 8:174–179. 2000.
View Article : Google Scholar
|
|
45
|
Münßinger JI, Halder S, Kleih SC, Furdea
A, Raco V, Hösle A and Kübler A: Brain painting: First evaluation
of a new brain-computer interface application with ALS-patients and
healthy volunteers. Front Neurosci. 4:1822010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Coyle DH, Carroll A, Stow J, McCann A,
Ally A and McElligott J: Enabling control in the minimally
conscious state in a single session with a three channel BCI.
Proceedings of the First International DECODER Workshop. The 1st
international DECODER Workshop, Paris. pp. 1–4. 2012;
|
|
47
|
Kübler A: Brain-computer interfaces for
communication in paralysed patients and implications for disorders
of consciousnessTononi G and Laureys S: The neurology of
consciousness. Academic Press, Elsevier; pp. 217–234. 2008
|
|
48
|
Lulé D, Noirhomme Q, Kleih SC, Chatelle C,
Halder S, Demertzi A, Bruno MA, Gosseries O, Vanhaudenhuyse A,
Schnakers C, et al: Probing command following in patients with
disorders of consciousness using a brain-computer interface. Clin
Neurophysiol. 124:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Pan J, He Y, Wang F, Laureys S, Xie
Q and Yu R: Detecting number processing and mental calculation in
patients with disorders of consciousness using a hybrid
brain-computer interface system. BMC Neurol. 15:2592015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Giacino JT, Whyte J, Bagiella E, Kalmar K,
Childs N, Khademi A, Eifert B, Long D, Katz DI, Cho S, et al:
Placebo-controlled trial of amantadine for severe traumatic brain
injury. N Engl J Med. 366:819–826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang Y, Wang K, Zhou F, Zhang Y, Xia X, He
Y, Chen Y, Ni X, He J, Yu R, et al: Conditions of patients with
chronic disorders of consciousness and caregiver's attitude and
pressure in three cities in China. J Clin Neurosurg. 14:102–107.
2017.
|
|
52
|
Giacino J, Fins JJ, Machado A and Schiff
ND: Central thalamic deep brain stimulation to promote recovery
from posttraumatic minimally conscious state: Challenges and
opportunities. Neuromodulation. 15:339–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Magrassi L, Maggioni G, Pistarini C, Di
Perri C, Bastianello S, Zippo AG, Iotti GA, Biella GE and Imberti
R: Results of a prospective study (CATS) on the effects of thalamic
stimulation in minimally conscious and vegetative state patients. J
Neurosurg. 125:972–981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Angelakis E, Liouta E, Andreadis N,
Korfias S, Ktonas P, Stranjalis G and Sakas DE: Transcranial direct
current stimulation effects in disorders of consciousness. Arch
Phys Med Rehabil. 95:283–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oliveira L and Fregni F: Pharmacological
and electrical stimulation in chronic disorders of consciousness:
New insights and future directions. Brain Inj. 25:315–327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Panksepp J, Fuchs T, Garcia VA and Lesiak
A: Does any aspect of mind survive brain damage that typically
leads to a persistent vegetative state? Ethical considerations.
Philos Ethics Humanit Med. 2:322007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tresch DD, Sims FH, Duthie EH Jr and
Goldstein MD: Patients in persistent vegetative state attitudes and
reactions of family members. J Am Geriatr Soc. 39:17–21. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tzidkiahu T, Sazbon L and Solzi P:
Characteristic reactions of relatives of post-coma unawareness
patients in the process of adjusting to loss. Brain Inj. 8:159–165.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stern JM, Sazbon L, Becker E and Costeff
H: Severe behavioural disturbance in families of patients with
prolonged coma. Brain Inj. 2:259–262. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chiambretto P, Ferrario S Rossi and Zotti
AM: Patients in a persistent vegetative state: Caregiver attitudes
and reactions. Acta Neurol Scand. 104:364–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jox RJ, Bernat JL, Laureys S and Racine E:
Disorders of consciousness: Responding to requests for novel
diagnostic and therapeutic interventions. Lancet Neurol.
11:732–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bruno MA, Bernheim JL, Ledoux D, Pellas F,
Demertzi A and Laureys S: A survey on self-assessed well-being in a
cohort of chronic locked-in syndrome patients: Happy majority,
miserable minority. BMJ Open. 1:e0000392011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Boly M, Faymonville ME, Schnakers C,
Peigneux P, Lambermont B, Phillips C, Lancellotti P, Luxen A, Lamy
M, Moonen G, et al: Perception of pain in the minimally conscious
state with PET activation: An observational study. Lancet Neurol.
7:1013–1020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Coleman MR, Bekinschtein T, Monti MM, Owen
AM and Pickard JD: A multimodal approach to the assessment of
patients with disorders of consciousness. Prog Brain Res.
177:231–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Leonardi M, Giovannetti AM, Pagani M,
Raggi A and Sattin D: National Consortium Functioning And
Disability In Vegetative And In Minimal Conscious State Patients:
Burden and needs of 487 caregivers of patients in vegetative state
and in minimally conscious state: Results from a national study.
Brain Inj. 26:1201–1210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pan J, Xie Q, He Y, Wang F, Di H, Laureys
S, Yu R and Li Y: Detecting awareness in patients with disorders of
consciousness using a hybrid brain-computer interface. J Neural
Eng. 11:0560072014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cui Y, Zheng L, Wang X, Zhang W, Yuan D
and Wei Y: Marchiafava-Bignami disease with rare etiology: A case
report. Exp Ther Med. 9:1515–1517. 2015.PubMed/NCBI
|