Introduction
Acute spinal cord injury (ASCI) can bring about
mechanically structural damage and secondary neurological
dysfunction, in which the loss of neuron may be an important cause
for the permanent dysfunction of nerve after the onset of ASCI
(1). Inflammatory response, cell
autophagy and cell apoptosis are the important pathogeneses of
neuron loss (2–4). Cell autophagy is a major method by
which cells can maintain the survival, differentiation and
homeostasis. In a study by Sekiguchi et al (5) it was reported that autophagy
enhancement can alleviate the injury of spinal cord in rats, thus
promoting the recovery of neurological functions. Another study
from Erlich et al (6)
confirmed that autophagy could exert the neuroprotective effect
through inhibiting the already enhanced apoptosis. Peak level of
spinal cord injury is usually attained at 24–48 h after the injury,
which is coincident with the level of apoptosis in some tissue
(7). In eukaryotes, JAK2/STAT3
signal pathway is a significant intracellular pathway, through
which the expression of multiple cytokines and growth factors, cell
proliferation, differentiation and apoptosis occurs (8,9). In this
study, we aimed to analyze the occurrences of autophagy and
apoptosis mediated by JAK2/STAT3 signaling pathway after the ASCI
of rats to provide a new target for intervention of ASCI at an
early stage.
Materials and methods
ASCI model
We selected a total of 45 Sprague-Dawley adult rats
of either sex. The age range of rats was from 8 to 10 weeks, and
the average weight was 245 g. These rats were purchased from the
Experimental Animal Center of Sangon Biotech Co., Ltd. (Shanghai,
China) and were prepared for study after 1 week of regular feeding.
Modified Allen method was used to prepare the models through the
following procedures. After 8 h of fasting, rats were anesthetized
abdominally using 3% chloral hydrate (27 mg/100 g); rats were
fixated in prone position, and an incision (~2.5 cm in length) was
made in the middle of back; skin was cut layer by layer, and the T8
to T10 vertebral plates were exposed. Total laminectomy was
performed for T9 vertebral plate to expose the dura mater spinalis;
T8 and T10 spinous processes were fixated using forceps; Kirschner
wire (10 g) was inserted into the catheter with scale, freely
plummeted from 25 mm height, finally a semicircular slice (4 mm in
diameter and 2 mm in width) made from thin plastic, was hit and the
wire was immediately removed, leading to the incomplete injury of
spinal cord in the rat; incision was sutured layer by layer. After
the strike, rats with the signs of tail-wagging reflection,
retraction flutter in lower limbs and body, and flaccid paralysis
in lower limbs in awake state represented successful construction
of the model. This study was approved by the Animal Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University.
Research method and observation
indexes
These rats were randomly divided into three groups,
i.e. sham-operated group, model group, and the AG-490 intervention
group (AG-490 is an inhibitor of JAK2). Each group contained 15
rats. For rats in the sham-operated group, total laminectomy of T9
vertebral was only performed without any damage to spinal cord; for
rats in the AG-490 intervention group, AG-490 (40 µg/g) was
dissolved in 45% dimethyl sulfoxide (DMSO) and injected abdominally
at 20 min before the spinal cord injury. Five rats in each group
were sacrificed at 6, 12 and 24 h, respectively, and then we
detected the p-JAK2 and p-STAT3 expression levels in spinal cord
tissue via western blot analysis, and levels of interleukin-6
(IL-6) and tumor necrosis factor-α (TNF-α) via enzyme-linked
immunosorbent assay (ELISA), positive expression rate of light
chain 3 (LC3)-II of microtubule-associated protein 1 via
immunofluorescence labeling method, and mRNA expression levels of
caspase-3 and Bax/Bcl-2 via RT-PCR.
Detection method
Western blot analysis
Excessive anesthesia was performed for rats using 3%
chloral hydrate (54 mg/100 g). Thoracic cavity was opened, where
the catheter was inserted into the aorta, and fixated using
hemostatic forceps. Right auricle was incised, and the icy normal
saline was rapidly perfused until the liquid turned clear. Then,
the liquid was replaced by 4% paraformaldehyde for fixation for 40
min. Spinal cord was stripped bluntly through the incision on the
back, and we obtained the spinal cord ~1 cm in length with the
damaged part as the center. Spinal cord in 4 µm of thickness was
then acquired after fixation, dehydration, paraffin embedding and
serial section. Spinal cord was then prepared into the tissue
homogenate, in which the RIPA lysate was added for extraction of
total protein of cells. Crude quantification was performed via
Coomassie Brilliant Blue method, and the result was standardized
using antibody for β-actin. Thirty micrograms of total protein was
taken for separation via 8% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), and the separated strips were
electrically transferred onto the PVDF membrane. Rabbit monoclonal
JAK2 antibody (dilution, 1:500; cat. no. ab108596) and rabbit
monoclonal STAT3 antibody (dilution, 1:500; cat. no. ab68153) were
added onto the membrane for incubation overnight, and then
secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000;
cat. no. an6721), was added onto the membrane for incubation for 4
h at room temperature. Thereafter, membrane was washed by
phosphate-buffered saline (PBS) and colored using
electrochemiluminescence (ECL). Results were scanned and preserved,
and semi-quantitative analysis was carried out through Lab Works
4.5 gel imaging software (Invitrogen, Carlsbad, CA, USA) with the
results being presented by integral optical density (IOD).
ELISA
After the tissue homogenate was prepared,
centrifugation was performed at 2,500 × g for 30 min. Supernatant
was taken for later detection. Reagents were purchased from Jiangsu
Beyotime Biotech Co., Ltd. (Jiangsu, China). All procedures were
carried out in accordance with the manufacturer instructions.
Immunofluorescence labeling
Sections were blocked using normal goat serum for 1
h. Then goat monoclonal LC3-II antibody (1:500; cat. no. TA803577;
Beijing Zhongshan Golden Bridge Co., Ltd., Beijing, China) was
added for incubation at 4°C overnight, and later immunofluorescence
labeled rabbit anti-goat IgG secondary antibody (1:500; cat. no.
ZDR-5308; Beijing Zhongshan Golden Bridge Co., Ltd.) was added onto
the section for incubation at 4°C overnight. Sections were stained
using 4′,6-diamidino-2-phenylindole (DAPI) for 2 min, and sealed
using glycerol after being washed. Then the section was placed
under the fluorescence microscope for observation in the dark. In
each group, we randomly selected 5 sections, and we chose upper,
lower, left, right and central areas from the visions (×400) to
calculate the percentage of LC3-II positive cells.
RT-PCR
Total RNA in the cell was extracted using regular
TRIzol reagent, and the concentration and purity were assayed
through ultraviolet spectrometer. Reverse transcription kit was
used for cDNA synthesis, and the primer sequences were synthesized
by Sangon Biotech Co., Ltd. according to the sequences of Gene
Bank: Bax forward, 5′-GGTTTCATCCAGGATCGAGCAGG-3′ and reverse,
5′-ACAAAGATGGTCACGGTCTGCC-3′, 445 bp; Bcl-2 forward,
5′-ACTACTTCTCCCGCCGCTAC-3′ and reverse,
5′-GAAATCAAACAGAGGCCGCATG-3′, 332 bp; caspase-3 forward,
5′-TACCAGTGGAGGCCGACTTC-3′ and reverse,
5′-GCACAAAGCGACTGGATGAAC-3′, 103 bp; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′-CGCGAGAAGATGACCCAGAT-3′ and
reverse, 5′-GCACTGTGTTGGCGTACAGG-3′, 225 bp. Reaction system was
set as follows: cDNA 2 µl + upstream primer 3 µl and downstream
primer 3 µl + Taq polymerase 0.5 µl + dNTPs 1 µl + MgCl2
3 µl + 10X nuffer 5 µl + ddH2O2 2.5 µl.
Reaction conditions were set as follows: 95°C for 5 min, 95°C for
30 sec, 58°C for 30 sec, 72°C for 60 sec and for a total of 30
cycles followed by 72°C for 10 min. PCR products were identified
using 2% agarose gel electrophoresis. Ultraviolet images were
developed through gel imaging analysis system. Grey value analysis
was performed using the digital photographs. Results were analyzed
by the 2−ΔΔCq method.
Statistical analysis
SPSS 20.0 (IBM, Armonk, NY, USA) was used for
statistical analysis. Measurement data were presented by mean ±
standard deviation, single-factor ANOVA was performed for
intergroup comparison, and LSD t-test for paired comparison;
variance analysis of repeated measurements was used in the
comparison among data in different time-points. p<0.05 indicated
that the difference had statistical significance.
Results
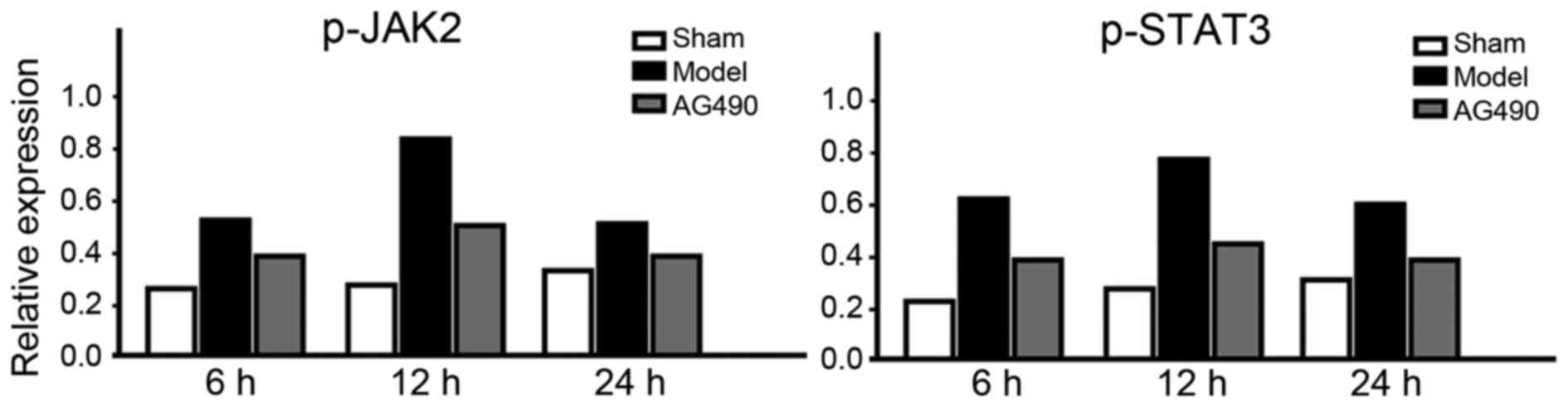
Expressions of p-JAK2 and p-STAT3
In the model group, expression levels of p-JAK2 and
p-STAT3 at every time-point were significantly higher than those in
the AG-490 intervention group, and the levels in the sham-operated
group were the lowest (p<0.05). In the model group, peak levels
of p-JAK2 and p-STAT3 were attained at 12 h, but a fall was seen at
24 h (Fig. 1).
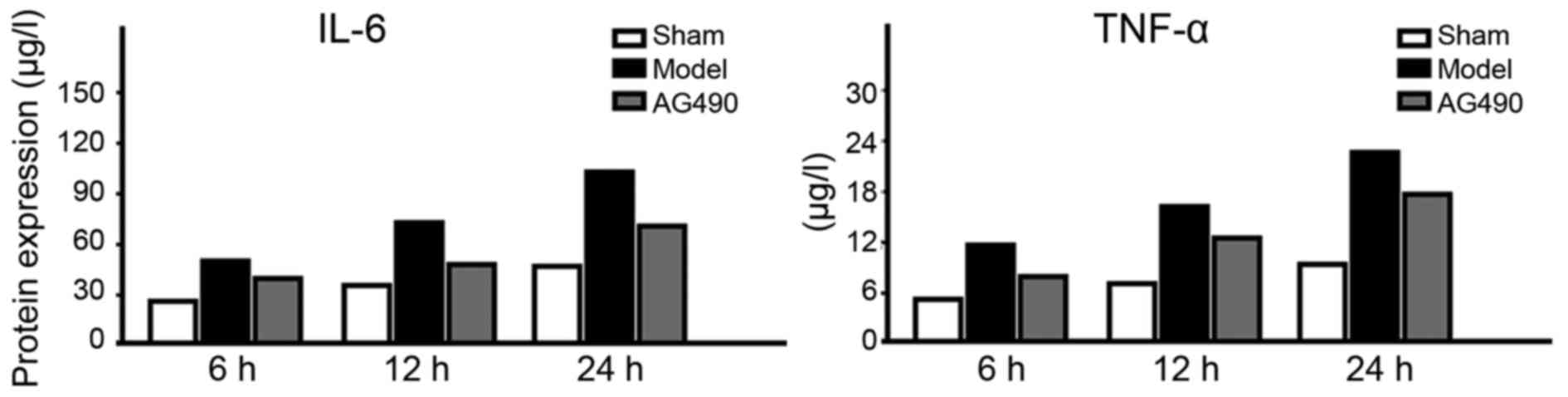
Levels of IL-6 and TNF-α
In the model group, the expression levels of IL-6
and TNF-α at every time-point were significantly higher than those
in the AG-490 intervention group, and the levels in the
sham-operated group were the lowest (p<0.05). In the model
group, an increasing trend was seen in the expression levels of
IL-6 and TNF-α (Fig. 2).
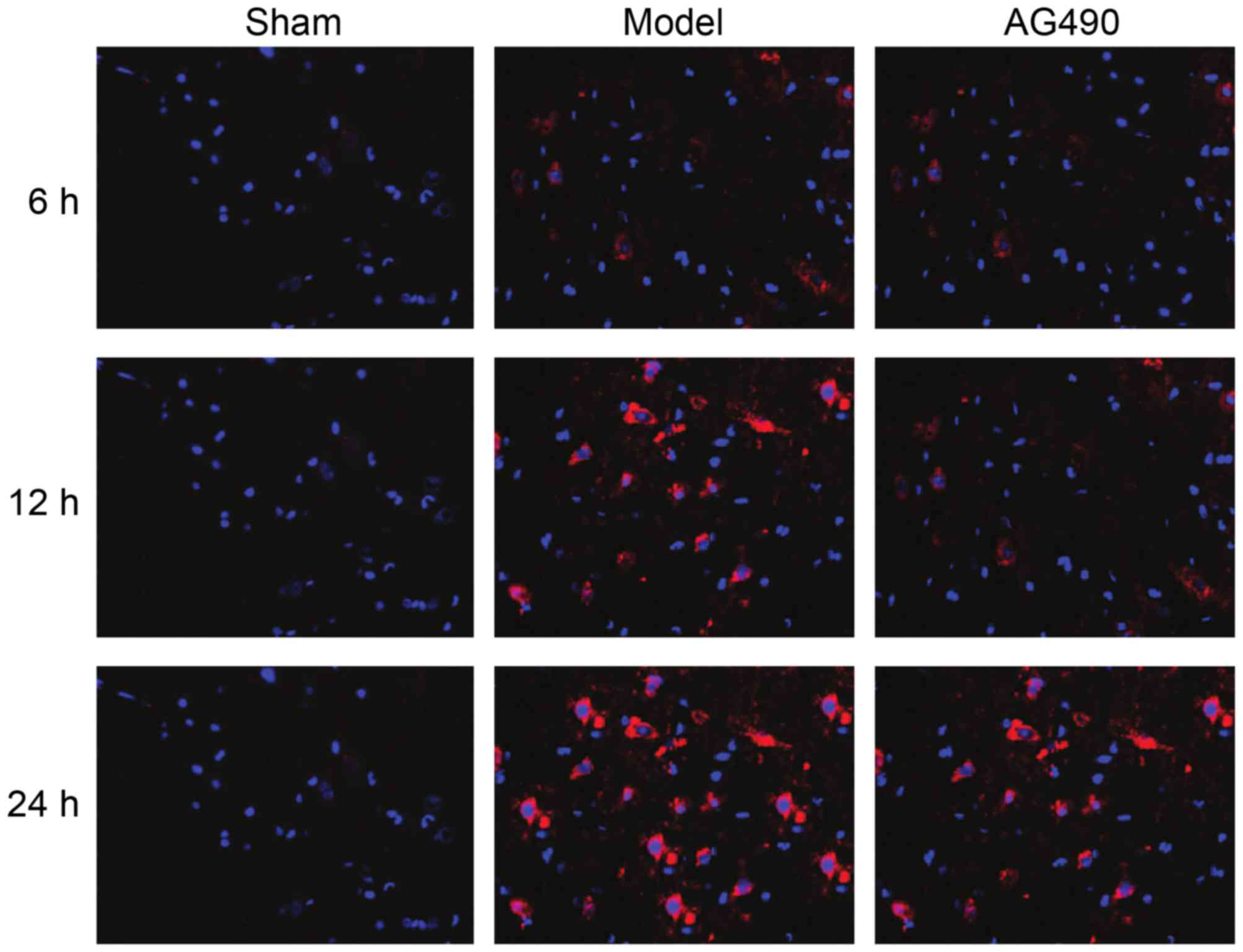
Positive expression of LC3-II
In the model group, positive expression rates of
LC3-II at every time-point were significantly higher than those in
the AG-490 intervention group, and the levels in the sham-operated
group were the lowest (p<0.05). In the model group, an
increasing trend was seen in the expression rates of LC3-II
(Fig. 3).
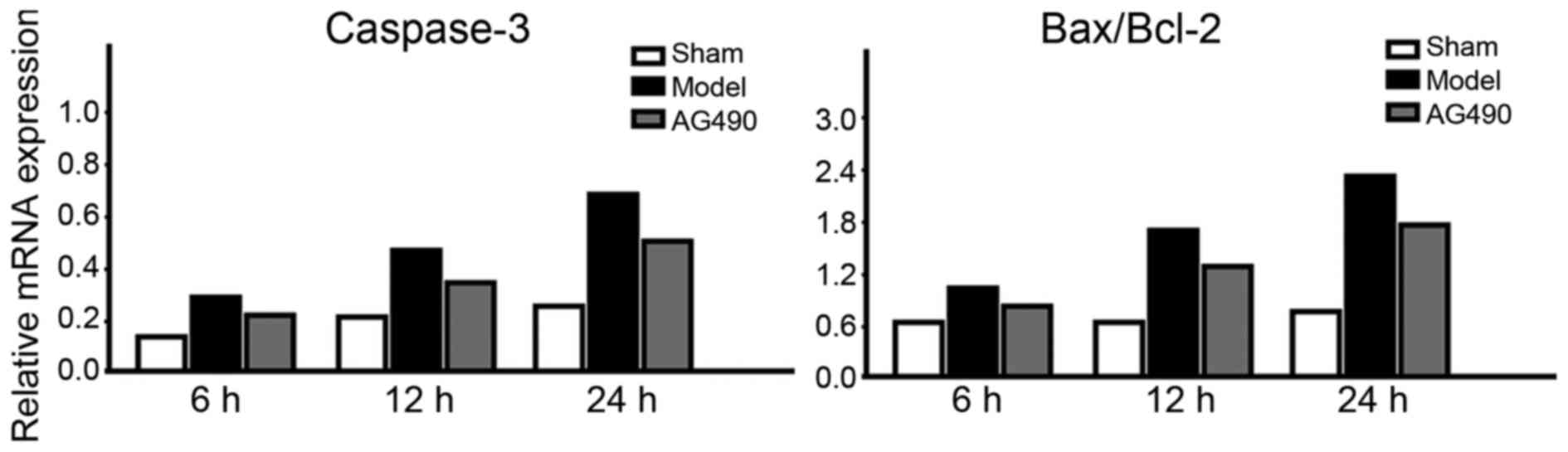
mRNA expression levels of caspase-3
and Bax/Bcl-2
In the model group, mRNA expression levels of
caspase-3 and Bax/Bcl-2 at every time-point were significantly
higher than those in the AG-490 intervention group, and the levels
in the sham-operated group were the lowest (p<0.05). In the
model group, an increasing trend was seen in the mRNA expression
levels of caspase-3 and Bax/Bcl-2 (Fig.
4).
Discussion
Under normal physiological condition, JAK2/STAT3 is
found in non-phosphorylated condition; but when the body is under
stress, phosphorylation of JAK2/STAT3 will be instantly completed,
last for a short period, ~6 to 12 h, and rapidly disappear
(10). Through the intracellular
binding site of tyrosine protein kinase, JAK2/STAT3 can bind with
the relevant receptors, such as IL-6, TNF-α, prolactin (PRL) and
colony stimulating factor (CSF), thus activating the tyrosine
residue of all downstream target proteins to exert the biological
effect (11). In the study by Suzuki
et al (12), it was found
that IL-6 can activate the JAK/STAT and p38-MAPK signal
transduction pathways in the central nervous system to mediate the
neurological injury and affect the neurological repair. Currently,
it has been proven that JAK2/STAT3 signal pathway plays an
important role in the immune reactions and the pathogenesis of
tumor (13). The study by Wang et
al (14) revealed that
JAK2/STAT3 signal pathway can also induce apoptosis of the cortical
neuron. As a substrate of JAK kinase, STAT, once being activated
can pass through the nuclear membrane in the form of polymers, such
as dimer or tetramer, to specifically bind with the response
element on DNA to initiate the transcription of target downstream
gene. Through this process, extracellular signals can be transduced
into the cells to regulate various processes, such as cell
proliferation, differentiation, apoptosis and immune regulations
(15). STAT3 is a kind of
bifunctional protein with key effect, and blocking JAK2/STAT3
signal pathway can significantly alleviate the focal cerebral
ischemic re-perfusion injuries of rats, decrease the apoptosis of
neurons, and ameliorate the impairment of neurologic functions
(16).
Through this study, we found that in the model
group, the expression levels of p-JAK2, p-STAT3, IL-6, TNF-α and
LC3-II, and the mRNA expression levels of caspase-3 and Bax/Bcl-2
at every time-point were significantly higher than those in the
AG-490 intervention group, and the levels in the sham-operated
group were the lowest. In the model group, peak levels of p-JAK2
and p-STAT3 were attained at 12 h, but a deline was seen at 24 h;
while increasing trend was seen in other indicators. This suggested
that in an early stage, JAK2/STAT3 signal pathway can mediate
autophagy and apoptosis activity after ASCI in rats. Activation of
JAK2/STAT3 signal pathway requires the induction of inflammatory
factors such as IL-6 and TNF-α, which can in turn aggravate the
release of inflammatory factors to induce the waterfall-like
cascade reactions of inflammation to participate in the impairment
of neurologic functions in the early and late stage of ASCI
(17). Marker proteins that can
reflect autophagy activity include LC3 and Atgl2-Atg5 complex, in
which LC3-I and LC3-II participate in the formation of
autophagosome, combination between LC3-I and
phosphatidylethanolamine can form LC3-II that is located on the
membrane of autophagosome, and the content of LC3-II is positively
correlated with the quantity of autophagosome (18). It was reported that the occurrence of
autophagy is closely related with the apoptosis level, in which
autophagy can inhibit the occurrence of excessively active
apoptosis to promote the recovery of neurologic functions (19).
Different from cell necrosis, cell apoptosis refers
to programmed cell death under precise regulation. Among various
mitochondrion-dependent or non-mitochondrion dependent apoptosis
mechanisms, apoptosis regulated by caspase family is a core member
(20). Apoptosis is a cascade
cleaving process of proteinase, and caspase-3, also known as the
death proteinase, cleaving the protein kinase, nuclease and
cytoskeleton, leading to a series of apoptotic activities, such as
nuclear shrinkage condensation and DNA fragmentation, thus
controlling the occurrence and progression of apoptosis (21). In addition, the orientation of
apoptosis is generally decided by the ratio of Bax to Bcl-2. If the
ratio of Bcl-2, the anti-apoptosis factor, to Bax, the
pro-apoptosis factor, is not <50%, significant anti-apoptosis
effect will emerge in the cells (22).
In conclusion, early intervention in autophagy,
apoptosis and the key target point in the JAK2/STAT3 signal
transduction pathway in ASCI may be of great significance for
effectively alleviating the neurologic injuries and promoting
recovery in neurologic functions.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81100906) and the National
Clinical Key Department Construction Programme of China (Cai She
[2011]170).
References
|
1
|
Krause JS, Newman JC, Clark JM and Dunn M:
The natural course of spinal cord injury: Changes over 40 years
among those with exceptional survival. Spinal Cord. 5:123–124.
2016.
|
|
2
|
Kwan T, Floyd CL, Kim S and King PH: RNA
binding protein HuR is translocated in astrocytes following spinal
cord injury and promotes the inflammatory response. J Neurotrauma.
6:125–126. 2016.
|
|
3
|
Kanno H, Ozawa H, Sekiguchi A and Itoi E:
Spinal cord injury induces upregulation of Beclin 1 and promotes
autophagic cell death. Neurobiol Dis. 33:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li G, Jia Z, Cao Y, Wang Y, Li H, Zhang Z,
Bi J, Lv G and Fan Z: Mitochondrial division inhibitor 1
ameliorates mitochondrial injury, apoptosis, and motor dysfunction
after acute spinal cord injury in rats. Neurochem Res.
40:1379–1392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekiguchi A, Kanno H, Ozawa H, Yamaya S
and Itoi E: Rapamycin promotes autophagy and reduces neural tissue
damage and locomotor impairment after spinal cord injury in mice. J
Neurotrauma. 29:946–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erlich S, Alexandrovich A, Shohami E and
Pinkas-Kramarski R: Rapamycin is a neuroprotective treatment for
traumatic brain injury. Neurobiol Dis. 26:86–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Liu Y, Wu C, Zhao W, Zhang J, Bao
G, Xu G, Sun Y, Chen J and Cui Z: The expression of IGFBP6 after
spinal cord injury: Implications for neuronal apoptosis. Neurochem
Res. 5:154–155. 2016.
|
|
8
|
Morales JK, Falanga YT, Depcrynski A,
Fernando J and Ryan JJ: Mast cell homeostasis and the JAK-STAT
pathway. Genes Immun. 11:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai SY and Johnson FM: Defining the role
of the JAK-STAT pathway in head and neck and thoracic malignancies:
Implications for future therapeutic approaches. Drug Resist Updat.
13:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Yao YM, Yu Y, Dong N, Yin HN and
Sheng ZY: Role of Janus kinase/signal transducer and activator of
transcription pathway in regulation of expression and
inflammation-promoting activity of high mobility group box protein
1 in rat peritoneal macrophages. Shock. 27:55–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang MK and Kang SK: Interleukin-6 induces
proliferation in adult spinal cord-derived neural progenitors via
the JAK2/STAT3 pathway with EGF-induced MAPK phosphorylation. Cell
Prolif. 41:377–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki S, Tanaka K and Suzuki N:
Ambivalent aspects of interleukin-6 in cerebral ischemia:
Inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab.
29:464–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong LY, Abou-Ghazal MK, Wei J,
Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA,
Schmittling RJ, et al: A novel inhibitor of signal transducers and
activators of transcription 3 activation is efficacious against
established central nervous system melanoma and inhibits regulatory
T cells. Clin Cancer Res. 14:5759–5768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Zhou D, Wang C, Gao Y, Zhou Q,
Qian G and DeCoster MA: Hypoxic preconditioning suppresses group
III secreted phospholipase A2-induced apoptosis via JAK2-STAT3
activation in cortical neurons. J Neurochem. 114:1039–1048.
2010.PubMed/NCBI
|
|
15
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang QZ, Lei C, Lu ZH, Wang BR and Xiong
LZ: Neuroprotective effects of combined application of JAK-STAT
signal pathway inhibitor and free radical scavenger on focal
cerebral ischemia/reperfusion injury in rats. Zhongguo Wei Zhong
Bing Ji Jiu Yi Xue. 20:641–644. 2008.(In Chinese). PubMed/NCBI
|
|
17
|
Song Y, Zeng Z, Jin C, Zhang J, Ding B and
Zhang F: Protective effect of ginkgolide B against acute spinal
cord injury in rats and its correlation with the Jak/STAT signaling
pathway. Neurochem Res. 38:610–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou H, Zhang L, Zhang L and Tang P: Acute
spinal cord injury in rats should target activated autophagy. J
Neurosurg Spine. 20:568–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YB, Li SX, Chen XP, Yang L, Zhang
YG, Liu R and Tao LY: Autophagy is activated and may protect
neurons from degeneration after traumatic brain injury. Neurosci
Bull. 24:143–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Chen S, Zhao H, Wang C, Gao K, Guo
Y, Shen Z, Wang Y, Wang H and Mei X: Probucol inhibits neural cell
apoptosis via inhibition of mTOR signaling pathway after spinal
cord injury. Neuroscience. 329:193–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gökce EC, Kahveci R, Gökce A, Cemil B,
Aksoy N, Sargon MF, Kısa Ü, Erdoğan B, Güvenç Y, Alagöz F, et al:
Neuroprotective effects of thymoquinone against spinal cord
ischemia-reperfusion injury by attenuation of inflammation,
oxidative stress, and apoptosis. J Neurosurg Spine. 24:949–959.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li CM, Xie SJ, Wang T, Du WB, Yang ZB and
Quan RF: Effects of electro-acupuncture on neuronal apoptosis and
associative function in rats with spinal cord injury. Zhongguo Gu
Shang. 28:733–738. 2015.(In Chinese). PubMed/NCBI
|