Introduction
With the incidence of cancer on the rise and the
increasing use of immunosuppressant in recent years,
Aspergillus infection has become the second most prevalent
deep fungal infection following monilial infection (1,2).
Aspergillus bloodstream infection (BSI) is a rarely seen
critical illness in the clinic, and most cases reported in the
literature have dissemination of invasive pulmonary aspergillosis
and end-stage infection in critical patients (3–5), with
few patients having infection without organ dissemination. The
present study reported on a case of gastric cancer with massive
hemorrhage in the gastrointestinal tract, and the patient developed
Aspergillus niger (A. niger) BSI after common hepatic
artery (CHA) embolization.
Case study
A 62-year-old man with intermittent abdominal pain
and 400 ml of hematochezia was admitted to the Department of
Emergency of the Affiliated Hospital of the Academy of Military
Medical Sciences (Beijing, China) on March 13, 2012. After
admission, the patient experienced dizziness, but without
hematemesis.
The patient had received a total gastrectomy under
general anesthesia for gastric cancer at Peking University People's
Hospital (Beijing, China) on December 9th, 2011. Post-operative
pathology had revealed moderately- and poorly-differentiated
adenocarcinoma invasion in gastric tissue (gastric angle and
cardia). Part of the lesion was manifested as mucinous
adenocarcinoma (3.7×3.2×0.5 cm), and the other part of it was
differentiated into signet ring cell carcinoma (4×3×1 cm). The
tumor invaded the entire peritoneum and surrounding fat. Vascular
tumor thrombus was visible, with negative upper and lower margins.
Lymph node metastasis occurred in the greater and lesser curvature
of the stomach (5/23 and 6/19), and metastatic carcinoma was seen
in the lymph nodes submitted for detection (12A; 1/1). There was no
tumor invasion in fibrofatty tissue (12P) or the greater omentum.
Immunohistochemical analysis revealed the following: Creatine
kinase(−), cytokeratin 20(+), CDX2(+++), villin(++), Ki-67(50%+),
P53(++), CerbB-2(−), glycoprotein hormones, alpha polypeptide(−),
synaptophysin (Syn)(−) and CD56(−). Specific alcian blue/periodic
acid Schiff staining was positive on December 12, 2011.
The patient received paclitaxel [150 mg day (d)1,
120 mg d8], oxaliplatin (200 mg d1) plus Xeloda [1,500 mg twice a
day (bid) d1-d14] from February 7, 2012 following strength
recovery, which enables individuals to withstand chemotherapy, as a
post-operative adjuvant chemotherapy for the first cycle. The
paclitaxel scheme was withdrawn on March 1, 2012 during the second
cycle due to self-reported fatigue that could not be tolerated.
Physical examination revealed a body temperature of 36.8°C, a heart
rate of 58 beats per minute and a blood pressure of 120/80 mmHg.
The patient was conscious without any signs of peritoneal
irritation or Murphy's sign, and bowel sounds were normal. Routine
blood test indicated hemoglobin (Hb) levels of 97 g/l.
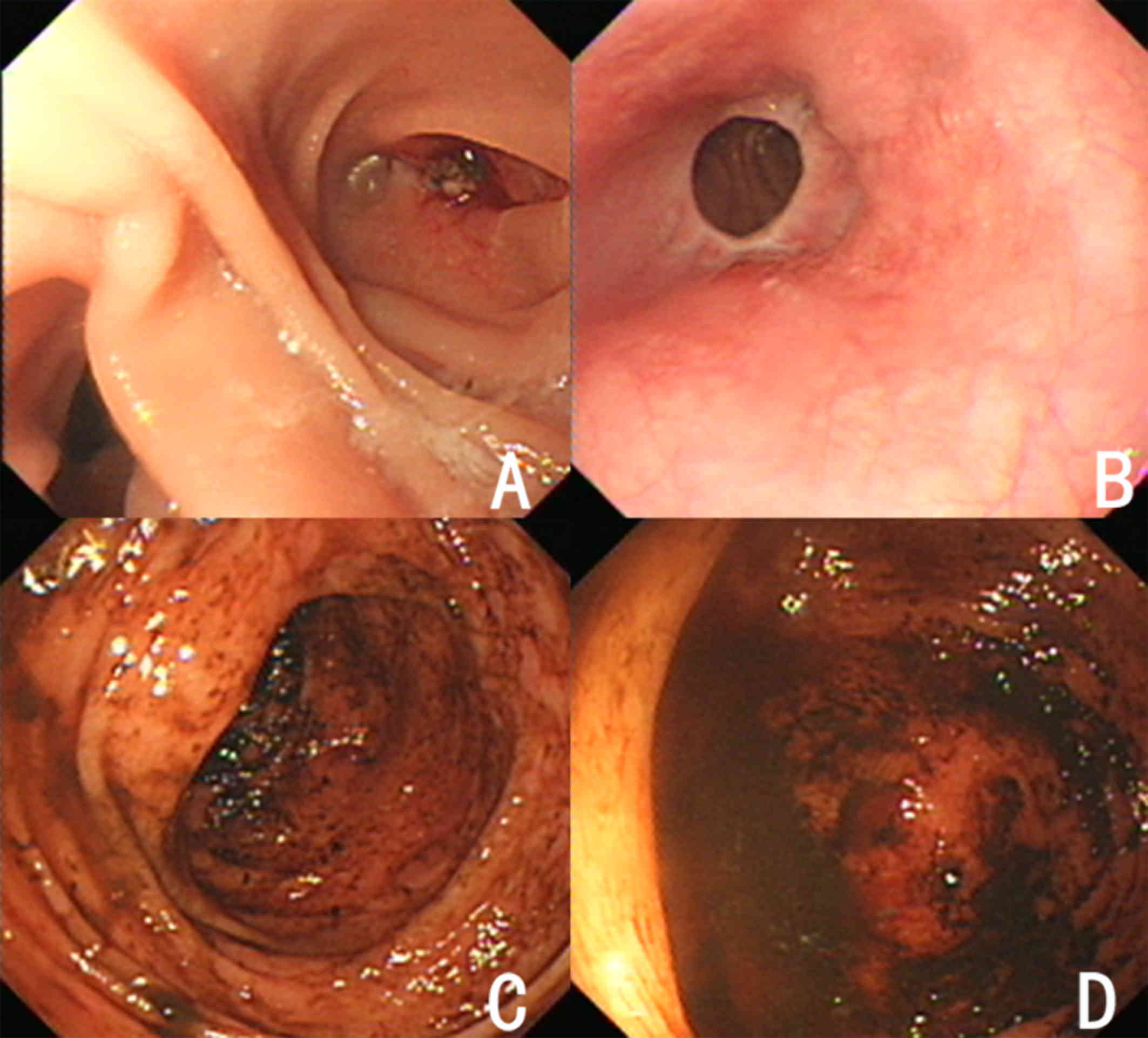
Gastrointestinal endoscopy indicated no hemorrhagic spots (Fig. 1) and there was no hematochezia within
3 days after admission.
On March 16, 2012, the patient had intermittent
hematochezia of up to 3,000 ml and his blood pressure dropped to as
low as 60/30 mmHg. Treatment by blood volume supplementation,
hemostasis and blood transfusion proved ineffective, and digital
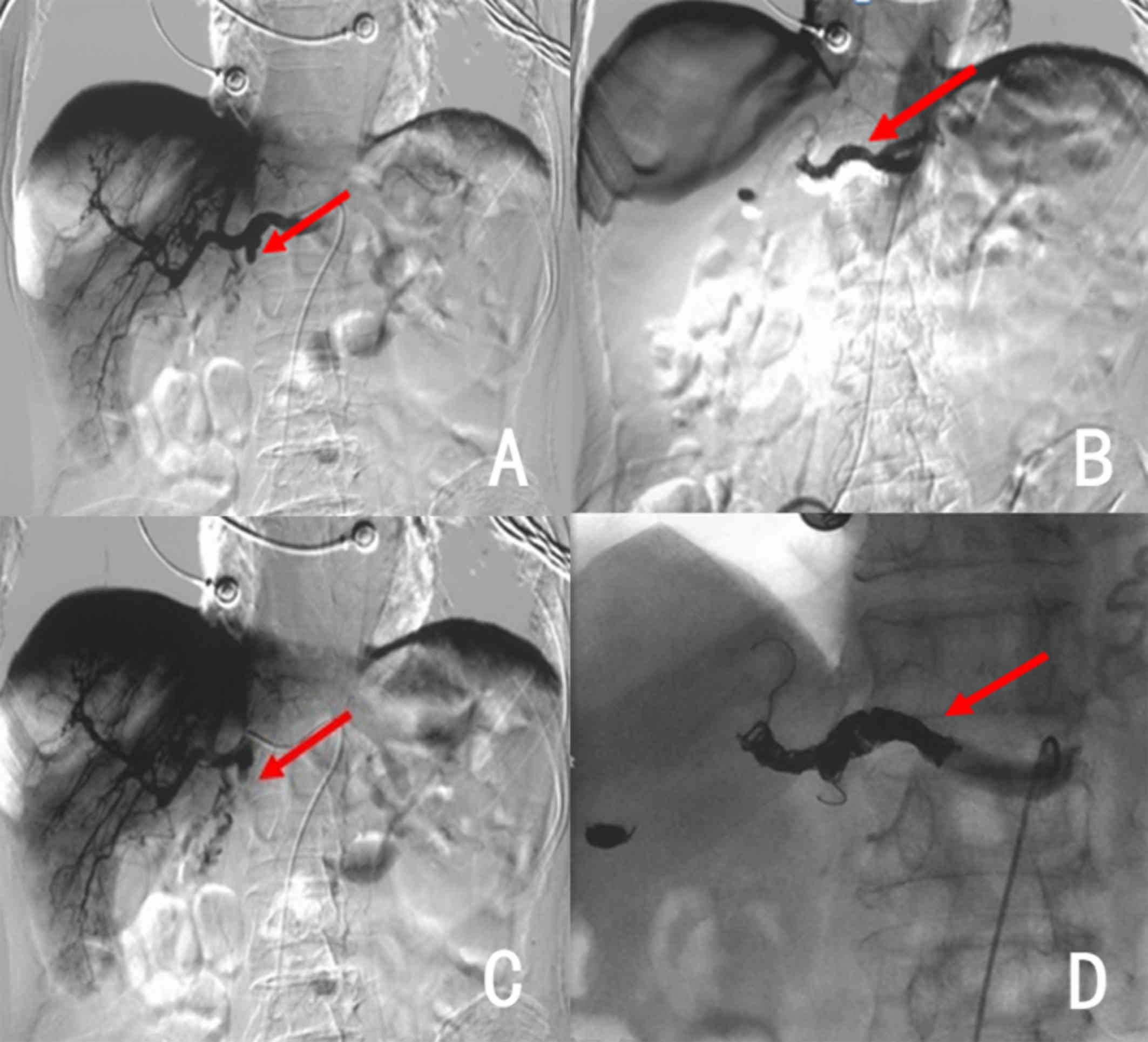
subtraction angiography (DSA; Siemens AG, Munich, Germany) on March
17 revealed the development of a gastroduodenal artery stump
(Fig. 2A). A microcatheter
(Progreat; Terumo Corp., Tokyo, Japan) was subsequently inserted
into the artery after super selection and dozens of absorbable
gelatin sponges (Jinling Pharmaceutical Ltd., Nanjing, China) were
used on their own for embolization to prevent gastrointestinal
infarction, which was found successful based on angiography
(Fig. 2B).
Six h later, the patient had massive hematochezia
again of about 4,000 ml, and Hb levels were as low as 32 g/l. After
multidisciplinary discussion, surgeons did not recommend an
additional operation. DSA was performed again on March 18, which
revealed bleeding in the same location (Fig. 2C). A total of 27 micro-coil springs
(Beijing Cook Medical Equipment Co., Ltd., Beijing, China) and
>20 gelatin sponges were used to perform CHA embolization based
on re-examination (Fig. 2D).
The patient underwent transfusion of red blood cells
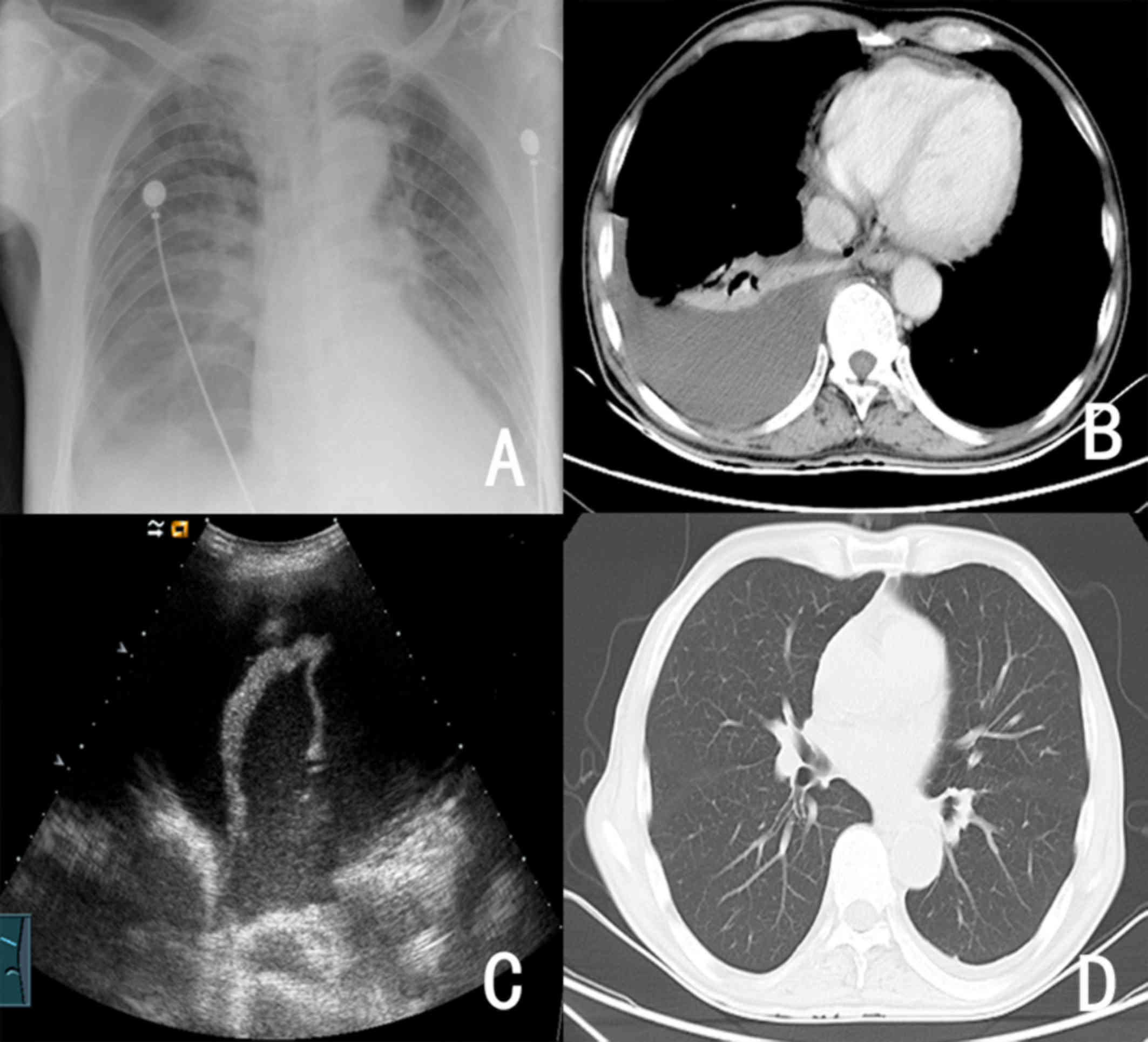
and other blood products (nearly 8,000 ml) within 48 h. Two days
after embolization, severe liver function damage, endogenous
Escherichia coli blood infection, urinary tract infection,
pulmonary infection and right pleural effusion occurred
sequentially (Fig. 3). The
pathological examination was completed using hemotoxylin and eosin
staining (H&E) to assess pleural effusion and no tumor cells
were observed (Fig. 4). The patient
was discharged from hospital after a 15-day course of support
nutrition therapy and antibiotic treatment including moxifloxacin,
cefatriaxone, meropenem, as well as right pleural effusion drainage
(500 ml), with no malignant cells.
From April 17, 2012, the patient began to show
intermittent rigors and fever, and his body temperature rose to
39.4°C, which was associated with frequent and urgent urination and
odynuria. A urine routine test revealed 2+ leukocyte levels and a
procalcitonin (PCT) level of 3.74 ng/ml, which was indicative of a
urinary system infection. After 3 days of antibiotic treatment with
ciprofloxacin, the patient's body temperature further increased to
40.0°C, and he was admitted to the hospital again on April 20.
Blood, urine and stool specimens were obtained from
the patient on admission to perform bacterial and fungal culture
tests. Four sets of venous blood specimens (10 ml per bottle) were
aseptically collected from bilateral arms of the patient during
chills and fever. The specimens were rapidly injected into special
blood culture bottles, mixed immediately and submitted for
detection. To culture and isolate pathogenic microorganisms, the
blood culture bottles were incubated in the BacT/Alert 3D automated
blood culture instrument (Organon Teknika LLC., Durham, NC,
USA).
When the instrument alarm turned positive, the
culture was immediately transferred to blood agar plates, a
MacConkey agar plate and chocolate agar plates for bacterial
culture. The culture was also inoculated to Sabouraud dextrose agar
(SDA) and potato dextrose agar (PDA) for fungal culture. For
bacteriological examination, Gram staining and microscopic
examination were performed, and the preliminary results were
provided to the clinicians. Fungal morphology was examined using
the steel ring method, and the culture was grown on PDA plates at
25°C for 7 days, followed by lactic acid-phenol-cotton blue
staining.
After collecting bacterial samples, the patient was
given meropenem, and his body temperature returned to normal 2 days
later, with negative blood, urine and stool analysis results. The
treatment was continued until April 26, when the medication was
replaced with cefuroxime sodium (1.5 g b.i.d). On April 30, the
fever without chilling occurred again with a maximum temperature of
39.6°C. Chemical examination revealed high-sensitivity C-reactive
protein (CRP) of 66 mg/l, PCT of 72.67 ng/ml and galacto-mannan of
Aspergillus antigens of 4.66 ng/ml. On the same day, only
fungus was seen in blood culture samples obtained at different
time-points on admission and from different parts of his upper
limbs. No bacteria were detected either by direct microscopic
observation or culture examination upon instrument alert.
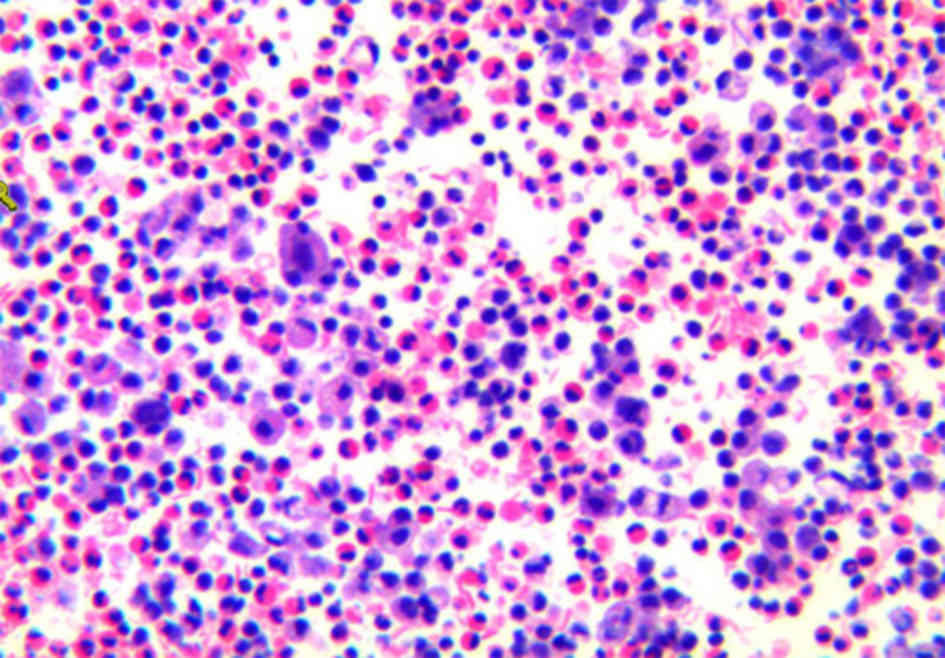
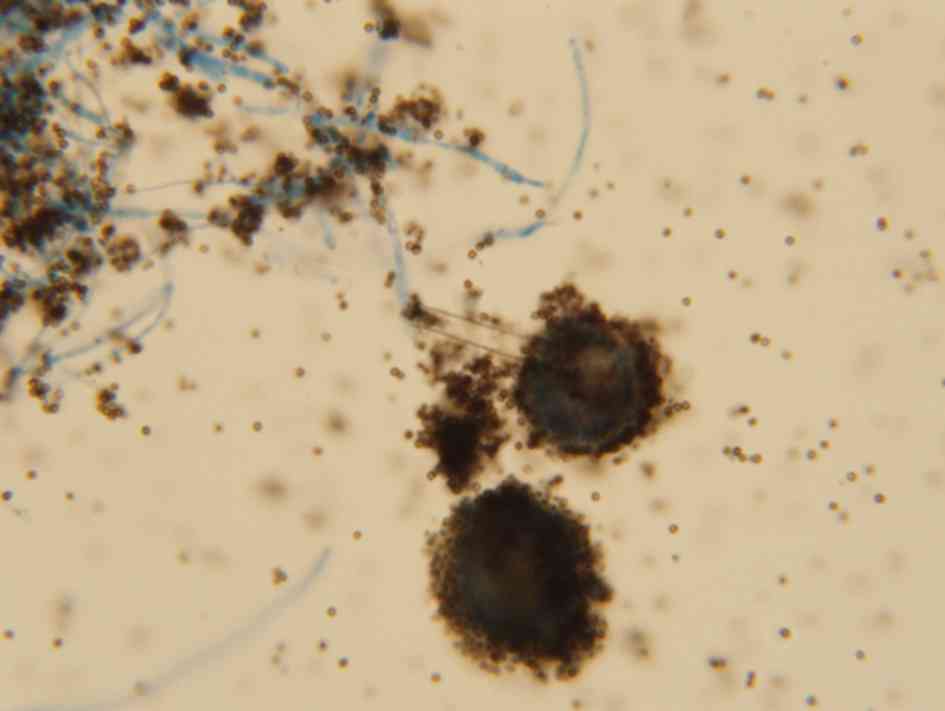
Identification of pathogenic fungus was according to
morphological characteristics: i) Microscopic examination: After
instrument alarm, blood culture was extracted from the bottle with
a syringe; hyaline and 45°-branching hyphae were seen on direct
smear; ii) Culture examination: The colonies grew fast on SDA
medium and developed a black powder surface; under a microscopic
view, conidial heads consisting of spherical vesicles, globose or
subglobose, were visible; the entire spherical vesicle was covered
with two layers of sterigma, one layer thick and the other layer
thin; the sterigma was radially arranged, black and bore chains of
spores (Fig. 5). According to the
above characteristics, the fungus was identified as A.
niger.
An anti-fungal drug sensitivity test against four
types of anti-fungal drugs was performed. The minimum inhibitory
concentrations for the fungus were as follows: Voriconazole 0.25
µg/ml, amphotericin B 4.0 µg/ml, itraconazole 0.5 µg/ml and
caspofungin 0.06 µg/ml.
After Aspergillus BSI was diagnosed,
intravenous therapy with voriconazole (400 mg bid d1, 200 mg bid
d2-d14) was performed. The patient's body temperature returned to
normal 2 days later, PCT fell to 4.04 ng/ml 6 days later, and other
inflammatory markers fell significantly as well (Table I). Two weeks later, sequential
therapy with oral voriconazole (200 mg bid) was applied for 4
weeks. At the two-year follow-up on July 2015, no evidence of
recurrent fungal BSI, gastrointestinal bleeding or metastatic
relapse was identified.
 | Table I.Changes in indicators of infection at
different exam dates. |
Table I.
Changes in indicators of infection at
different exam dates.
| Item | Normal value | D1a | D5 | D8 | D11b | D15 | D20 |
|---|
| WBC
(109/l) | 3.5–9.5 | 7.7 | 5.69 | 6.14 | 10.86 | 7.41 | 7.57 |
| Neutrophils (%) | 40–75 | 65.7 | 63.7 | 54.9 | 85.3 | 67.7 | 71.5 |
| Hemoglobin (g/l) | 130–175 | 85 | 74 | 82 | 78 | 78 | 78 |
| Platelets
(109/l) | 125–350 | 208 | 143 | 290 | 264 | 269 | 225 |
| CRP (mg/l) | <3 | 144 | 45 | 17 | 66 | 30 | 41 |
| G (pg/ml) | <100.5 | ND | ND | ND | 62.3 | 131.8 | ND |
| GM (ng/ml) | <0.5 | ND | ND | ND | 4.66 | 0.16 | ND |
| PCT (ng/ml) | <0.25 | 3.74 | 9.45 | ND | 72.67 | 4.04 | ND |
The patient provided written informed consent
regarding the publication of his data and images in the present
study.
Discussion
Selective arterial embolization is an important
treatment for gastrointestinal bleeding of unknown primary origin
(6–8). Superselection of the bleeding arteries
is mostly used in the clinic (9–11), but
embolization of the CHA is rarely used, since it may severely
damage liver function (12).
Gastrointestinal bleeding in the present case was likely due to
digestive system reconstruction after total gastrectomy and
duodenal residual corrosion by digestive juice (e.g., gastric acid
bile and pancreatin), which led to mucosal damage and ulceration,
which may have also been aggravated by the adverse reactions of
nausea and vomiting following chemotherapy. All of the above
mentioned factors may have led to the rupture of the vascular
branch of the gastroduodenal artery supplying residual duodena to
cause gastrointestinal bleeding.
In addition, possible vascular malformation was
caused by the change in anatomical structure after surgery, and it
later developed into a pseudoaneurysm (13–16),
which ruptured and bled massively after systemic chemotherapy. All
factors contributed to the bleeding, resulting in a
life-threatening condition. Surgical operation at this juncture is
extremely risky, and gastroduodenal artery bleeding was diagnosed
twice using DSA. The first attempt of embolization failed, likely
as a result of the fact that the bleeding artery was close to the
CHA, and the rapid blood flow led to re-canalization. The second
embolization was performed on the CHA using permanent embolic
micro-coil springs in addition to gelatin sponges, which stopped
the bleeding and saved the patient's life. Gelatin sponge particles
may be absorbed in the short term, and if the bleeding recurs, the
use of gelatin sponge particles alone should be avoided due to
permanent embolism. CHA embolization changed the blood supply of
the liver and caused severe embolism syndrome, which was later
cured by liver protection and anti-biotic treatment.
The patient presented with chill, fever and urinary
irritation 1 month after embolization, which was probably due to a
urinary infection. The influence of the contrast agent during
continuous DSA could not be ruled out. The fever recurred during
anti-biotic therapy and blood culture indicated growth of A.
niger, which is rarely seen in the clinic. Caused mostly by
Candida and occasionally by Cryptococcus neoformans
(2,17), fungemia has a poor prognosis, and it
is usually an end-stage symptom of an immunocompromised patient.
Aspergillus grows naturally, and the majority of reported
Aspergillus BSIs are induced by exogenous infection due to
large-area burns (18,19). Invasive Aspergillus infection
generally occurs in the lungs in the early stages, and then spreads
to the central nervous system and other organs. However, it seldom
enters the blood and Aspergillus BSI is therefore rare in
the clinic.
Imageological examination of the patient indicated
no translocation of Aspergillus infection, and therefore,
the cause of the A. niger BSI remains unknown. The following
factors are potential causes for the infection: i) Test
contamination: A. niger was not detected in any of the
multiple blood samples submitted on the same day, so test
contamination is excluded; ii) blood sample contamination during
collection: Two blood samples with positive results were collected
from different parts of the body at different time-points by
different nurses, so blood sample contamination during collection
is excluded; ii) low immunity: Surgery, post-operative
chemotherapy, massive hemorrhage of the gastrointestinal tract,
severely damaged liver function after CHA embolization and BSI with
Gram-negative bacteria, as well as the application of strong
anti-biotics, may all significantly lower immunity and result in
exogenous Aspergillus entering the blood and cause
infection; and iv) invasive operation contamination: The patient
received invasive DSA and embolization twice within 24 h, and the
embolization required various embolic materials. As the only
invasive operation with a long exposure duration in the course of
the disease, embolization of the CHA cannot be totally excluded as
the cause underlying A. niger BSI, even though there was an
extended time period between the operation and infection, and none
of the other patients undergoing interventional therapy within 1
week prior to and after the patient were diagnosed with A.
niger BSI.
Within 3–4 months after gastric cancer surgery, the
patient had successive massive hemorrhage of the gastrointestinal
tract and A. niger BSI, which are critical illnesses, and he
was cured by CHA embolization and anti-fungal therapy with
voriconazole. Serious infections such as those with
Aspergillus tend to occur in patients with hematological
diseases and cancer who are immunocompromised (20–22). It
is important to provide active protection in order to prevent
infection during invasive tests and treatments. In addition, it is
worth noting that Aspergillus culture takes a long time by
continuous passage culture (23).
Thus, D-glucose, GM, PCT and CRP assessments should be combined to
achieve a timely anti-fungal treatment.
References
|
1
|
Rosa C, Araujo R, Rodrigues AG,
Pinto-de-Sousa MI and Pina-Vaz C: Detection of Aspergillus species
in BACTEC blood cultures. Med Microbiol. 60:1467–1471. 2011.
View Article : Google Scholar
|
|
2
|
Arendrup MC, Fuursted K, Gahrn-Hansen B,
Jensen IM, Knudsen JD, Lundgren B, Schønheyder HC and Tvede M:
Seminational surveillance of fungemia in Denmark: Notably high
rates of fungemia and numbers of isolates with reduced azole
susceptibility. J Clin Microbiol. 43:4434–4440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Person AK, Chudgar SM, Norton BL, Tong BC
and Stout JE: Aspergillus niger: An unusual cause of invasive
pulmonary aspergillosis. J Med Microbiol. 59:834–838. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali S, Malik A, Bhargava R, Shahid M and
Fatima N: Aspergillus colonization in patients with bronchogenic
carcinoma. Asian Cardiovasc Thorac Ann. 22:460–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gifford AH, Lahey T and Von Reyn C
Fordham: Fatal hemoptysis from invasive Aspergillus niger in a
patient with cavitary lung disease and Mycobacterium avium complex
infection. Med Mycol. 44:557–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renzulli P, Candinas D and Seiler CA:
Acute ‘difficult’ gastrointestinal bleeding. Ther Umsch.
63:311–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Remy-Jardin M, Bouaziz N, Dumont P,
Brillet PY, Bruzzi J and Remy J: Bronchial and nonbronchial
systemic arteries at multi detector row CT angiography: Comparison
with conventional angiography. Radiology. 233:741–749. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boufi M, Hashemi AA, Azghari A, Hartung O,
Ramis O, Moutardier V and Alimi YS: Endovascular management of
severe bleeding after major abdominal surgery. AnnVasc Surg.
27:1098–1104. 2013.
|
|
9
|
Lee JH, Hwang DW, Lee SY, Hwang JW, Song
DK, Gwon DI, Shin JH, Ko GY, Park KM and Lee YJ: Clinical features
and management of pseudoaneurysmal bleeding after
pancreatoduodenectomy. Am Surg. 78:309–317. 2012.PubMed/NCBI
|
|
10
|
Li Z, Jie Z, Liu Y and Xie X: Management
of delayed hemorrhage following radical gastrectomy for gastric
carcinoma patients. Hepatogastroenterology. 59:2016–2019.
2012.PubMed/NCBI
|
|
11
|
Anil G, Tan AG, Cheong HW, Ng KS and Teoh
WC: Emergency gastroduodenal artery embolization by sandwich
technique for angiographically obvious and oblivious, endotherapy
failed bleeding duodenal ulcers. Clin Radiol. 67:468–475. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanada Y, Kondo H, Goshima S, Kanematsu M,
Tanaka Y, Tokuyama Y, Osada S and Yoshida K: Liver abscess after
common hepatic artery embolization for delayed hemorrhage following
pancreaticoduodenectomy: A case report. Case Rep Med.
2010:2804302010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HG, Heo JS, Choi SH and Choi DW:
Management of bleeding from pseudoaneurysms following
pancreaticoduodenectomy. World J Gastroenterol. 16:1239–1244. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loffroy R and Guiu B: Arterial
embolization is the best treatment for pancreaticojejunal
anastomotic bleeding after pancreatoduodenectomy. World J
Gastroenterol. 15:4090–4091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nonokuma M, Okazaki M, Higashibara H,
Kimura S, Kora S, Urakawa H, Shinagawa Y, Osame A, Ueki T and
Nakayama T: Successful embolization of pancreaticoduodenal artery
pseudoaneurysm in a patient with common hepatic arterial occlusion
after modified pancreatoduodenectomy with preservation of arteries
in the head of pancreas. Hepatogastroenterology. 56:245–248.
2009.PubMed/NCBI
|
|
16
|
Santoro R, Carlini M, Carboni F, Nicolas C
and Santoro E: Delayed massive arterial hemorrhage after
pancreaticoduodenectomy for cancer. Management of a
life-threatening complication. Hepatogastroenterology.
50:2199–2204. 2003.PubMed/NCBI
|
|
17
|
Guy GE, Shetty PC, Sharma RP, Burke MW and
Burke TH: Acute lower gastrointestinal hemorrhage: Treatment by
superselective embolization with polyvinyl alcohol particles. AJR
Am J Roentgenol. 159:521–526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shinohara MM, Miller CJ and Seykora JT:
Pigmented fruiting bodies and birefringent crystals in a surgical
wound: A clue to Aspergillus niger infection. J Cutan Pathol.
38:603–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singhal P, Usuda K and Mehta AC: Post-lung
transplantation Aspergillus niger infection. J Heart Lung
Transplant. 24:1446–1447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fianchi L, Picardi M, Cudillo L, Corvatta
L, Mele L, Trapè G, Girmenia C and Pagano L: Aspergillus niger
infection in patients with haematological diseases: A report of
eight cases. Mycoses. 47:163–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aksoy DY, Turker A, Altundag MK, Abali H,
Durusu M, Erman M, Uner A, Sungur AA, Unal S and Uzun O:
Concomitant Mycobacterium tuberculosis and Aspergillus niger
infection in a patient with acute myeloid leukemia. Chemotherapy.
49:264–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vermeulen E, Maertens J, Meersseman P,
Saegeman V, Dupont L and Lagrou K: Invasive Aspergillus niger
complex infections in a Belgian tertiary care hospital. Clin
Microbiol Infect. 20:O333–O335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia-Vidal C and Carratalà J:
Pathogenesis of invasive fungal infections. Enferm Infecc Microbiol
Clin. 30:151–158. 2012.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|