Introduction
Bone homeostasis is regulated through the functions
of osteoclasts and osteoblasts (1–3). Bone is
a dynamic tissue constantly remodeled by the sequential removal
(bone resorption) of mature tissue by osteoclasts and its
replacement (bone formation) through the deposition of newly formed
mineralized matrix by osteoblasts (1–3).
Osteoclasts are derived from hematopoietic progenitor cells and
osteoblasts develop from bone marrow mesenchymal stem cells
(1,2). Bone homeostasis is maintained through
the actions of various hormones, cytokines and bone marrow
environmental systems (1–3). This disturbance induces bone loss
(1). Aging leads to osteoporosis
associated with a deterioration of bone mass through suppressed
bone formation and promoted bone resorption (4,5).
Osteoporosis is widely recognized as a major public health problem
(4,5). A notable manifestation of this disease
is fracture of the proximal femur, the incidence of which increases
as the population ages (4,5). Decreased bone mass in females is
primarily due to reduced secretion of estrogen following the
beginning of the menopause (5).
Osteoporosis is an important cause of morbidity and mortality in
elderly women. Development of a new supplemental strategy will be
useful in the prevention and treatment of osteoporosis.
Bisphosphonates are a group of drugs that have a
structural similarity to pyrophosphate, a high affinity for
mineralized tissue, and were developed as agents for inhibiting
osteoclastic bone resorption (6,7). These
drugs have often been used as the first treatment option for
osteoporosis since 1960s, when the first bisphosphonates were
developed as drugs for human use (6,7). There
are two main classes of bisphosphonates which differ in potency and
mode of action, namely the low potency, non-nitrogen-containing
bisphosphonates including coronate and ternate, and the more
commonly used higher potency, nitrogen-containing bisphosphonates
including alendronate, ibandronate and zoledronate (6,7).
Bisphosphonates are widely used for their multimodal bone-sparing
action to prevent and treat osteoporosis in postmenopausal women,
bone pain and hypercalcemia of malignancy (6,8,9). Oral bisphosphonates such as
alendronate, risedronate and etidronate are used beneficially to
reduce the risk of skeletal fractures in patients with osteoporosis
and in metastatic bone cancer (10).
However, recent studies have suggested that the inhibitory effects
of bisphosphonates on osteoclasts lead to impaired bone remodeling,
bisphosphonate-related osteonecrosis of the jaw, gastrointestinal
side effects and risk of cancer (11–13).
Botanical isoflavones, including daidzin, daidzein,
genistein and genistein, are contained at relatively high
concentrations in soybeans (14,15).
Daidzin and genistin are hydrolyzed to daidzein and genistein,
respectively, by β-glucosidase in the gastrointestinal tract
(14,15). Among isoflavones, genistein has been
demonstrated to have potent direct anabolic effects on bone
metabolism in vitro (14–20),
suggesting a role in the prevention of osteoporosis. Therefore, it
is hypothesized that genistein may be useful in the prevention and
treatment of osteoporosis.
The present study was undertaken to determine
whether the bisphosphonate alendronate and the isoflavone genistein
synergistically suppress osteoclastic differentiation using
preosteoclastic RAW267.4 cells in vitro. The combination of
alendronate and genistein was found to exhibit a synergistic
suppressive effect on osteoclastic differentiation in vitro.
Thus, such combinations may provide a new strategy for the
prevention and treatment of osteoporosis with reduced
bisphosphonate toxicity.
Materials and methods
Materials and cells
Dulbecco's Modification of Eagle's Medium (DMEM)
with 4.5 g/l glucose, L-glutamine, sodium pyruvate and antibiotics
[penicillin and streptomycin (P/S)] was purchased from Mediatech,
Inc. (Corning, Manassas, VA, USA). Fetal bovine serum (FBS) was
from Hyclone (GE Healthcare Life Sciences, Logan, UT, USA).
Alendronate, genistein, leukocyte acid phosphatase kits for
tartrate resistant acid phosphatase (TRAP) staining and all other
reagents were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) unless otherwise specified. The receptor activator of
nuclear factor-κB ligand (RANKL) was from R&D Systems, Inc.
(Minneapolis, MN, USA). Reagents were dissolved in 100% ethanol and
sterilized distilled water. Mouse monocytic RAW267.4 cells were
obtained from the American Type Culture Collection (Rockville, MD,
USA) (21,22).
Cell proliferation
RAW267.4 cells (1×105/ml cells/well; 2 ml
medium added per well in 24-well plates) were cultured in DMEM
containing 10% FBS and 1% P/S for 3 days in a water-saturated
atmosphere containing 5% CO2 and 95% air at 37°C
(23). The cells were then cultured
in DMEM containing 10% FBS and 1% P/S in the presence or absence of
vehicle (ethanol; final concentration 0.1%), alendronate (0.1, 1,
10 or 100 µM), genistein (0.1, 1, 10 or 100 µM), or alendronate
(0.1, 1, 10 or 100 µM) plus genistein (0.1, 1, 10, or 100 µM).
After culture, cells were detached from each culture dish and
counted. Following trypsinization of each of culture dish using
0.2% trpysin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C, cells
detached from the dish were collected following centrifugation at
150 × g and 4°C for 5 min (23).
Cells were resuspended in PBS solution and stained with eosin. Cell
numbers were counted under a microscope (Olympus MTV-3; Olympus
Corporation, Tokyo, Japan) using a hemocytometer plate. For each
dish, the average of two counts was calculated. Cell numbers are
presented as the number per well.
Cell death
RAW267.4 cells (1×105/ml cells/well; 2 ml
medium added per well in 24-well plates) were cultured in DMEM
containing 10% FBS and 1% P/S for 3 days when confluence was
reached (24). The cells were then
cultured for an additional 2 days in the presence or absence of
alendronate (0.1, 1, 10 or 100 µM), genistein (0.1, 1, 10 or 100
µM), or alendronate (0.1, 1, 10 or 100 µM) plus genistein (0.1, 1,
10 or 100 µM). After culture, cells were detached from each culture
dish and counted as described for the cell proliferation assay.
Osteoclastogenesis assays and TRAP
staining
RAW264.7 cells were cultured in 96-well plates in
DMEM supplemented with 10% FBS and 1% P/S at a density of
1×104 cells/well. Cells were cultured for 6 days with
RANKL (30 ng/ml) pre-incubated for 10 min with crosslinking
anti-poly-histidine antibody (2.5 µg/ml; IC050P; R&D Systems,
Inc., Minneapolis, MN, USA) to induce osteoclast formation
(21,22), in the presence or absence of vehicle
(ethanol; final concentration 0.1%), alendronate (0.1, 1, 10 or 100
µM), genistein (0.1, 1, 10 or 100 µM), or alendronate (0.1, 1, 10
or 100 µM) plus genistein (0.1, 1, 10 or 100 µM). After 6 days of
culture, the cells were fixed and stained for TRAP, a specific
marker of the osteoclast phenotype, using a leukocyte acid
phosphatase kit. Briefly, cells were washed with PBS, fixed with
10% neutralized formalin-phosphate (pH 7.2) for 10 min, dried and
stained with a leukocyte acid phosphatase kit (387A; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for staining of tartrate resistant
acid phosphatase (TRAP) at room temperature for 90 min.
TRAP-positive multinucleated cells (MNCs with at least three
nuclei) were considered to be osteoclast-like cells, and the cells
were counted using light microscopy (Olympus MTV-3). MNC scores are
expressed as the mean ± standard deviation of six cultures with two
replicate wells per data set using different dishes and cell
preparation.
Statistical analysis
Statistical analysis was performed using GraphPad
InStat software (version 3; GraphPad Software, Inc., La Jolla, CA,
USA). Multiple comparisons were performed by one-way analysis of
variance, followed by a post hoc Tukey's range test for parametric
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
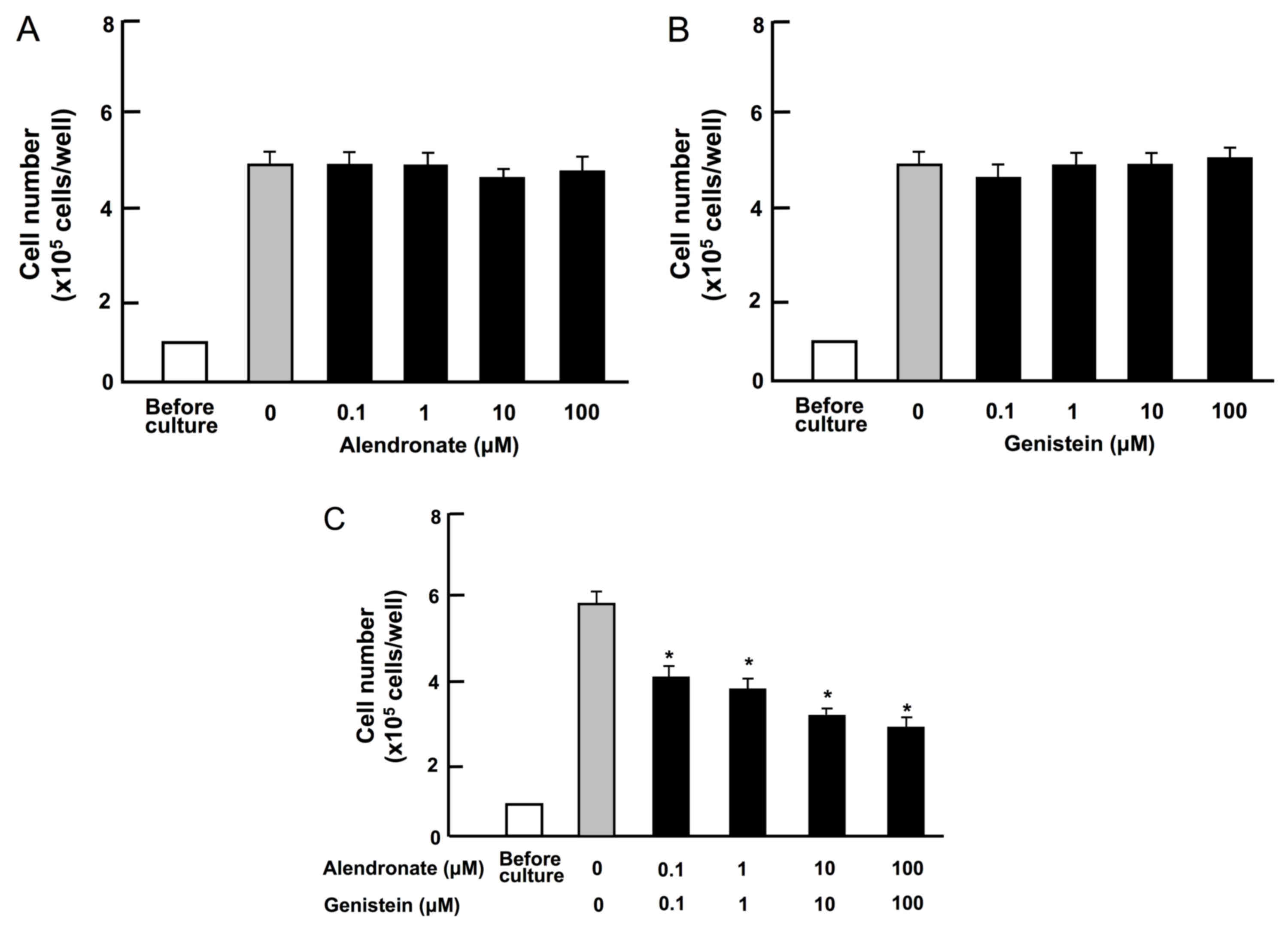
Combination of alendronate and
genistein reveals a synergistic suppressive effect on the
proliferation of RAW267.4 cells
The effects of the bone metabolism regulators
alendronate and genistein on the proliferation of RAW267.4 cells
in vitro were examined. RAW267.4 cells were cultured for 3
days in the presence or absence of each compound. Culture with
alendronate (0.1, 1.0, 10 and 100 µM) or genistein (0.1, 1.0, 10
and 100 µM) individually did not have a significant effect on the
proliferation of RAW267.4 cells as compared with that of the
control (0.1% ethanol vehicle; Fig. 1A
and B). Next, the effects of various combinations of
alendronate and genistein on the proliferation of RAW267.4 cells
in vitro were determined. Notably, the combinations of
alendronate and genistein with concentrations (0.1, 1.0, 10 and 100
µM) that did not independently reveal a significant effect on cell
proliferation were found to synergistically suppress cell
proliferation (Fig. 1C). Thus, the
combination of alendronate and genistein was shown to possess a
potent and synergistic suppressive effect on RAW267.4 cells in
vitro.
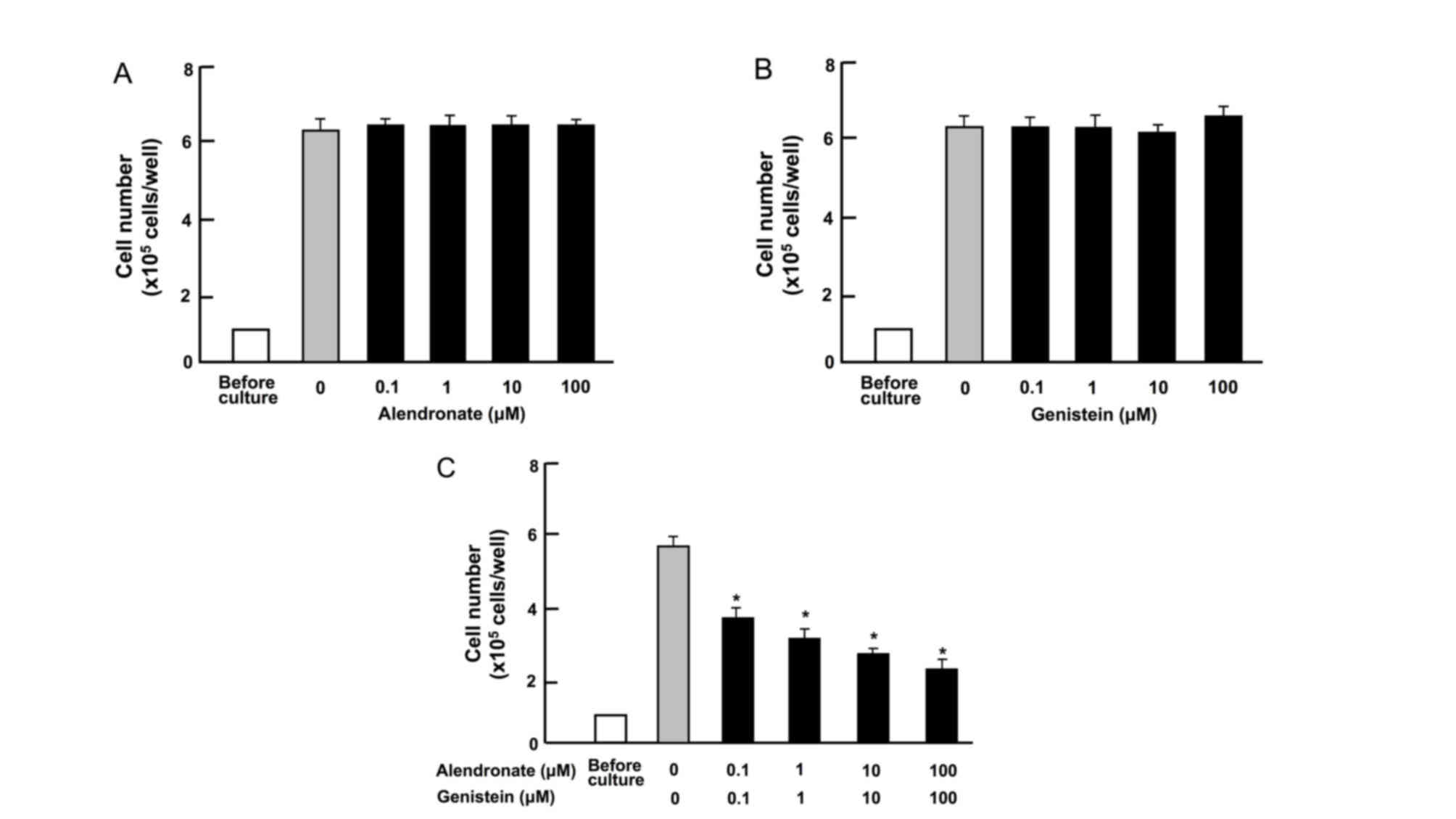
Combination of alendronate and
genistein synergistically stimulates the death of RAW267.4 cells in
vitro
The effects of alendronate and genistein on the
death of RAW267.4 cells in vitro were determined. Cells were
cultured for 3 days until they reached confluence, and then the
cells were additionally cultured for 2 days in the presence of 0.1%
ethanol, alendronate (0.1, 1.0, 10 and 100 µM) or genistein (0.1,
1.0, 10 and 100 µM). Alendronate (0.1, 1.0, 10 and 100 µM) or
genistein (0.1, 1.0, 10 and 100 µM) alone did not cause a
significant alteration in the number of RAW267.4 cells (Fig. 2A and B). However, combinations of
alendronate (0.1, 1.0, 10 and 100 µM) and genistein (0.1, 1.0, 10
and 100 µM) significantly reduced the cell number, indicating that
this combination treatment induces cell death (Fig. 2C).
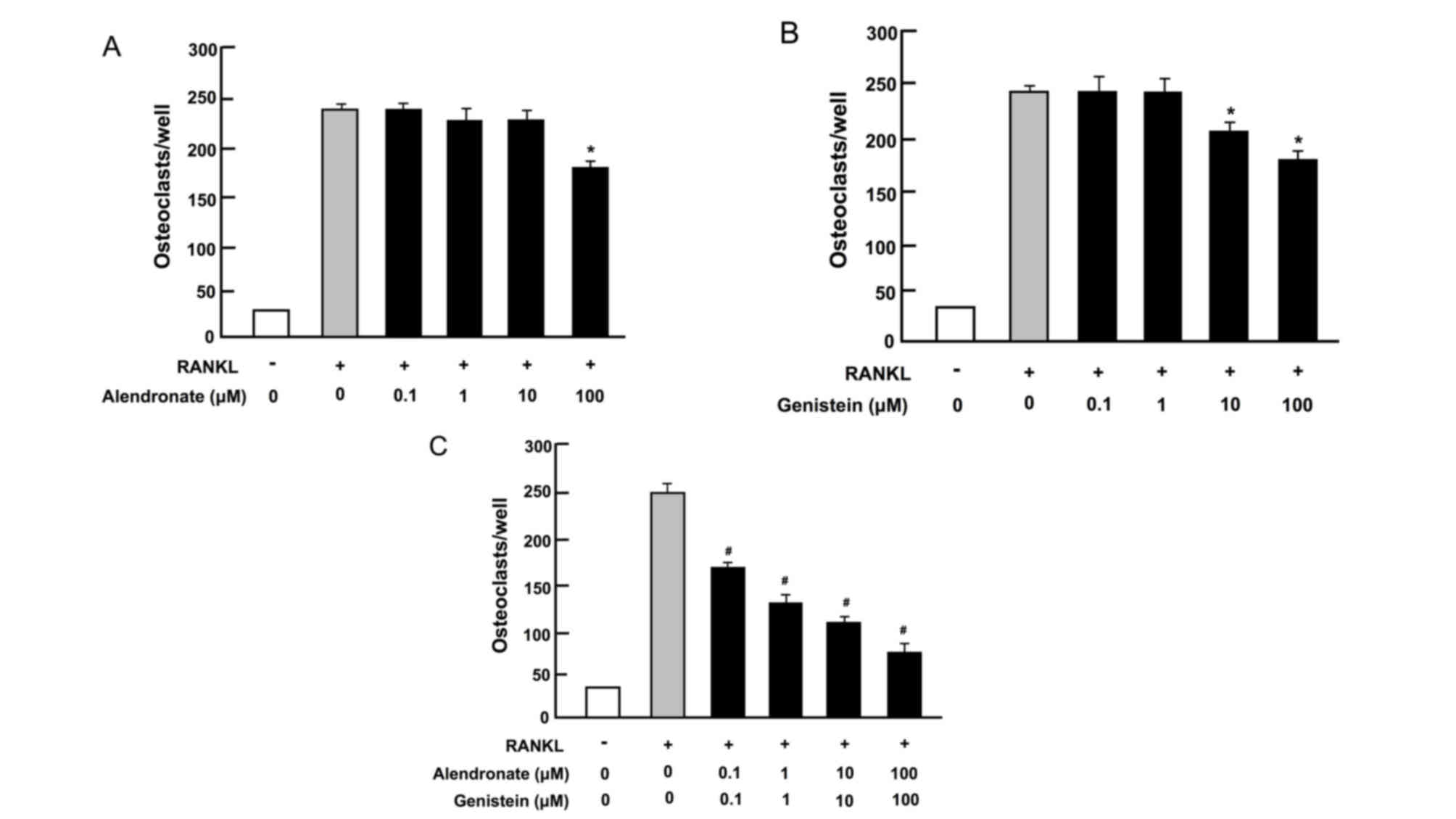
Combination of alendronate and
genistein synergistically suppresses osteoclast differentiation of
RAW267.4 cells in vitro
To establish an in vitro osteoclastogenesis
model suitable for investigation of the activity of combined
alendronate and genistein, RAW264.7 monocytic cells were induced to
differentiate into osteoclasts by the addition of the key
osteoclastogenic cytokine RANKL (2,3). The
effects of alendronate and genistein on osteoclast differentiation
were tested over a dose range from 0.1 to 100 µM; the cultures were
stained with TRAP 6 days later and osteoclast formation was
quantified. RANKL induced robust osteoclast formation (Fig. 3). Culture with alendronate (0.1, 1.0
and 10 µM) alone had no significant effect on the RANK-induced
enhancement of osteoclastic differentiation (Fig. 3A). Genistein (0.1 and 1.0 µM) also
did not reveal an effect on RANKL-stimulated osteoclastic
differentiation. However, this stimulatory effect of RANKL was
suppressed by addition of higher concentrations of alendronate (100
µM; Fig. 3A) and genistein (10 and
100 µM; Fig. 3B). Notably, the
combinations of alendronate (0.1, 1.0, 10 and 100 µM) and genistein
(0.1, 1.0, 10 and 100 µM) were found to exhibit synergistic
suppressive effects on the RANKL-induced enhancement of
osteoclastogenesis in RAW267.4 cells in vitro (Fig. 3C).
Discussion
The present study demonstrates the ability of
combination of alendronate and genistein to synergistically
suppress the proliferation and stimulate the death of
preosteoclastic RAW264.7 murine monocytic cells in vitro.
Moreover, the combination of alendronate and genistein was found to
synergistically suppress the osteoclastic differentiation enhanced
by RANKL, a previously demonstrated stimulator of osteoclastic
differentiation in RAW264.7 cells (21,22). To
the best of our knowledge, this is novel that has not been reported
previously, and it may provide a new strategy for the prevention
and treatment of bone deterioration induced by osteoclastic bone
resorption.
Alendronate and genistein when used separately did
not show significant effects on the proliferation and death of
RAW267.4 cells in vitro. Notably, the combination of the two
agents acted synergistically to suppress the proliferation and
increase the death of RAW267.4 cells, and subsequently suppress the
differentiation of preosteoclastic RAW267.4 cells to mature
osteoclasts. Furthermore, this combination exhibited a synergistic
suppressive effect on RANKL-induced osteoclastic differentiation as
compared with the effect of each agent. This suppressive effect on
osteoclastic differentiation may be partly based on the
proliferation-inhibiting and death-inducing effects of the
combination on RAW267.4 cells, which decreased the number of
preosteoclastic cells.
Direct effects of genistein on osteoclast precursor
differentiation (25,26) and mature osteoclasts (27) have shown that, in addition to
stimulating osteoblast function (20), genistein may protect bone by reducing
mature osteoclast formation. These effects were observed with
genistein concentrations of >10 µM (25–27).
Genistein has been shown to have a potent suppressive effect at the
later stage of RANKL-induced osteoclastic differentiation in mouse
bone marrow cultures (17).
Moreover, genistein has been shown to suppress bone resorption in
bone tissue culture (16). In the
present study, it was demonstrated that genistein negatively
regulates the RANKL-induced osteoclastic differentiation of
RAW264.7 cells in vitro.
Bisphosphonates have been shown to have a direct
suppressive effect on osteoclasts (6,7,28). This effect was observed with an
alendronate concentration of 100 µM, which induced the apoptosis of
rabbit osteoclasts in vitro (28). In the present study, combinations of
alendronate and genistein with lower concentrations were found to
synergistically inhibit the RANKL-induced osteoclastic
differentiation of RAW267.4 cells in vitro. These results
indicate that this combination may have a potent suppressive effect
on osteoclastic bone resorption. Genistein has been indicated to
exhibit suppressive effects at the later stage of osteoclastic
differentiation and stimulatory effects on the apoptosis of mature
osteoclasts (17,25–27), and
alendronate may directly stimulate the apoptosis of mature
osteoclasts (16). Thus, a
combination of alendronate and genistein is speculated to regulate
multiple steps associated with osteoclastogenesis and mature
osteoclasts. However, additional studies are required to elucidate
the mechanism.
There is evidence indicating that bisphosphonate
drugs have toxic effects, leading to impaired bone remodeling,
bisphosphonate-related osteonecrosis of the jaw, gastrointestinal
side effects and risk of cancer (11–13). The
present study demonstrated that a combination comprising a
relatively low effective dose of a bisphosphonate-type drug and
genistein exhibits an inhibitory effect on osteoclastic
differentiation. Furthermore, genistein possesses a potent
stimulatory effect on osteoblastic bone formation in vitro
and in vivo (15,16). The current study findings may provide
a new strategy for the prevention and treatment of osteoclastic
bone loss in osteoporosis. Combining alendronate with genistein may
be useful for reducing the toxicity of the bisphosphonate drug, and
may provide potent therapeutic effects.
In conclusion, the present study demonstrates that
combinations of alendronate and genistein exhibit potent and
synergistic suppressive effects on the osteoclastic differentiation
of preosteoclastic RAW267.4 cells in vitro. These
combinations could potentially inhibit osteoclastic bone
resorption. However, further studies are required to investigate
the clinical aspects.
References
|
1
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone remodeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaidi M, Blair HC, Moonga BS, Abe E and
Huang CL: Osteoclastogenesis, bone resorption, and osteoclast-based
therapeutics. J Bone Miner Res. 18:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chambers TJ and Fuller K: How are
osteoclasts induced to resorb bone? Ann N Y Acad Sci. 1240:1–6.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper C and Melton LJ III: Epidemiology
of osteoporosis. Trends Endocrinol Metab. 3:224–229. 1995.
View Article : Google Scholar
|
|
5
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fleisch H: Bisphosphonates: Mechanism of
action. Endocr Rev. 19:80–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mottaghi P: Intravenous bisphosphonates
for postmenopausal osteoporosis. J Res Med Sci. 15:175–184.
2010.PubMed/NCBI
|
|
8
|
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G
and Naujoks C: The impact of compliance with osteoporosis therapy
on fracture rates in actual practice. Osteoporos Int. 15:1003–1008.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng Z, Zeng S, Wang Y, Zheng Z and Chen
Z: Bishosphonates for the prevention and treatment of osteoporosis
in patients with rheumatic diseases: A systematic review and
meta-analysis. PLoS One. 8:e808902013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Newcomb PA, Trentham-Dietz A and Hampton
JM: Bisphosphonates for osteoporosis treatment are associated with
reduced breast cancer risk. Br J Cancer. 102:799–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh YH, Yoon C and Park SM: Bisphosphonate
use and gastrointestinal tract cancer risk: Meta-analysis of
observational studies. World J Gastroentero. 18:5779–5788. 2012.
View Article : Google Scholar
|
|
12
|
Faiman B, Pillai AL and Benghiac AG:
Bisphosphonate-related osteonecrosis of the Jaw: Historical,
ethical, and legal issues associated with prescribing. J Adv Pract
Oncol. 4:25–35. 2013.PubMed/NCBI
|
|
13
|
Cardwell CR, Abnet CC, Veal P, Hughes CM,
Cantwell MM and Murray LJ: Exposure to oral bisphosphonates and
risk of cancer. Int J Cancer. 131:E717–E725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi M and Gao-Balch YH: Role of
dietary soybean genistein in osteoporosis prevention. Int J Food
Sci Nutr Diet. 2:27–34. 2013. View Article : Google Scholar
|
|
15
|
Yamaguchi M: Nutritional factors and bone
homeostasis: Synergistic effect with zinc and genistein in
osteogenesis. Mol Cell Biochem. 366:201–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi M and Gao YH: Inhibitory effect
of genistein on bone resorption in tissue culture. Biochem
Pharmacol. 55:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao YH and Yamaguchi M: Inhibitory effect
of genistein on osteoclast-like cell formation in mouse marrow
cultures. Biochem Pharmacol. 58:767–772. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao YH and Yamaguchi M: Anabolic effect of
daizein on cortical bone in tissue culture: Comparison with
genistein effect. Mol Cell Biochem. 194:93–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M and Sugimoto E: Stimulatory
effect of genistein and daizein on protein synthesis in
osteoblastic MC3T3-E1 cells: Activation of aminoacyl-tRNA
synthetase. Mol Cell Biochem. 214:97–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi M and Weitzmann MN: The estrogen
17beta-estradiol and phytoestrogen genistein mediate differential
effects on osteoblastic NF-kappaB activity. Int J Mol Med.
23:297–301. 2009.PubMed/NCBI
|
|
21
|
Yamaguchi M and Weitzmann MN: The intact
strontium ranelate complex stimulates osteoblastogenesis and
suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol
Cell Biochem. 359:399–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi M and Weitzmann MN: Vitamin K2
stimulates osteoblastogenesis and suppresses osteoclastogenesis by
suppressing NF-κB activation. Int J Mol Med. 27:3–14.
2011.PubMed/NCBI
|
|
23
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
García Palacios V, Robinson LJ, Borysenko
CW, Lehmann T, Kalla SE and Blair HC: Negative regulation of
RANKL-induced osteoclastic differentiation in RAW264.7 cells by
estrogen and phytoestrogens. J Biol Chem. 280:13720–13727. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW267.4 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao YH and Yamaguchi M: Suppressive effect
of genistein on rat bone osteoclasts: Involvement of protein kinase
inhibition and protein tyrosine phosphatase activation. Int J Mol
Med. 5:261–267. 2000.PubMed/NCBI
|
|
28
|
Sutherland KA, Rogers HL, Tosh D and
Rogers MJ: RANKL increases the level of Mcl-1 in osteoclasts and
reduces bisphosphonate-induced osteoclast apoptosis in vitro.
Arthritis Res Ther. 11:R582009. View
Article : Google Scholar : PubMed/NCBI
|