Introduction
Pyogenic hepatic abscess (PHA) is a rare, but
potentially life-threatening condition. Population-based studies
have reported that the annual average incidence rates of PHA range
between 2.3 and 3.6 cases per 100,000 individuals, and the
in-hospital case-fatality rates range between 0.0 and 10.0% in
western countries (1–5). In China, the annual average incidence
rate is 11.9 per 100,000 individuals and the in-hospital
case-fatality rates range between 2.1 and 11.7% (6–9). At
present, ultrasound (US) or computed tomography-guided percutaneous
needle aspiration (PNA) or catheter drainage (PCD) is appropriate
as a first-line treatment based on systemic antibiotic therapy
(5,8,10–15).
Although PNA or PCD are equally safe and effective for managing
PHA, it remains unclear which procedure should be preferred.
However, it is difficult to aspirate or drain pus and to select the
appropriate antibiotic treatment in cases where the abscess has
thick pus and polymicrobial co-infection, or its pathogenic
bacterium is multidrug resistant and cryptogenic in origin
(8,16,17).
Absolute alcohol (AA) has the functions of
dehydration and fixation. AA has been demonstrated to unselectively
cause coagulation necrosis to human and Echinococcus
granulosus cells, while it also inactivates the inflammatory
mediators and toxins secreted. AA has been demonstrated to
unselectively cause coagulation necrosis to human and
Echinococcus granulosus cells, while it has also been
demonstrated to inactivate the inflammatory mediators and toxins
secreted (18–22). Therefore, small hepatocellular
carcinoma (18–20), viable hydatid liver cyst (21) and benign hepatic cyst (16,22) can
all be safely and effectively managed with alcoholization.
To the best of our knowledge, alcoholization as a
possible treatment for PHA has previously been reported in three
studies, including two cases with chronic granulomatous disease
treated by alcohol instillation (23,24) and
a study reporting the treatment in animals by alcohol infusion
(25). The alcoholization may result
in complete resolution of the abscess, since it resolves the issues
of aspiration of thick pus and selection of the appropriate
antibiotic treatment (23–28). However, its efficacy and safety
should be further evaluated.
The present study established a PHA model in Bama
minipigs (29), and the animals were
then prospectively treated with the alcoholization. The results
demonstrated that the alcoholization is a safe, effective and
well-tolerated method to manage PHA.
Materials and methods
Animals
A total of 6 Chinese clinically healthy Bama
minipigs (2 females and 4 males; mean body weight, 23.8±4.1 kg;
weight range, 19–29 kg) were used in the present study. The
minipigs were 9–10 weeks of age and purchased from Animal Science
and Technology of Guangxi University (Nanning, China). Under
specific pathogen-free (SPF) conditions, the animals were kept at
21°C in a humidity of 50%, with a 12 h light/dark cycle from 6:00
to 18:00 and fed with combination diet of 0.36 kg food/kg weight
with ad libitum access to water. The study was performed in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of the Chinese PLA
General Hospital (Beijing, China).
Percutaneous transhepatic
alcoholization procedure
Subsequent to exposing the abdominal cavity of the
animals, a mixture of methicillin-sensitive S. aureus (ATCC
29213; American Type Culture Collection, Manassas, VA, USA) and
autologous venous blood clot was quickly injected into the liver
parenchyma. Autologous venous blood was acquired from the splenic
vein when the animal abdominal cavity was exposed and autologous
venous blood clot was acquired when the blood was automatically
coagulated, as previously described (29). After day 21, the animals were
diagnosed with PHA and were at abscess-formation stage (29). Next, they were randomly allocated
into two groups (15), including the
alcoholization treatment and the physiological saline (PS) control
groups.
Food was withdrawn 24 h prior to the intervention
with AA. The mini pigs were sedated (29) using intramuscular injection of a
solution containing a mixture of 0.83 mg/kg body weight of
zolazepam, tiletamine, xylazine and ketamine and 0.17 mg/kg body
weight butorphanol [all purchased from Department of Pharmacy,
Chinese PLA General Hospital (Beijing, China)]. The body weight and
rectal temperature were respectively measured with a health meter
and mercury thermometer. Venous blood was obtained from the vena
cava anterior in order to examine the white blood cell (WBC) count;
a catheter (22G) was inserted into the right ear vein to infuse an
anesthetic (29).
All animals were placed in dorsal recumbency, and
the abdominal area was clipped and prepared aseptically.
Intervention was performed with AA in the alcoholization group. PS
was used in the other group, which acted as a negative control. All
animals were sacrificed by exsanguination through the femoral
artery under full sedation using intramuscular injection of a
solution containing a mixture of zolazepam, tiletamine, xylazine
and ketamine (0.83 mg/kg BW of each drug) and butorphanol (0.17
mg/kg BW) (29). After 21 days of
the alcoholization, autopsy was performed for assessing the
therapeutic efficacy of the treatment. Samples from the caseous pus
of the hepatic abscess were assessed for the bactericidal effect of
AA by Gram staining, bacterial cultivation, polymerase chain
reaction (PCR) identification and histopathology (29).
Percutaneous transhepatic US-guided
interventions
All percutaneous interventions were performed under
color Doppler US guidance with an Aloka SSD-650 machine and a 3.5
MHz curvilinear transducer (Hitachi Aloka, Tokyo, Japan). A
free-hand technique using an 18G × 20 cm disposable PTC needle
(Hakko, Nagano-Ken, Japan) was employed for puncturing the
abscesses.
The interventions were performed with the PTC needle
attached to a 10-ml syringe. The needle was introduced into the
abscess cavity through normal hepatic tissue at a depth of at least
1 cm. When the tip of the needle was in the lesion, as much as
possible of the purulent material was removed. If the abscess
material was not drained at all, then alcoholization was performed
by slowly instilling sterile AA (≥99.7%; 6.17±0.753 ml) until the
lesion progressively became diffuse hyperechogenic (23,24). The
procedure was repeated 7 days later when required.
Bactericidal effect of AA
Samples from the caseous pus of the hepatic abscess
were assessed for the bactericidal effect of AA by Gram staining,
bacterial cultivation, PCR identification and histopathology
(29). The bactericidal effect of AA
was also assessed by cultural method in AA or penicillin
solution.
First caseous pus of mung bean from a PHA was spread
on glass slides and subjected to Gram staining following
air-drying. Caseous pus was inoculated into LB broth and incubated
overnight at 37°C with agitation (200 rpm). The bacterial
suspension of 0.1 ml was inoculated on an LB agar plate and
immovably incubated overnight at 37°C. The bacterial precipitate
was generated by the centrifugation of 3 ml bacterial suspension
for 10 min at 4°C and 4,000 × g then the chromatin DNA was
extracted using the E.Z.N.A.® Bacterial DNA kit (Omega
Bio-Tek, Inc., Norcross, GA, USA) and lysostaphin (Sangon Biotech,
Co., Ltd., Shanghai, China) following the manufacturers protocol,
as previously described (29). The
primer pairs of the thermostable nuclease A gene (nucA)
were, 5′-GCGATTGATGGTGATACGGTT-3′ and
5′-AGCCAAGCCTTGACGAACTAAAGC-3′. The cycling conditions were as
follows: 94°C for 45 sec followed by 30 cycles at 94°C for 30 sec,
55°C for 30 sec and 72°C for 60 sec; 72°C for 120 sec and held at
4°C. The desired fragment length was 279 bp following PCR. S.
aureus strains ATCC 29213 served as positive controls, and
deionized water served as the negative control. All samples were
analyzed in triplicate.
The samples of PHA were fixed for 24 h with 4%
formaldehyde in phosphate-buffered saline, and then processed
through graded concentrations of ethanol (75, 85, 95 and 100%) and
xylene, embedded in paraffin wax, cut into 3–5 µm sections,
rehydrated and finally stained with hematoxylin and eosin
(H&E).
Caseous pus was then respectively cultured in AA and
penicillin solutions (diluted with LB broth) overnight at 37°C with
agitation (200 rpm) and observed. A bacterial suspension of 0.1 ml
was inoculated into the LB agar plate and incubated overnight at
37°C and observed.
Outcome measurement
A successful percutaneous intervention was defined
as improvement with decreased temperature if the animals initially
had fever, decreased WBC if they presented leukocytosis (30), and total or partial organization of
purulent material as observed by pathology.
Statistical analysis
Statistical analysis was performed with SPSS
statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA).
All continuous variables are expressed as the mean ± standard
deviation. Categorical variables are reported as percentages.
Quantitative variables were compared by one-way analysis of
variance (ANOVA) and the F-test, while multiple comparisons were
executed by one-way ANOVA and the least significant difference
method. All significance tests were two-tailed, and differences
with P<0.05 were considered to be statistically significant.
Results
Percutaneous transhepatic
alcoholization in Bama minipig with PHA
During PHA treatment with AA or PS all animals
presented anorexia, however none had pyrexia. Leukocytosis was
identified in all animals treated with PS (control group). Normal
reference values of rectal temperature (RT) and WBC count in
minipigs are 38.0–39.5°C and 7.53–16.82×109/l
respectively (31).
The average mean WBC count when the animals were
treated for 7 days with PS was significantly higher compared with
the healthy state, being the WBC counts prior to the experiment
[P=0.015; 95% confidence interval (CI) of 4.05–31.35] and that of
animals treated for 14 days with AA (P=0.034; CI, 1.64–35.06). The
WBC count of the six minipigs under the healthy state was
respectively 12.7, 7.3, 7.9, 7.0, 8.3 and 13.6×109/l.
The mean was 9.467×109/l and standard deviation was
2.903. No significant difference was observed between PS and AA on
day 7 of treatment (P=0.055). In addition, the average WBC count in
animals treated for 14 days with PS was significantly higher
compared with the healthy state value (P=0.012; CI, 4.80–32.10) and
that of animals treated for 7 and 14 days with AA [P=0.046 (CI,
0.39–33.81) and P=0.028 (CI, 2.39–35.81), respectively]. However,
the average body weight (F=1.028, P=0.434) and the abscess size
(F=1.116, P=0.411) were not significantly different between the two
groups (Fig. 1).
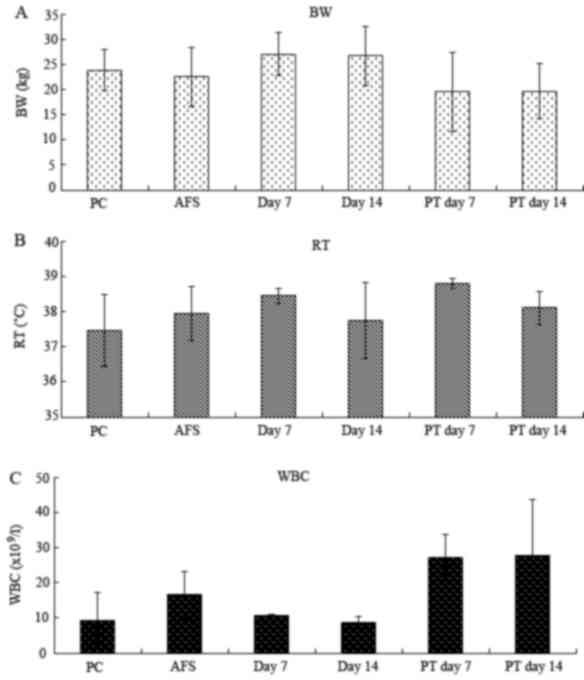 | Figure 1.Changes in (A) BW, (B) RT and (C) WBC
in Bama minipig with PHA subsequent to management by
ultrasound-guided instillation with PS or AA. The WBC in PS-treated
minipigs was significantly increased compared with the normal
physiological condition and AA-treated minipigs (P<0.05).
However the difference between PS and AA on day 7 of treatment was
not significant (P>0.05). However, BW and RT were not
significantly different between the two groups (P=0.484 and
P=0.434, respectively). WBC counts of day 7 of PS treatment vs. PC
and day 14 of AT. WBC counts of day 14 of PS treatment vs. PC, day
7 and 14 of AT. BW, body weight; RT, rectal temperature; WBC, white
blood cell count; PS, physiological saline; AA, absolute alcohol;
PT, PS-treated minipigs; PC, normal physiological condition; AT,
AA-treated minipigs. |
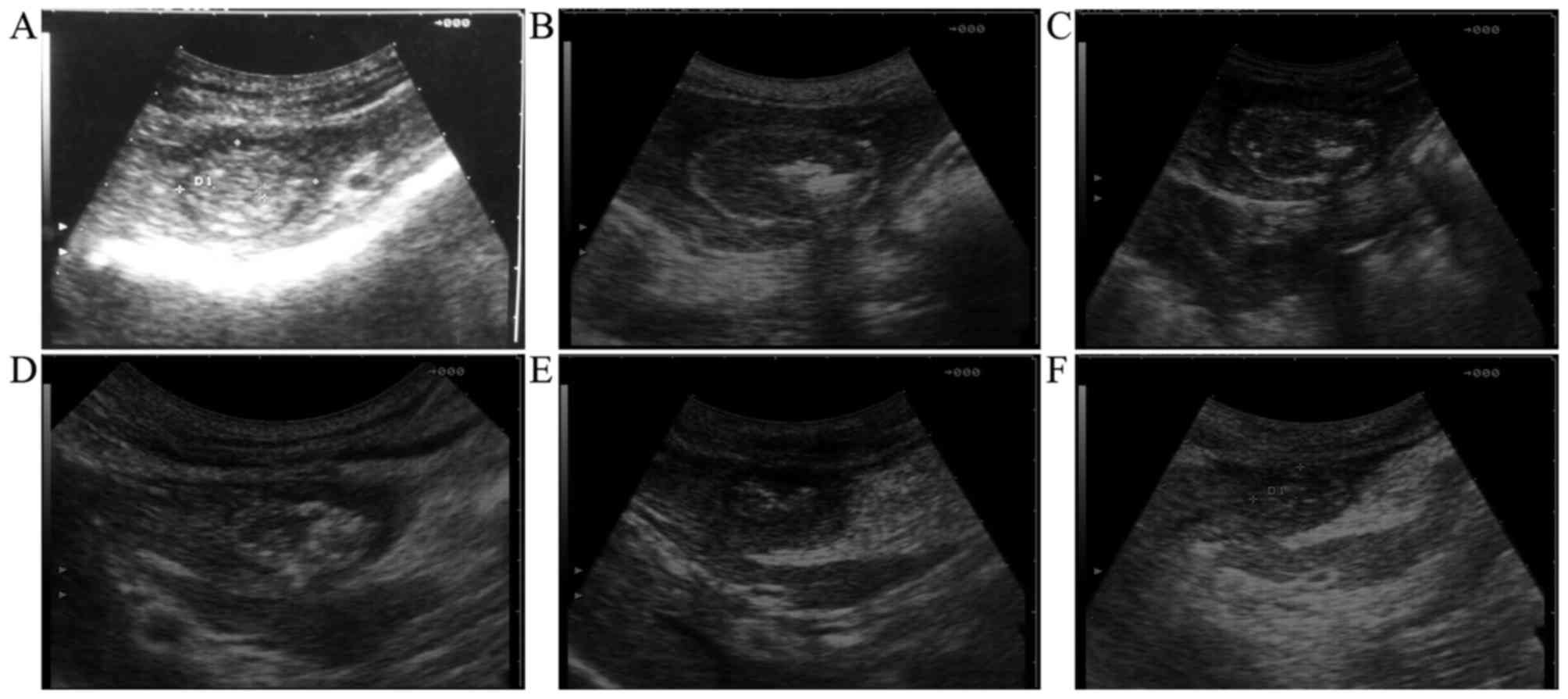
As observed, all 6 animals had a single lesion
(cross section, 4.86±2.47 cm2) at the left hepatic lobe,
which included round to oval mixed echogenicity with a ring of
hypoechogenic liver edema surrounding the lesion. On day 7
following the therapeutic procedure, the ring of hypoechogenic
liver edema disappeared in the AA group not in the PS group
(Fig. 2). On day 14 following the
therapeutic procedure, the mixed echogenicity remained apparent,
but the ring of hypoechogenic liver edema had disappeared in the
two groups. The autopsy results subsequent to sacrifice on day 21
revealed that the abscess wall attached to thick gray-yellow pus
was thin, pale and tenacious (Fig.
3A). Histopathology indicated that the abscess wall was
compact, thick and even, and its WBC count was lower in the AA
group. The abscess wall was all lax, thin and uneven, and its WBC
count was more in the PS group.
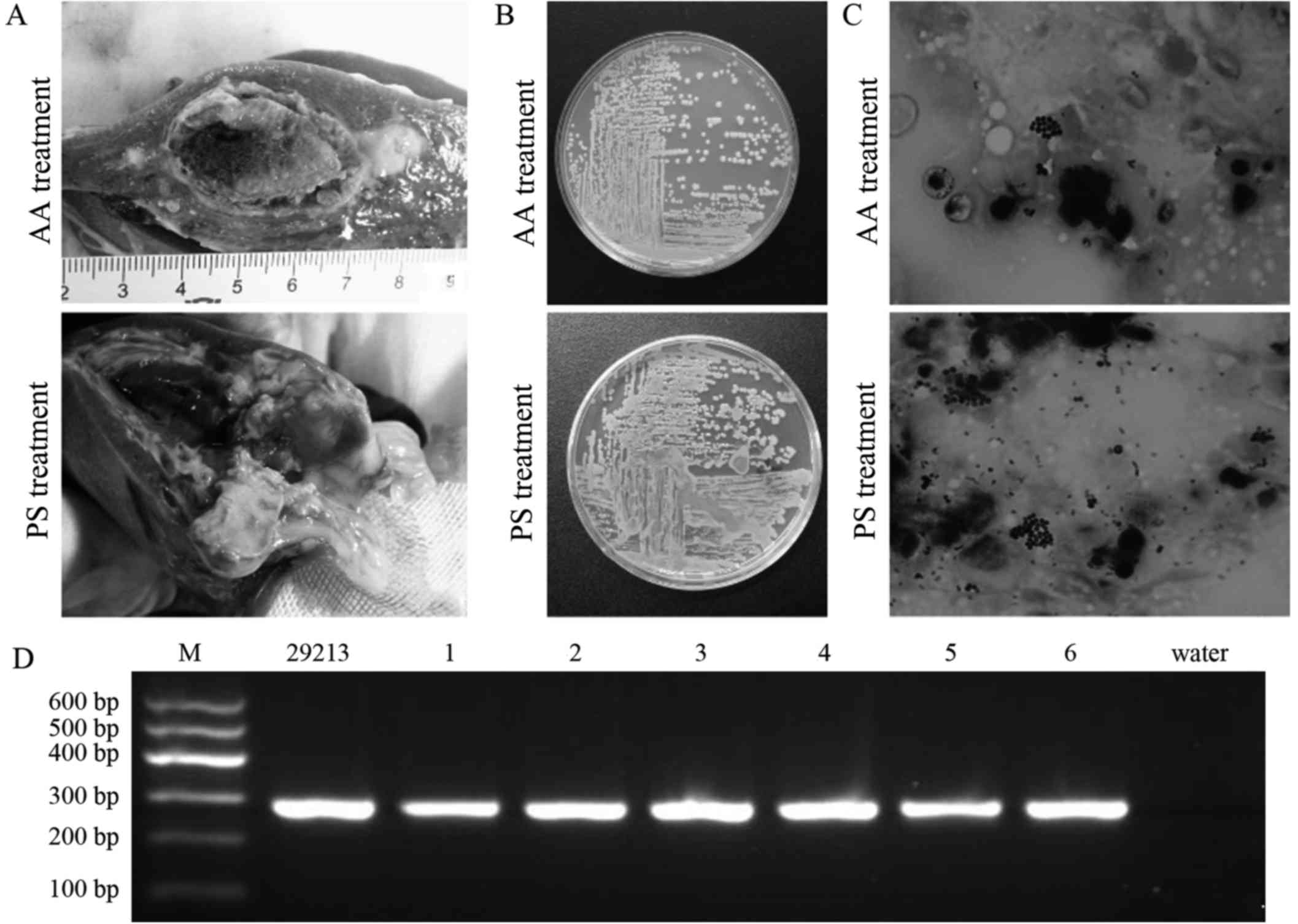 | Figure 3.Results obtained from autopsy, pus
culture, Gram staining and PCR identification in Bama minipig with
PHA. (A) Thick gray-yellow pus attached to thin and pale abscess
wall, with fully organized abscess content at 14 days after
alcoholization (cross section, 2.5×2.0 cm) and partly organized
abscess content at 14 days after treatment with PS (cross section,
2.1×1.6 cm). (B) Typical colonies of S. aureus identified
following culture of caseous pus on LB agar plate at 14 days after
alcoholization or PS treatment. (C) Gram-positive thyrsiform cocci
identified in caseous pus by Gram staining at 14 days after
alcoholization or PS treatment, with a higher number of cocci
observed in the PS treatment group (magnification, ×1,000). (D)
Pathogenic bacteria of PHA identified by PCR with nucA
oligonucleotide primers. From left to right, the gel shows the
results for standard molecular marker (M), S. aureus ATCC
29213, minipigs 1–6 and deionized water. PHA, pyogenic hepatic
abscess; PS, physiological saline; AA, absolute alcohol; PCR,
polymerase chain reaction. |
Bactericidal effects of AA
S. aureus bacteria from the caseous pus of
the hepatic abscess were killed following incubation in AA for 20
and 30 min in AA; however, the bacteria were still alive when
incubated for 10 min in AA or in penicillin G sodium (9.6 mg/ml in
PS). The color of the suspension was evenly yellowish after the
caseous pus was inoculated into the Luria-Bertani (LB) broth and
incubated at 37°C overnight with agitation. Typical colonies of
S. aureus were identified after the bacterial suspension was
inoculated into the LB agar plate and incubated overnight.
Typical colonies of S. aureus were observed
following the caseous pus of the hepatic abscess, on day 14 of
alcoholization or PS treatment cultured in LB broth then on an LB
agar plate (Fig. 3B). Numerous
Gram-positive thyrsiform cocci were detected after the caseous pus
of the liver abscess was smeared on glass slides and Gram staining
was performed (Fig. 3C). In
addition, the desired fragment was identified following PCR
amplification (Fig. 3D). According
to histopathological analysis results, the abscess contents were
organized fully in 2 minipigs and partially in 1 minipig in the AA
group, and fully in 1 minipig and partially in 2 minipigs in the PS
group.
The success rate of PHA treatment was 100% in the AA
group subsequent to alcoholization twice, with full or partial
organization of the abscess materials and normal WBC count.
However, none of the animals was consistent with the criteria of
successful percutaneous intervention in the PS group. No
complications, such as hepatic hemorrhage, hepatonecrosis and
mortality, were detected in any of the animals during AA or PS
treatment.
Discussion
Image-guided PNA or PCD based on systemic antibiotic
therapy is currently the preferred first-line management over
surgical drainage, as in open or laparoscopic drainage, treating
liver abscesses in the majority cases. However, it is difficult to
aspirate or drain pus, and to select the appropriate antibiotic
treatment when the abscess has thick pus and polymicrobial
co-infection, or its pathogenic bacteria are multidrug resistant
and cryptogenic in origin (8,16,17).
Thick pus is a known characteristic in the Bama
minipig PHA model and cannot be directly aspirated (29). Therefore, AA instillation was
selected in the present study for treating PHA. Subsequent to
instillation of AA twice, the abscess contents were fully or
partially organized, and the WBC content was restored to the normal
values. These results suggested that AA may not only kill the super
and inner bacteria in the caseous pus, but also inactivate their
toxins and inflammatory mediators secreted. Although the
achievement ratio of treatment was 100%, S. aureus bacteria
were identified in the caseous pus of all minipigs, along with a
number of Gram-positive thyrsiform cocci, when cultured
microbiologically. Furthermore, S. aureus bacteria in the
caseous pus were not completely killed after incubation in AA for
10 min. Thus, the experimental results of the present study
suggested that AA can only perform limited osmosis and bactericidal
effect in the caseous pus (17).
The body weight and the size of the abscess cavity
were not significantly different between the AA and PS groups
subsequent to the alcoholization, which may be associated with no
systemic antibiotic therapy (23–25,30). The
rectal temperature was not significantly different between the two
treatment groups, which was possibly associated with the cold and
dry ambient air (26°C) in the operating room and the applied
general anesthesia, since the anesthesia and surgical procedure can
synergistically interfere with normal thermoregulation (32).
During the alcoholization, the procedure was well
tolerated in the minipigs. Following the alcoholization, there were
no alcoholic adverse effects, procedure-associated complications or
mortality observed in the minipigs. Therefore, the present study
suggests that the US-guided percutaneous alcoholization is a safe
and effective procedure for PHA treatment, as also stated in
previous reports (23–25).
In the present study, the criteria for determining a
successful intervention were met following alcoholization with AA
rather than with 95% alcohol, as used previously (23–25). AA
was used in the current experiments since the thick pus from PHA
was not drained at all. In order to avoid the attenuation of
alcohol density injected and obtain the maximal function of
dehydration and fixation (22), PHA
was managed with AA rather than 95% alcohol.
The present study has three limitations. Firstly,
measuring the temperature of minipigs was difficult since they were
easily agitated. Thus, a more simple and accurate method to record
the temperature, such as biochips, should be used in future
studies. Furthermore, the present results should be confirmed in
study including a larger number of Bama minipigs. Finally,
significantly different results could be obtained between the AA
and PS groups if the observable period was longer and systemic
antibiotic therapy was added. These shortcomings may be addressed
in upcoming studies.
In conclusion, US-guided percutaneous alcoholization
is a safe and effective procedure to manage PHA. The alcoholization
may shorten the treatment period and become a new method to manage
PHA.
Acknowledgements
The current research was supported by the China
Postdoctoral Science Foundation (grant no. 20100471817) and Army
11th Five Important Special Financial Assistance (grant no.
08Z037).
References
|
1
|
Kaplan GG, Gregson DB and Laupland KB:
Population-based study of the epidemiology of and the risk factors
for pyogenic liver abscess. Clin Gastroenterol Hepatol.
2:1032–1038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meddings L, Myers RP, Hubbard J, Shaheen
AA, Laupland KB, Dixon E, Coffin C and Kaplan GG: A
population-based study of pyogenic liver abscesses in the United
States: Incidence, mortality, and temporal trends. Am J
Gastroenterol. 105:117–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Farrell N, Collins CG and McEntee GP:
Pyogenic liver abscesses: Diminished role for operative treatment.
Surgeon. 8:192–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alkofer B, Dufay C, Parienti JJ, Lepennec
V, Dargere S and Chiche L: Are pyogenic liver abscesses still a
surgical concern? A Western experience. HPB Surg. 2012:3160132012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heneghan HM, Healy NA, Martin ST, Ryan RS,
Nolan N, Traynor O and Waldron R: Modern management of pyogenic
hepatic abscess: A case series and review of the literature. BMC
Res Notes. 4:802011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Wang S, Jacob R, Fan Z, Zhang F and
Ji G: A 10-year retrospective analysis of clinical profiles,
laboratory characteristics and management of pyogenic liver
abscesses in a chinese hospital. Gut Liver. 5:221–227. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lok KH, Li KF, Li KK and Szeto ML:
Pyogenic liver abscess: Clinical profile, microbiological
characteristics, and management in a Hong Kong hospital. J
Microbiol Immunol Infect. 41:483–490. 2008.PubMed/NCBI
|
|
8
|
Chen SC, Tsai SJ, Chen CH, Huang CC, Lin
DB, Wang PH, Chen CC and Lee MC: Predictors of mortality in
patients with pyogenic liver abscess. Neth J Med. 66:196–203.
2008.PubMed/NCBI
|
|
9
|
Tsai FC, Huang YT, Chang LY and Wang JT:
Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect
Dis. 14:1592–1600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mezhir JJ, Fong Y, Jacks LM, Getrajdman
GI, Brody LA, Covey AM, Thornton RH, Jarnagin WR, Solomon SB and
Brown KT: Current management of pyogenic liver abscess: Surgery is
now second-line treatment. J Am CollSurg. 210:975–983. 2010.
|
|
11
|
Balint TD, Bailey BM, Mendelson KG and
Pofahl W: Hepatic abscess: Current concepts in diagnosis and
treatment. Curr Surg. 58:381–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferraioli G, Garlaschelli A, Zanaboni D,
Gulizia R, Brunetti E, Tinozzi FP, Cammà C and Filice C:
Percutaneous and surgical treatment of pyogenic liver abscesses:
Observation over a 21-year period in 148 patients. Dig Liver Dis.
40:690–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung YF: Pyogenic liver
abscess-predicting failure to improve outcome. Neth J Med.
66:183–184. 2008.PubMed/NCBI
|
|
14
|
Yu SC, Ho SS, Lau WY, Yeung DT, Yuen EH,
Lee PS and Metreweli C: Treatment of pyogenic liver abscess:
Prospective randomized comparison of catheter drainage and needle
aspiration. Hepatology. 39:932–938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onder A, Kapan M, Böyük A, Gümüş M, Tekbaş
G, Girgin S and Tacyildiz IH: Surgical management of pyogenic liver
abscess. Eur Rev Med Pharmacol Sci. 15:1182–1186. 2011.PubMed/NCBI
|
|
16
|
Lohela P: Ultrasound-guided drainages and
sclerotherapy. Eur Radiol. 12:288–295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laborda A, De Gregorio MA, Miguelena JM,
Medrano J, Gómez-Arrue J, Serrano C, de Blas I, Gimenez M and
D'Agostino H: Percutaneous treatment of intrabdominal abscess:
Urokinase versus saline serum in 100 cases using two surgical
scoring systems in a randomized trial. Eur Radiol. 19:1772–1779.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiina S, Tateishi R, Imamura M, Teratani
T, Koike Y, Sato S, Obi S, Kanai F, Kato N, Yoshida H, et al:
Percutaneous ethanol injection for hepatocellular carcinoma:
20-year outcome and prognostic factors. Liver Int. 32:1434–1442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahnken AH, Bruners P and Günther RW:
Local ablative therapies in HCC: Percutaneous ethanol injection and
radiofrequency ablation. Dig Dis. 27:148–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SR, Imoto S, Nakajima T, Ando K, Mita
K, Taniguchi M, Sasase N, Matsuoka T, Kudo M and Hayashi Y:
Well-differentiated hepatocellular carcinoma smaller than 15 mm in
diameter totally eradicated with percutaneous ethanol injection
instead of radiofrequency ablation. Hepatol Int. 3:411–415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giorgio A, Tarantino L, de Stefano G,
Francica G, Esposito F, Perrotta A, Aloisio V, Farella N,
Mariniello N, Coppola C and Caturelli E: Complications after
interventional sonography of focal liver lesions: A 22-year
single-center experience. J Ultrasound Med. 22:193–205. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan-Hong F, Lin-Xue Q, Hai-Ma G, Qing Z,
Yu G and Xiangdong H: Sclerotherapy of simple hepatic cysts by
repeated aspiration and alcohol instillation. Turk J Gastroenterol.
23:359–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alberti D, Borsellino A, Locatelli C, Nani
R, Cheli M, Torre G, Notarangelo L and Locatelli G: Percutaneous
transhepatic alcoholization: A new therapeutic strategy in children
with chronic granulomatous disease and liver abscess. Pediatr
Infect Dis J. 21:1081–1083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angelino G, Natali GL, Falappa P, Folgori
L, Moretti R, Cantarutti N, Di Matteo G, Chiriaco M, Rossi P, Roos
D, Aiuti A and Finocchi A: Successful treatment with percutaneous
transhepatic alcoholization of a liver abscess in a child with
chronic granulomatous disease. Pediatr Infect Dis J. 30:819–820.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zatelli A, Bonfanti U, Zini E, D'Ippolito
P and Bussadori C: Percutaneous drainage and alcoholization of
hepatic abscesses in five dogs and a cat. J Am Anim Hosp Assoc.
41:34–38. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lublin M, Bartlett DL, Danforth DN,
Kauffman H, Gallin JI, Malech HL, Shawker T, Choyke P, Kleiner DE,
Schwartzentruber DJ, et al: Hepatic abscess in patients with
chronic granulomatous disease. Ann Surg. 235:383–391. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hussain N, Feld JJ, Kleiner DE, Hoofnagle
JH, Garcia-Eulate R, Ahlawat S, Koziel DE, Anderson V, Hilligoss D,
Choyke P, et al: Hepatic abnormalities in patients with chronic
granulomatous disease. Hepatology. 45:675–683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen LE, Minkes RK, Shackelford PG,
Strasberg SM, Kuo EY and Langer JC: Cut it out: Managing hepatic
abscesses in patients with chronic granulomatous disease. J Pediatr
Surg. 38:709–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang RG, Wang XD, Zhang XL and Yang YS:
An experimental model for Staphylococcus aureus hepatic abscess in
Bama minipig. Genet Mol Res. 13:7113–7122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CH, Gervais DA, Hahn PF, Arellano RS,
Uppot RN and Mueller PR: Percutaneous hepatic abscess drainage: Do
multiple abscesses or multiloculated abscesses preclude drainage or
affect outcome? J Vasc Interv Radiol. 20:1059–1065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu JH, Yao M and Cui SF: Laboratory animal
science course [M]. Shanghai: Shanghai Scientific & Technical
Publishers; pp. 107–113. 2009
|
|
32
|
Carpenter L and Baysinger CL: Maintaining
perioperative normothermia in the patient undergoing cesarean
delivery. Obstet Gynecol Surv. 67:436–446. 2012. View Article : Google Scholar : PubMed/NCBI
|