Introduction
Dilated cardiomyopathy (DCM), which is one of
reasons for heart failure, is a type of cardiac disease featuring
enlargement of the heart chamber and dysfunction of the left or
right ventricle and myocardial systole. At present, the prognosis
of affected patients is poor and effective treatments are lacking
(1). Studies have demonstrated that
ventricular remodeling is a critical process of heart failure from
compensation to decompensation and from reversible to irreversible,
and that a key step for preventing the development of heart failure
is to control the progression of ventricular remodeling (2–4).
The morphological characteristics associated with
ventricular remodeling in DCM are ventricular dilation, a thinned
ventricular wall and increased ventricular sphericity. At the
cellular level, ventricular remodeling is characterized by the
elongation and slippage of myocardial cells (5,6). It is
well known that intercalated disks are the connecting structures to
integrate myocardial cells into a whole tissue. The development of
ventricular remodeling is closely associated with the abnormal
structure and function of intercalated discs (7). N-type cadherin (N-cadherin) is an
important medium among cells and matrix, which forms the N-type
cadherin/catenin complex (cadherin-catenin complex) and functions
as an important connection component. The cadherin-catenin complex
is the molecular basis for the formation of intercalated disk
structure to maintain the normal structure and function of the
heart (8). Numerous studies have
indicated that ventricular remodeling in DCM is closely associated
with abnormal expression and distribution of the cadherin-catenin
complex (9–11). In a hamster model of DCM, it was
found that the expression of the cadherin-catenin complex in
myocardial cells was significantly decreased, and the structure of
intercalated discs was abnormal (12). After specific knockout of the
N-cadherin gene in mature mice, no intercalated disks were present
and similar ventricular remodeling to that in DCM was observed in
these mice (13).
The normal functioning of the cadherin-catenin
complex depends on its integrity, and abnormality of any component
affects the cell adhesion function mediated by N-cadherin (14,15). A
disintegrin and metalloproteases (ADAMs) have an important role in
the processing of N-cadherin in fibroblast and nerve cells
(16). ADAMs hydrolyze N-cadherin at
the R714-I715 site of the extracellular domain in the N-terminus,
which then affects the cadherin-catenin complex, resulting in loss
of function and corresponding pathophysiological changes, such as
depressed neuronal cell adhesion and neurite outgrowth (16,17). It
has been demonstrated that the normal function of the processing of
N-cadherin by ADAMs is important in fibroblasts and nerve cells
(16). ADAM9, −10 and −17
participate in the processing of N-cadherin, with ADAM10 having the
most important role (18).
A previous study by our group found that ADAM10 had
important roles in processing N-cadherin substrate in myocardial
cells (19). Furthermore, ADAM10
expression was reported to be abnormally high in myocardial cells
of DCM patients (20). In the
present study, a single chain variable fragment antibody (ScFv)
with the ability to specifically block the ADAM10 hydrolysis site
on N-cadherin was designed and used to interfere with the
hydrolysis of N-cadherin by ADAM10. The effect of ScFv on
N-cadherin levels and ventricular remodeling was assessed.
Materials and methods
Synthesis of ScFv
According to the conserved sequence of the variable
framework regions in the antibody-encoding gene, primers for
amplification and splicing of the heavy chain variable domain
(VH) and light chain variable domain (VL)
were designed and synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The following primers were
used: VH upstream (PHA),
5′-CAGGTSMARCTGCAGSAGTCWGG-3′, downstream without linker sequence
(PHB1), 5′-TGAGGAGACGGTGACCGTGGTCC-3′ and downstream
containing linker sequence PHB,
5′-GCCAGAGCCACCTCCGCCTGAACCGCCTCCACCTGAGGAGACGGTGACCGTGGTCC-3′;
VL downstream primer (PLC),
5′-CCGTTTGATTTCCAGCTTGGTCCC-3′, upstream without linker sequence
(PLD1), 5′-GACATCGAGCTCACCCAGTCTC-3′ and upstream
containing linker sequence (PLD),
5′-CAGGCGGAGGTGGCTCTGGCGGTGGCGGATCGGACATCGAGCTCACCCAGTCTC-3′. As it
was not easy to amplify the VH and VL regions
with primers containing the linker sequences, nucleotides without
linker sequences (named VH1 and VL1) were
synthesized using the primers PHA, PHB1,
PLC and PLD1. The purified VH and
VL fragments were linked together to obtain ScFv by gene
splicing through overlap extension polymerase chain reaction
(SOE-PCR). Finally, ScFv was amplified using
PHA/PLC primers with the SOE-PCR product as a
template. Through Tag enzyme amplification, ScFv was linked into
pMD-19T (both from Takara Biotechnology Co., Ltd., Dalian, China),
and Sanger DNA sequencing technology (Thermo Fisher Scientific,
Inc.) was used to identify successfully constructed pMD-19T-ScFv.
The ScFv was amplified by PCR using pMD-19T-ScFv as a template, and
the following primers were used: upstream primer
(5′-ATAGGTACCGCCACCATGCAGGTGCAACTGCAGGA-3′ containing the
KpnI site, GGTACC) and downstream primer
(5′-GGCAAGCTTTTAGTTTGATTTCCAGCTTGGTC-3 containing the
HindIII site, AAGCTT). Taq DNA polymerase was purchased from
Takara Biotechnology Co., Ltd. The PCR reaction program started
with 5 min at 94°C, which was followed by 30 cycles of 30 sec at
94°C, 30 sec at 56°C and 1 min at 72°C, and a final extension at
72°C for 7 min. The ScFv fragment was then linked into pAdTrack-CMV
(plasmid no. 16405; Addgene, Inc., Cambridge, MA, USA), a shuttle
adenoviral plasmid (pAd). The recombinant pAdTrack-ScFv shuttle
plasmid was identified by PCR, KpnI and HindIII
enzyme (Takara Biotechnology Co., Ltd.) restriction digestion and
sequencing.
Construction and packaging of
recombinant adenoviral vector pAd-ScFv
PmeI enzyme (New England BioLabs, Inc.,
Ipswich, MA, USA) was used to linearly cut pAdTrack-ScFv shuttle
plasmid. After purification, it was transformed into competent
BJ5183 bacteria with pAdEasy-1 (both from Agilent Technologies,
Inc., Santa Clara, CA, USA) for homogenous recombination. After
recovery at 37°C for 1 h, bacteria were cultured on a plate with
kanamycin and ampicillin. The positive clone was collected for
identifying correctly constructed recombinant pAd-ScFv by PCR and
PacI enzyme (New England BioLabs, Inc.) restriction
digestion.
A total of 5×106/ml HEK293 cells (Cell
Bank, Chinese Academy of Sciences, Shanghai, China) were seeded in
culture flasks. When the cell density reached 60–70%, pAd-ScFv
plasmid linearly cut by PacI endonuclease restriction enzyme
was transfected into the cells by using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). After 24 h, the fluorescence in the cells
was observed. When 1/3-1/2 of the cells became round and floated,
cells were collected, centrifuged and re-suspended.
Virus-containing supernatant was obtained by using the Adenovirus
Purification and Concentration kit (Merck KGaA, Darmstadt,
Germany). Recurrent infection into HEK293 cells was then performed
to obtain a large amount of pAd-ScFv recombinant adenovirus.
Viral transfection
H9C2 cells (Cell Bank, Chinese Academy of Sciences)
were cultured in Dulbecco's modified Eagle's medium with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.). Cells
(2×105/ml) were seeded into a 6-well plate at 2.5
ml/well and cultured for 2 h at 37°C in a humidified atmosphere
containing 5% CO2. The recombinant pAd-ScFv was
transfected into H9C2 cells (pAd-ScFv infection group). An empty
vector infection group and blank control group were also set up.
After transfection for 48 h, cells were collected.
Western blot analysis
Western blot analysis was used to detect the
expression of N-cadherin and C-terminal fragment of N-cadherin
(CTF1). In brief, proteins were extracted from transfected cells
and heart tissues using lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein concentration was
determined using the BCA protein assay kit (P0012A; Beyotime
Institute of Biotechnology). Protein (30–50 µg per lane) was
separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. After washing and blocking with bovine serum
albumin (BSA) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1
h, primary rabbit anti-human polyclonal anti-N-cadherin antibody
(ab18203; 1:1,000; Abcam, Cambridge, MA, USA) was added, followed
by incubation overnight at 4°C. The primary antibody for N-cadherin
also recognizes antigenic epitopes of CTF1, so the antibody for
CTF1 was the same as the antibody for N-cadherin. After washing the
membrane, a secondary horseradish peroxidase (HRP)-labeled goat
anti-rabbit antibody (sc-2004, 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was added, followed by incubation for 1 h at
room temperature. The membrane was then developed using enhanced
chemiluminescence plus reagent (PerkinElmer, Inc., Waltham, MA,
USA). β-actin was detected by anti-β-actin antibody (sc-69879,
1:1,000; Santa Cruz Biotechnology, Inc.), which was used as an
internal reference. Each sample was analyzed in three replicates.
Detection of HRP was performed by Chemiluminescent Imaging and
Analysis system (Beijing Sage Creation Science Co., Ltd., Beijing,
China).
Flow cytometric analysis
After digestion with trypsin, pAd-ScFv-infected H9C2
cells were collected and washed in PBS with centrifugation at 200 ×
g at 4°C for 3 min. Cells were stained with fluorescence-labeled
anti-N-cadherin antibody (ab195185, 1:1,000 dilution; Abcam) in PBS
for 30 min at 4°C, while cells in the control group were treated
with PBS only. After washing with PBS twice, the cells were
re-suspended in PBS at 4°C and immediately subjected to flow
cytometric analysis (BD FACSCalibur, BD Biosciences, Franklin
Lakes, NJ, USA). The average fluorescence of 50,000 cells was used
to determine the expression levels of N-cadherin.
Cell adhesion assay
Fibronectin (10 µg/ml, 100 µl per well, Merck KGaA)
was used to coat 96-well plates. Wells coated with 1% BSA and 100
µg/ml poly-lysine were used as reference for minimum and maximum
adhesion, respectively. Cell suspension (5×105 cells/ml;
100 µl) from the pAd-ScFv infection group, mock infection group or
blank control group was added, followed by incubation for 3 h at
37°C. PBS was used to rinse off non-adherent cells. Cells were then
fixed with paraformaldehyde and stained with 1% methylene blue for
20 min. Subsequently, 100 µl HCl (1 mol/l) was added, followed by
incubation at 37°C for 40 min. The absorbance (A) at 600 nm was
detected by using a microplate reader. The cell adhesion rate was
calculated by using the following formula: (Atest group
-ABSA well)/(Apoly-lysine well -ABSA
well) × 100%.
Animal grouping and treatment
A rat model of DCM was established by
intraperitoneal injection of 2 ml/kg adriamycin (Merck KGaA) once a
week for 6 weeks. Adult male Sprague-Dawley rats (purchased from
the Experimental Animal Center of Wuhan University, Wuhan, China)
were divided into 4 groups: Normal control group (n=10), DCM model
group (n=25), ScFv treatment group (n=25) and empty vector control
group (n=25). Two or three rats were housed per cage under specific
pathogen-free conditions (controlled temperature of 24±3°C and
humidity of 55±15%) with a 12-h light/dark cycle and ad
libitum access to food and tap water. In the normal control
group, intravenous injection with the same volume of saline once a
week for 8 weeks was performed. In the DCM model group, rats
received intraperitoneal injection of 2 ml/kg adriamycin once a
week for 6 weeks. In the ScFv treatment group, at the end of the
6-week adriamycin treatment, 30 µl pAd-ScFv at 1010
pfu/ml was injected into 6 sites on the right ventricle
diaphragmatic surface of DCM rats after exposing the heart
(21). In the empty vector treatment
group, an equal volume of empty vector was injected into the DCM
rats. At 72 h after injection, 3 rats from the pAd-ScFv
transfection group were used to isolate primary myocardial cells
and detect fluorescence. Rats in the other groups and the remaining
rats in the transfection group were used for examination of heart
function by ultrasonography. Subsequently, rats were sacrificed to
collect specimens for the other analyses. This study was carried
out in strict accordance with international, national and
institutional rules regarding animal experiments and all surgery
was performed under sodium pentobarbital anesthesia. All efforts
were made to minimize suffering. The protocol was approved by the
Committee on the Ethics of Animal Experiments of the Wuhan
University (permit no. WDRY2013-L016).
Measurement of heart function
Rats in each group were anesthetized by
intraperitoneal injection of 10% chloral hydrate (3 ml/kg;
Sigma-Aldrich; Merck KGaA). Cardiac color Doppler ultrasound VIVID7
(GE Healthcare, Little Chalfont, UK) with a 12-MHz probe was used
to detect the heart chamber diameter and cardiac function. The left
ventricular end diastole (LVED), left ventricular end systole
(LVES), fraction shortening (FS), left ventricular ejection
fraction (LVEF) and interventricular septal thickness (IVS) were
recorded.
Hematoxylin and eosin (H&E)
staining
Ventricular myocardium of rats in each group was
collected and fixed with 4% paraformaldehyde. The tissue was
paraffin-embedded and sliced for H&E staining. The stained
tissues were observed under a microscope and images were
captured.
Immunohistochemistry
Paraffin-embedded tissues were serially sectioned
and the routine streptavidine-avidine-biotin complex method was
used for immunohistochemistry based on the standard protocol of the
SABC-kit (SP-9000, Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China). The primary polyclonal antibody for
N-cadherin was same as that used for western blot analysis
(dilution, 1:150; 4°C; overnight). Samples were further processed
using a Goat anti-rabbit IgG IHC kit (PV-9001, Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.), and the sections were
counterstained with hematoxylin. In the negative control, PBS was
used to replace the primary antibody. Cells with brown staining or
granules in the cytoplasm or nucleus were defined as expressing
N-cadherin. Ten fields of view were randomly selected under high
magnification (×200). Cells with N-cadherin staining were counted
and their percentage was determined. The average percentage was
used as the relative protein expression levels in myocardial cells.
The same brown was used as a standard to estimate the relative
expression for each section and analyze the integrated optical
density values (IOD). The average IOD of the images for each group
was represented as the mean ± standard deviation (SD).
Statistical analysis
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Values are expressed as the mean
± SD. If there was homogeneity of variance, a paired Student's
t-test was used. If there was heterogeneity, differences among
groups were determined by analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of pAdTrack-ScFv
adenoviral shuttle plasmid and recombinant adenoviral pAd-ScFv
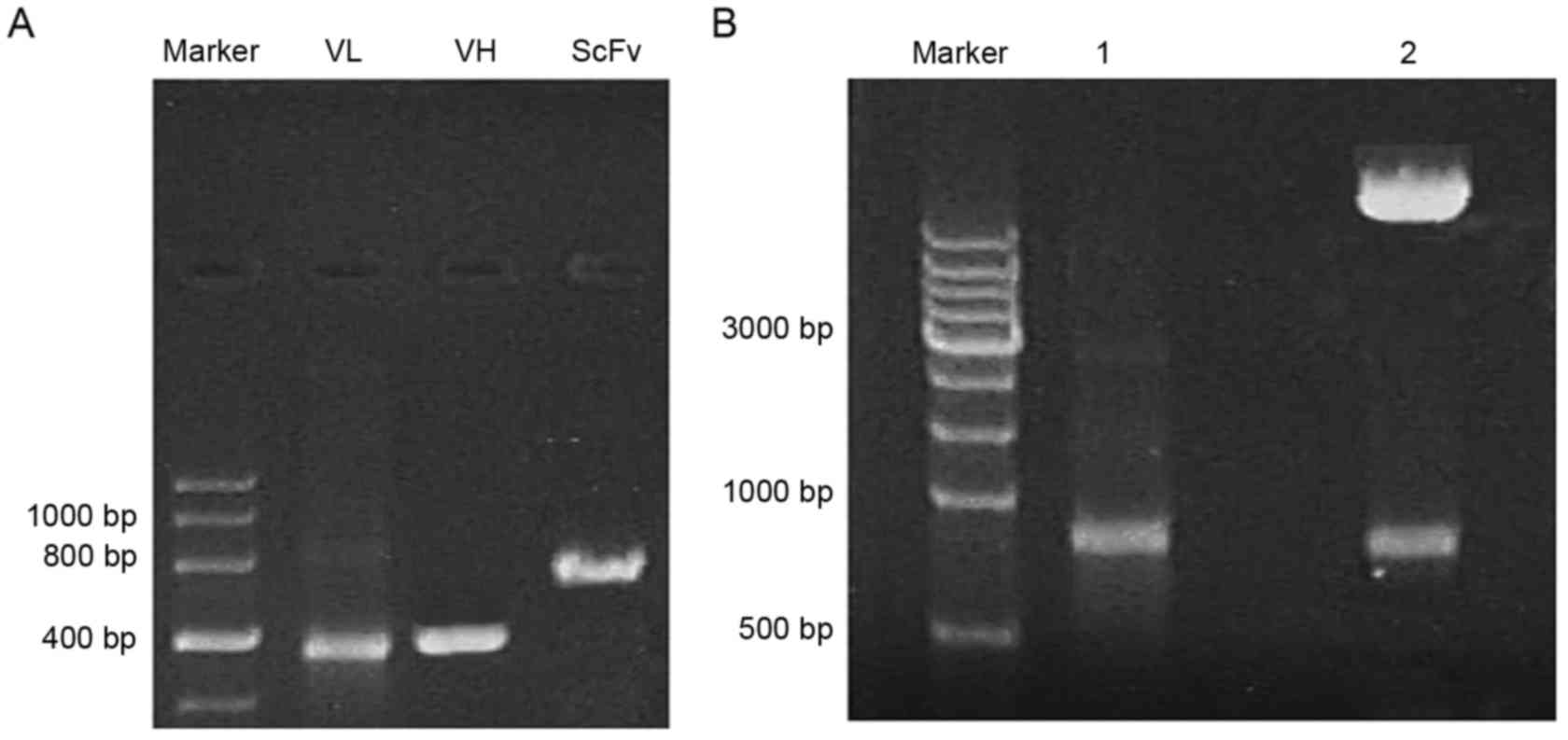
To obtain the ScFv fragment, the VH1 (410
bp) and VL1 (380 bp) templates were first amplified in
the 2B3 cell line to secrete monoclonal antibody recognizing a
sequence of N-cadherin containing the ADAM10 hydrolysis site.
SOE-PCR was used to obtain the ScFv fragment of ~750 bp in length,
which was identified by gel electrophoresis (Fig. 1A). As presented in Fig. 1B, the full-length ScFv gene was
inserted into the adenoviral shuttle plasmid pAdTrack-CMV to
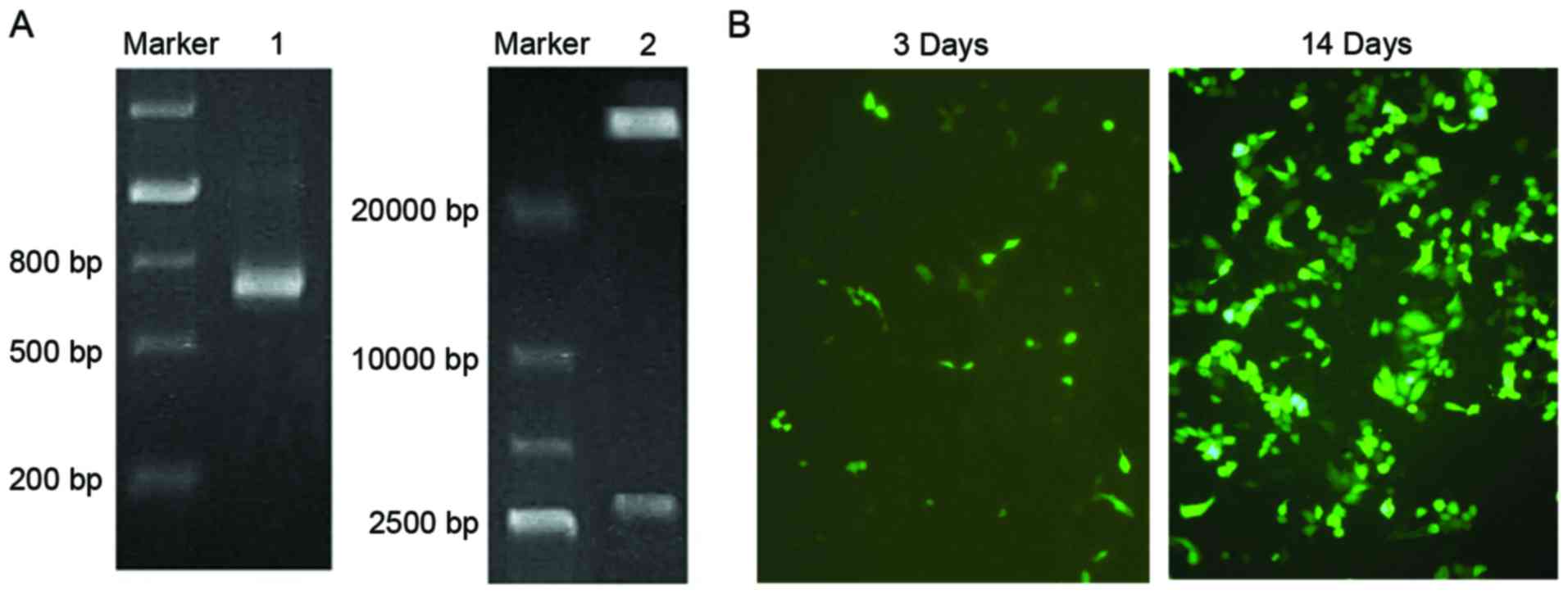
construct pAdTrack-ScFv. After linearization by digestion with
PmeI, pAdTrack-ScFv adnoviral shuttle plasmid was
homologously recombined with a pAdEasy-1 backbone vector. Following
identification by PCR, the specific band of a nucleotide with the
length of 750 bp was amplified (Fig.
2A). The adenoviral plasmid was then digested by PacI
and electrophoretic analysis was used for further confirmation
(Fig. 2A).
The Lipofectamine method was used for transfecting
pAd-ScFv into HEK293 cells. After 72 h, fluorescence was observed
in the HEK293 cells, which gradually increased when the culture
time was extended. After 14 days of transfection, the cells became
enlarged and formed clusters (Fig.
2B). These cells were used to obtain recombinant pAd-ScFv
adenovirus.
pAd-ScFv increases N-cadherin and
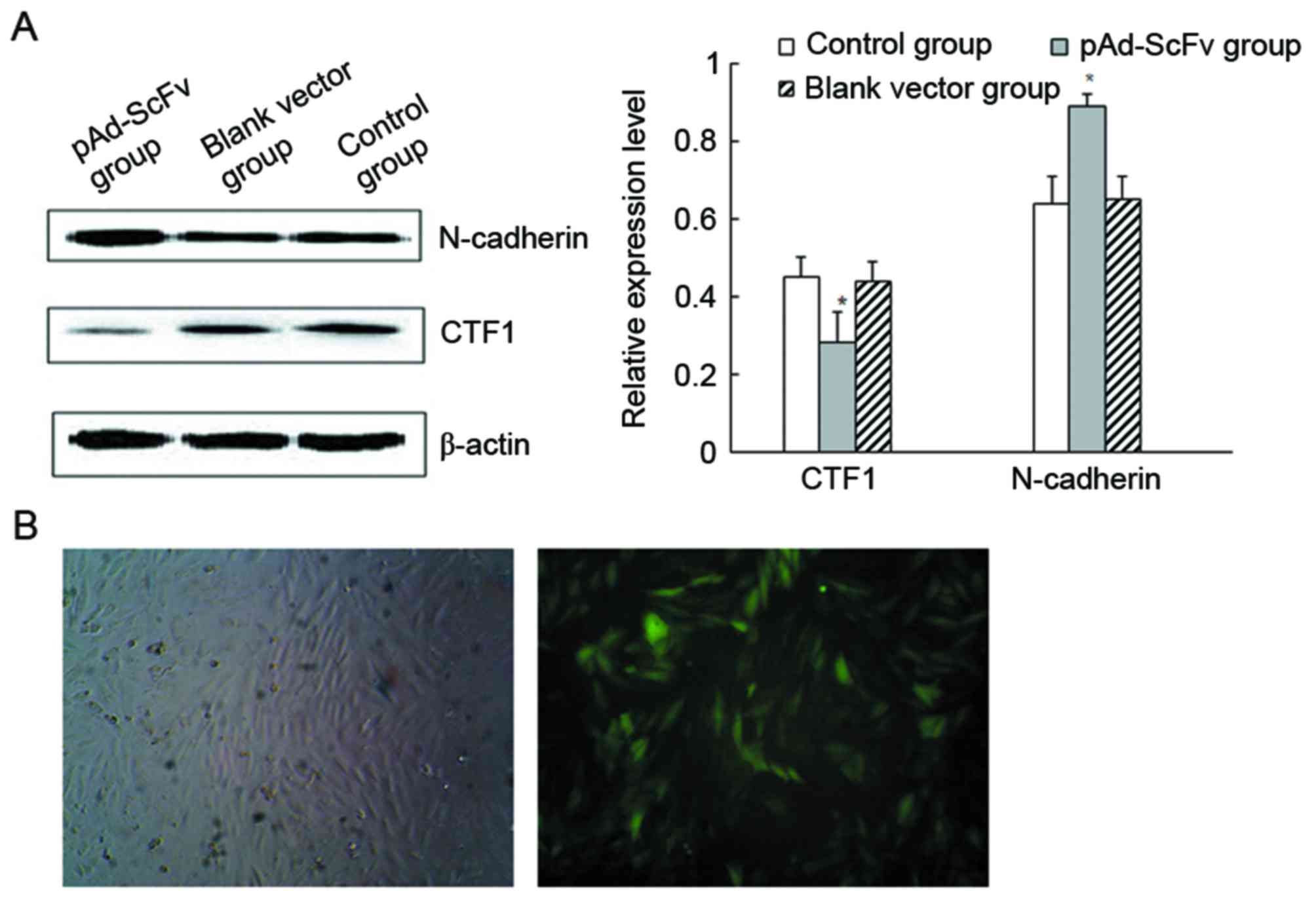
decreases CTF1 protein levels in cardiomyocytes
To detect the levels of N-cadherin and CTF1 protein
in H9C2 cells after transfection, western blot analysis was used.
As presented in Fig. 3A, N-cadherin
protein levels were significantly increased in the transfection
group compared with those in the blank control group, while CTF1
protein levels were significantly decreased. The results indicated
that transfection of the recombinant adenoviral vector decreased
CTF1 and increased N-cadherin protein levels. As demonstrated in
Fig. 3B, pAd-ScFv was successfully
transfected into H9C2 cells.
pAd-ScFv increases N-cadherin on the
surface of myocardial cells in vitro
To detect the changes of N-cadherin protein on the
surface of myocardial cells, flow cytometry was used. As presented
in Fig. 4, N-cadherin protein on the
myocardial cell surface was significantly increased in the pAd-ScFv
infection group compared with that in the control groups
(P<0.05). These results indicated that pAd-ScFv decreased the
hydrolysis process on N-cadherin by ADAM10 through blocking the
ADAM10 hydrolysis site in N-cadherin.
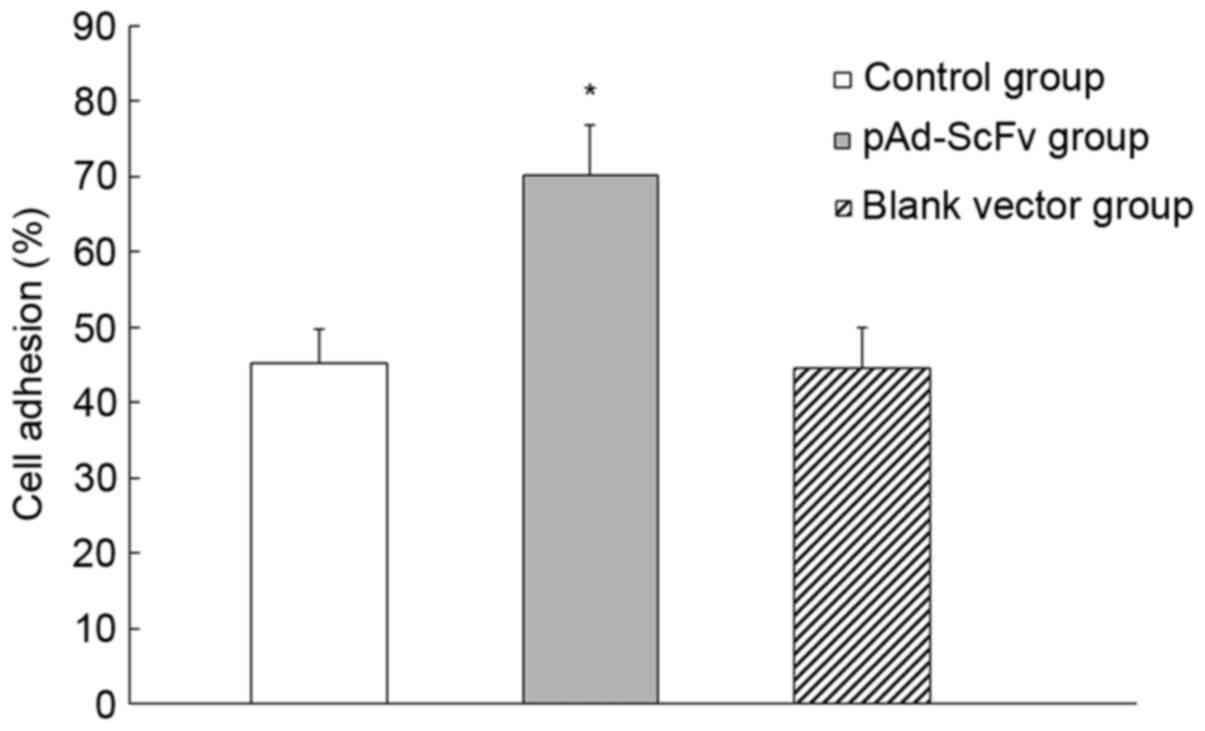
pAd-ScFv increases myocardial cell
adhesion
The influence of pAd-ScFv on the adhesion of H9C2
cells was assessed. As presented in Fig.
5, the cell adhesion rate in the pAd-ScFv group was 70.23±6.5%,
while it was 45.21±4.5% in the blank group and 44.58±5.7% in the
empty plasmid group. The pAd-ScFv group had a significantly higher
adhesion rate compared with that in the control groups (P<0.05),
which indicated that pAd-ScFv increased the adhesion capability of
myocardial cells.
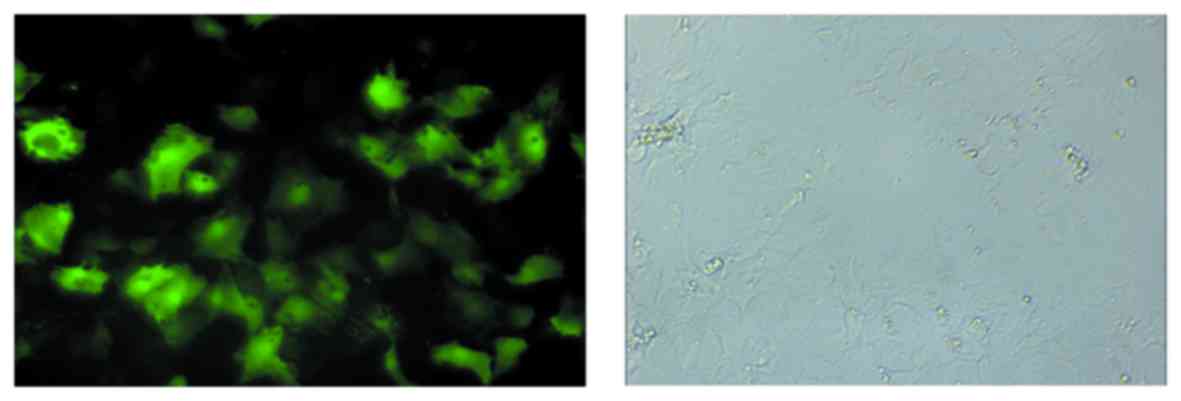
Fluorescence detection of pAd-ScFv in
primary myocardial cells of rats in the transfection group
To confirm that transfection was successful, the
fluorescence in primary myocardial cells isolated from rats in the
transfection group was detected. As presented in Fig. 6, a large amount of fluorescence was
observed in isolated primary myocardial cells, which indicated that
the transfection of the rat model was successful.
pAd-ScFv improves heart function in a
rat model of DCM
In the DCM model group, a total of 10 rats had died
(mortality rate, 40%), the main reason of which was congestive
heart failure. In the treatment group, the mortality rate was 12%,
which was significantly lower than that in the model group
(P<0.01), while it was 36% in the empty vector group (P>0.05
vs. model group). In the blank normal control group, none of the
rats died. The heart function was compared between the different
groups (Table I). Compared with
those in the control group, the LVED, LVES and IVS were
significantly increased in the DCM model group, while the LVEF and
FS were significantly decreased. In the pAd-ScFv treatment group,
the myocardial structure and heart function were significantly
improved. Compared with those in the model group, the LVED, LVES
and IVS were significantly decreased, while LVEF and FS were
increased.
 | Table I.Parameters of heart function in the
experimental groups of rats. |
Table I.
Parameters of heart function in the
experimental groups of rats.
| Group | LVED (mm) | LVES (mm) | IVS (mm) | LVEF (%) | FS (%) |
|---|
| Normal control | 5.62±0.45 | 2.83±0.36 | 1.95±0.32 | 92±2 | 58±4 |
| Model |
6.73±0.52a |
4.05±0.22a |
3.05±0.43a | 80±5a | 44±3a |
| pAd-ScFv
treatment |
5.82±0.33b |
3.28±0.40b |
2.03±0.24b | 88±6b | 55±2b |
| Empty vector
control |
6.65±0.42a |
4.03±0.54a |
2.98±0.42a | 81±4a | 46±3a |
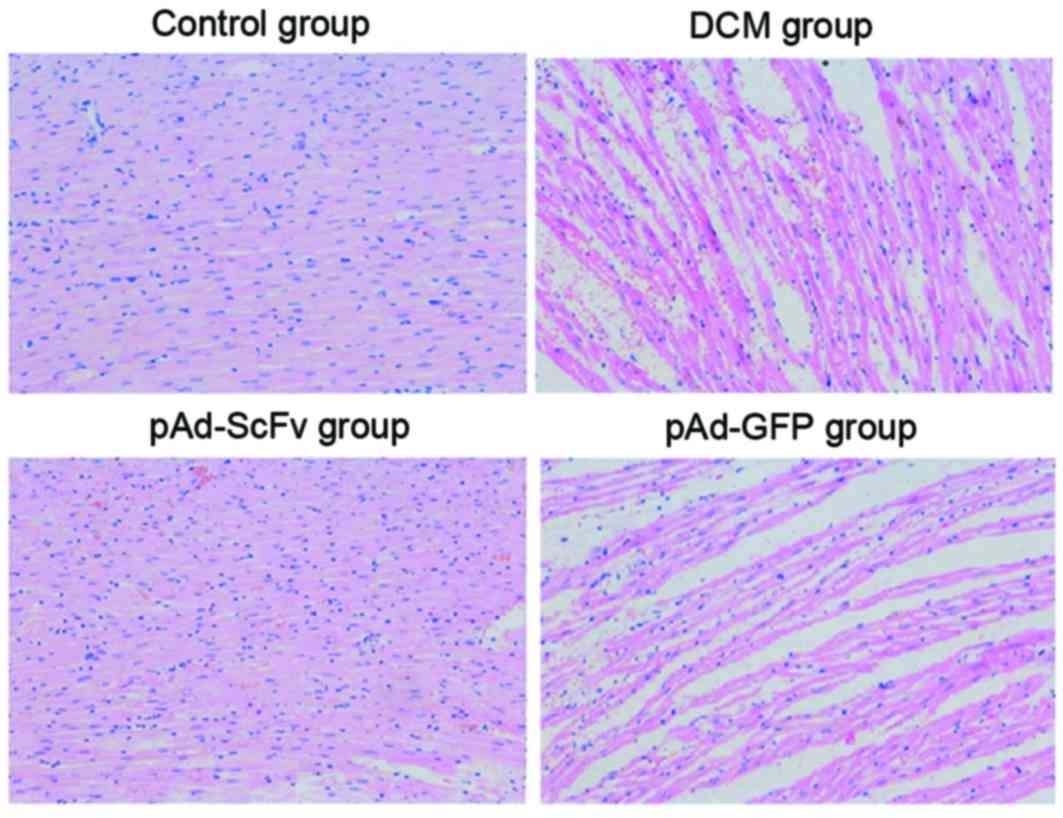
pAd-ScFv reduces DCM-induced
pathological changes in rat myocardial tissues
To explore the pathological changes of myocardial
tissues among different groups, H&E staining was performed. As
demonstrated in Fig. 7, a normal
morphology of cardiomyocytes was observed in the normal control
group and the cytoplasm was clear. In the model group and the empty
vector control group, necrosis was observed. Cardiomyocytes were
enlarged with widened gaps. Myocardial fibers were fractured, and
hyperplasia of endocardial collagen and elastic fibers was
observed. Certain parts of myocardial tissues had been replaced by
fibrous tissues and certain areas of the cardiac sarcoplasm were
condensed. Cell nuclei were condensed, distorted or had
disappeared, and fat was degenerated. Cytoplasmic vacuolization in
cells, lymphocyte infiltration, fibroblast proliferation and
capillary hyperplasia were also observed. In the pAd-ScFv treatment
group, all of the above pathological changes were obviously
improved.
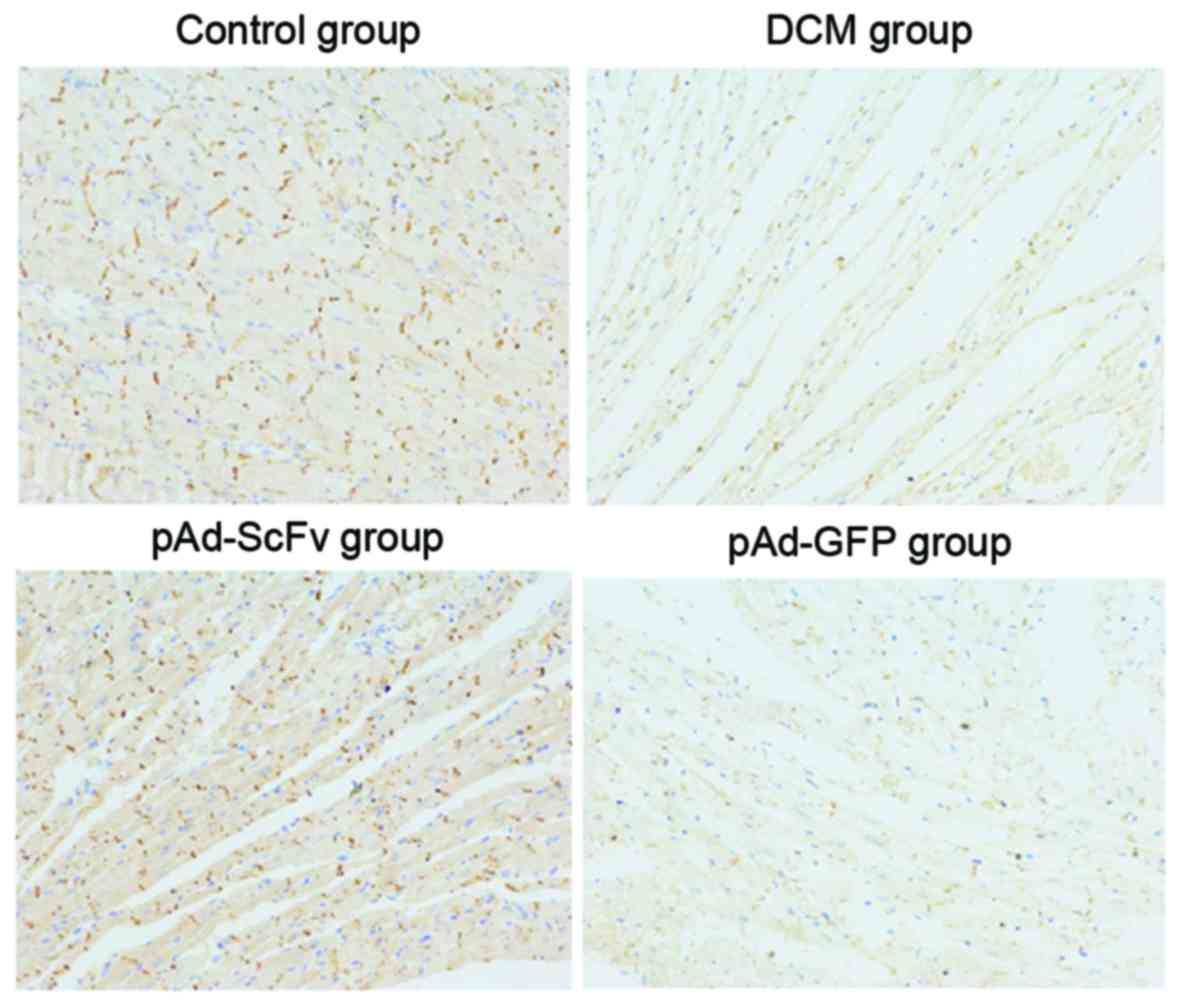
pAd-ScFv increases N-cadherin on the
surface of myocardial cells of DCM model rats
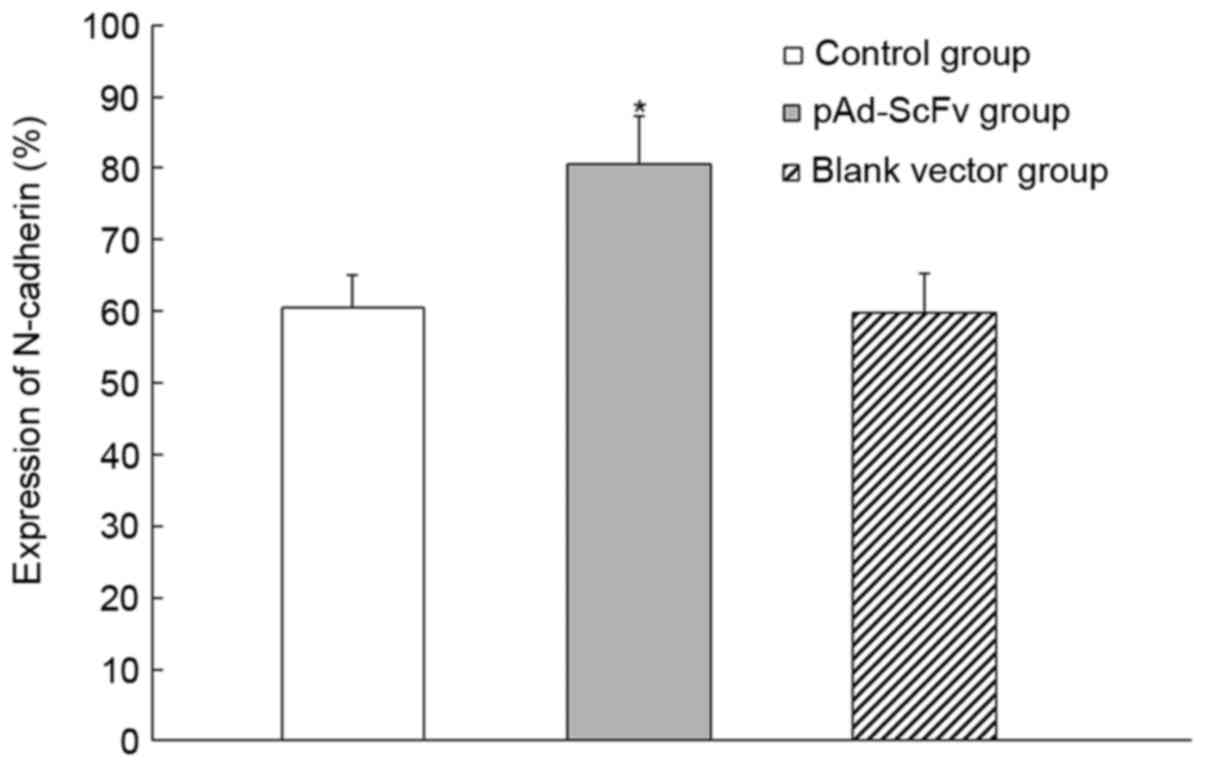
To assess the effect of pAd-ScFv on N-cadherin on
the surface of myocardial cells of DCM rats, immunohistochemical
staining was performed. As presented in Fig. 8 and Table
II, N-cadherin was obviously decreased in the model group
compared with that in the normal control group. In the pAd-ScFv
treatment group, N-cadherin was increased, as ScFv blocked the
ADAM10 hydrolysis site in N-cadherin.
 | Table II.Expression of N-cadherin in the
myocardial cells of different groups. |
Table II.
Expression of N-cadherin in the
myocardial cells of different groups.
| Group | Integrated optical
density |
|---|
| Normal control |
4129.73±1154.72 |
| Model |
1073.55±509.44a |
| pAd-ScFv
treatment |
3664.50±1713.91b |
| Empty vector
control |
1127.25±679.87b |
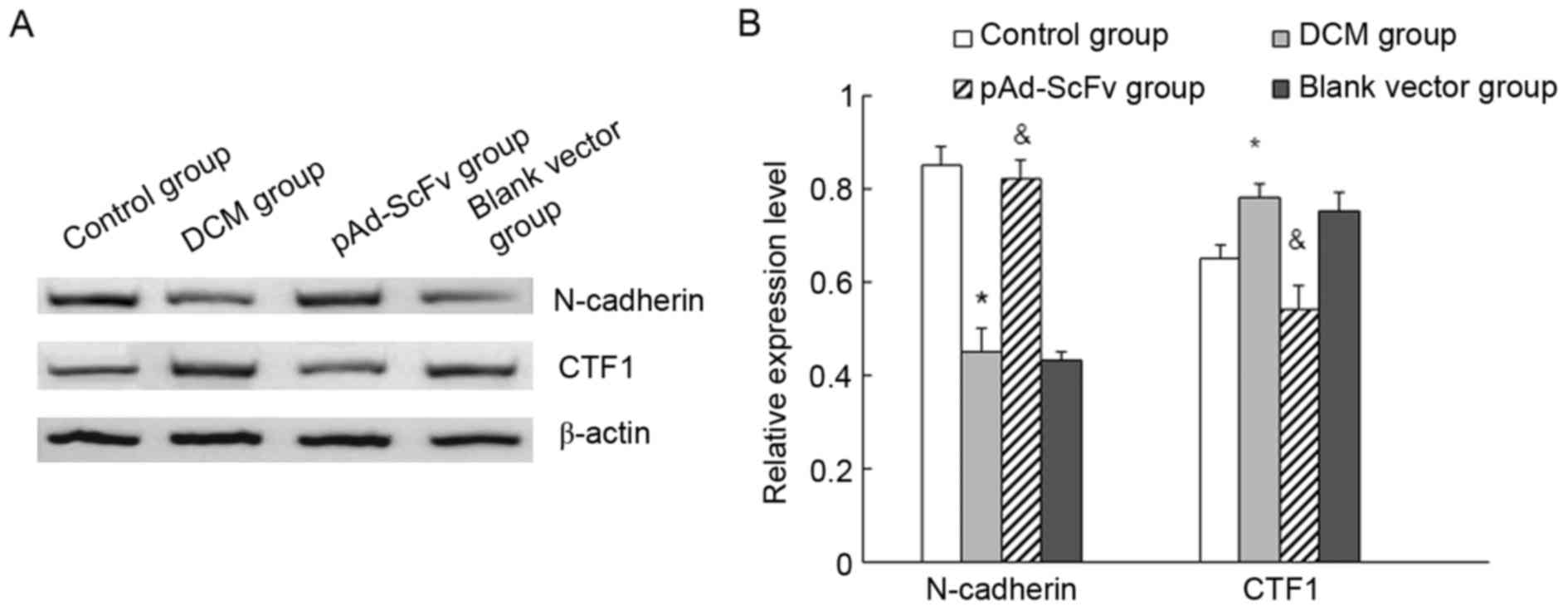
pAd-ScFv increases N-cadherin and
decreases CTF1 in the myocardial tissues of DCM rats
The levels of N-cadherin and CTF1 in heart tissues
of the different groups of rats were assessed by western blot
analysis. As displayed in Fig. 9,
N-cadherin protein levels were significantly decreased in the model
group compared with those in the control group, while they were
significantly increased in the treatment group compared with those
in the model group. Furthermore, CTF1 protein was significantly
increased in the model group compared with that in the control
group, while it was significantly decreased in the transfection
group compared with that in the model group.
Discussion
DCM is characterized by left ventricle or
dysfunction of the left and right ventricular dilatation and
systole, which is a disease representative of chronic congestive
heart failure. A previous study reported that ventricular
remodeling is important in cardiac dysfunction (22). Ventricular remodeling includes
mechanical and electrical remodeling. Changes in the number of
connections of intercalated discs to cardiac cells as well as their
distribution and functional disorders have important roles in
processes of ventricular mechanical remodeling (23). N-cadherin-mediated connections are
essential for cardiomyocyte adhesion. N-cadherin combines with
catenin to form the cadherin-catenin complex, which is an important
structure in maintaining myocardial morphology, substance transport
and trans-membrane signal transfer (24). Studies have demonstrated that
abnormal structure and function of the cadherin-catenin complex are
closely associated with ventricular remodeling (25). ADAMs have important roles in
processing N-cadherin in fibroblast cells and nerve cells (16), thereby regulating the cellular
distribution of N-cadherin and the formation of the
cadherin-catenin complex. ADAM10, one member of the family of
ADAMs, has the most critical role in the proteolytic processing of
N-cadherin. ADAM10 hydrolyzes the N-terminal R714-I715 site in the
extracellular domain of N-cadherin, thus producing CTF1 and NTF
fragments. The integrity of N-cadherin molecules is destroyed,
which further affects the formation of the cadherin-catenin
complex. Previous studies by our group found that this hydrolysis
pathway for processing N-cadherin was present in cardiomyocytes
(19,26). Current research focuses on the
interaction of ADAMs and N-cadherin in cardiomyocytes and their
participation in processes of ventricular remodeling, with a
specific focus on ADAM10 inhibitors, including RNA interference and
antibody-mediated blocking. However, direct inhibition of ADAM10
has certain flaws and shortcomings: Besides N-cadherin, ADAM10 has
numerous other substrates, which have important roles in normal
physiological processes, and therefore, inhibition of the activity
of ADAM10 has severe side effects, including changes of cell
adhesion, chemokine function, immune disorders and developmental
defects of the nervous and cardiovascular systems (27,28).
In the present study, ScFv antibody was used to
block the ADAM10 hydrolysis site in N-cadherin. This strategy has
two advantages: First, it specially interferes with the interaction
of N-cadherin with ADAM10 and does not affect the hydrolysis of any
of the other substrates of ADAM10, thereby avoiding effects not
specific for N-cadherin processing. Furthermore, N-cadherin prefers
to form the cadherin-catenin complex to exert its physiological
functions in response to blocking of the hydrolysis site. To pursue
this strategy, the recombinant adenoviral pAd-ScFv vector was
constructed. After transfection into H9C2 cells, the protein levels
of N-cadherin were significantly increased, while CTF1 was
significantly decreased. Flow cytometric analysis demonstrated that
N-cadherin on the cell surface was significantly increased in
transfected myocardial cells compared with that in the control
group, which indicated that ADAM10 had important roles in the
hydrolysis of N-cadherin in myocardial cells. A cell adhesion assay
indicated that the adhesion ability of myocardial cells was
significantly increased by the antibody. The results further
indicated that N-cadherin hydrolysis by ADAM10 destroyed the
integrity of N-cadherin and influenced the formation of the
cadherin-catenin complex, which induced associated pathological and
physiological changes.
In addition to the in vitro cell-based
experiments, the recombinant pAd-ScFv adenovirus was also injected
into the hearts of rats with adriamycin-induced DCM. Compared with
that in the model group, the structure and function of the heart
was significantly improved in the ScFv treatment group. Western
blot analysis indicated that N-cadherin protein was significantly
increased in the ScFv treatment group and that CTF1 protein was
significantly reduced compared with that in the control group.
Immunohistochemical analysis revealed that N-cadherin protein on
the myocardial cell surface was significantly increased by
pAd-ScFv. These results indicated that N-cadherin was increased by
ScFv through blocking the ADAM hydrolysis site on N-cadherin, which
promoted the formation of the cadherin-catenin complex, thereby
enhancing cardiomyocyte adhesion to inhibit myocardial remodeling
and delay heart failure.
In conclusion, pAd-ScFv adenovirual plasmid was
successfully constructed and transfected into cardiomyocytes. The
ADAM10 hydrolysis site in N-cadherin was specifically blocked,
leading to high levels of N-cadherin. Intracardial injection of
pAd-ScFv was found to ameliorate DCM in rats. The present findings
broadened the current understanding of the pathogenesis of DCM; it
was indicated that the degradation of N-cadherin by highly
expressed ADAM10 in cardiomyocytes is one of the reasons for
ventricular remodeling in DCM. The results of the present study may
also provide an experimental foundation for the prevention and
treatment of DCM.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 8100094), the Natural
Science Foundation of Hubei Province (no. 2012FFB04418) and the
Wuhan Health and Family Planning Commission Project (no.
WX14C71).
References
|
1
|
Ye XZ, Zhi H, Cao K, Li J, Li XL, Gu K,
Zhang HF, Xu F, Zhou YL, Zhou L and Xu DJ: A research about the
relationship between cardiac and renal function in dilated
cardiomyopathy patients with heart failure. Lin Chuang Xin Xue Guan
Bing Za Zhi. 26:104–108. 2010.(In Chinese).
|
|
2
|
Konstam MA, Kramer DG, Patel AR, Maron MS
and Udelson JE: Left ventricular remodeling in heart failure:
Current concepts in clinical significance and assessment. JACC
Cardiovasc Imaging. 4:98–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruiz-Zamora I, Rodriguez-Capitan J,
Guerrero-Molina A, Morcillo-Hidalgo L, Rodriguez-Bailon I,
Gomez-Doblas JJ, de Teresa-Galvan E and Garcia-Pinilla JM:
Incidence and prognosis implications of long term left ventricular
reverse remodeling in patients with dilated cardiomyopathy. Int J
Cardiol. 203:1114–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon JH, Son JW, Chung H, Park CH, Kim YJ,
Chang HJ, Hong GR, Kim TH, Ha JW, Choi BW, et al: Relationship
between myocardial extracellular space expansion estimated with
post-contrast T1 mapping MRI and left ventricular remodeling and
neurohormonal activation in patients with dilated cardiomyopathy.
Korean J Radiol. 16:1153–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Ni XD, Hu YP, Song ZW, Yang WY
and Xu R: Left ventricular longitudinal rotation changes in
patients with dilated cardiomyopathy detected by two-dimensional
speckle tracking imaging. Zhonghua Xin Xue Guan Bing Za Zhi.
39:920–924. 2011.(In Chinese). PubMed/NCBI
|
|
6
|
Pahl E, Sleeper LA, Canter CE, Hsu DT, Lu
M, Webber SA, Colan SD, Kantor PF, Everitt MD, Towbin JA, et al:
Incidence of and risk factors for sudden cardiac death in children
with dilated cardiomyopathy: A report from the pediatric
cardiomyopathy registry. J Am Coll Cardiol. 59:607–615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ram R, Wescott AP, Varandas K, Dirksen RT
and Blaxall BC: Mena associates with Rac1 and modulates connexin 43
remodeling in cardiomyocytes. Am J Physiol Heart Circ Physiol.
306:H154–H159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hertig CM, Butz S, Koch S,
Eppenberger-Eberhardt M, Kemler R and Eppenberger HM: N-cadherin in
adult rat cardiomyocytes in culture. II. Spatio-temporal appearance
of proteins involved in cell-cell contact and communication.
Formation of two distinct N-cadherin/catenin complexes. J Cell Sci.
109:11–20. 1996.PubMed/NCBI
|
|
9
|
Frank D, Rangrez AY, Poyanmehr R, Seeger
TS, Kuhn C, Eden M, Stiebeling K, Bernt A, Grund C, Franke WW and
Frey N: Mice with cardiac-restricted overexpression of Myozap are
sensitized to biomechanical stress and develop a
protein-aggregate-associated cardiomyopathy. J Mol Cell Cardiol.
72:196–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen SN, Gurha P, Lombardi R, Ruggiero A,
Willerson JT and Marian AJ: The hippo pathway is activated and is a
causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy.
Circ Res. 114:454–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asimaki A, Kapoor S, Plovie E, Arndt Karin
A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, et al:
Identification of a new modulator of the intercalated disc in a
zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med.
6:240ra742014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Qu J, Yi XP, Graber K, Huber L,
Wang X, Gerdes AM and Li F: Up-regulation of gamma-catenin
compensates for the loss of beta-catenin in adult cardiac myocytes.
Am J Physiol Heart Circ Physiol. 292:H270–H276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sodian R, Hoerstrup SP, Sperling JS,
Daebritz S, Martin DP, Moran AM, Kim BS, Schoen FJ, Vacanti JP and
Mayer JE Jr: Early in vivo experience with tissue-enginered
trileaflet heart valves. Circulation. 102 (19 Suppl 3):III22–III29.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jannesari-Ladani F, Hossein G, Monhasery
N, Shahoei SH and Mood Izadi N: Wnt5a influences viability,
migration, adhesion, colony formation, E- and N-cadherin expression
of human ovarian cancer cell line SKOV-3. Folia Biol (Praha).
60:57–67. 2014.PubMed/NCBI
|
|
15
|
Vega LJC, Lee MK, Jeong JH, Smith CE, Lee
KY, Chung HJ, Leckband DE and Kong H: Recapitulating cell-cell
adhesion using N-cadherin biologically tethered to substrates.
Biomacromolecules. 15:2172–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reiss K, Maretzky T, Ludwig A, Tousseyn T,
de Strooper B, Hartmann D and Saftig P: ADAM10 cleavage of
N-cadherin and regulation of cell-cell adhesion and beta-catenin
nuclear signaling. EMBO J. 24:742–752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuda T, Fujio Y, Nariai T, Ito T,
Yamane M, Takatani T, Takahashi K and Azuma J: N-cadherin signals
through Rac1 determine the localization of connexin 43 in cardiac
myocytes. J Mol Cell Cardiol. 40:495–502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uemura K, Kihara T, Kuzuya A, Okawa K,
Nishimoto T, Ninomiya H, Sugimoto H, Kinoshita A and Shimohama S:
Characterization of sequential N-cadherin cleavage by ADAM10 and
PS1. Neurosci Lett. 402:278–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XO, Huang W, Chen YJ and Zhou LR:
Silencing of ADAM10 gene by siRNA inhibits the ADAMs cleavage of
N-cadherin in cardiac myocytes. Zhongguo Bing Li Sheng Li Za Zhi.
9:1702–1704. 2012.(In Chinese).
|
|
20
|
Fedak PW, Moravec CS, McCarthy PM,
Altamentova SM, Wong AP, Skrtic M, Verma S, Weisel RD and Li RK:
Altered expression of disintegrin metalloproteinases and their
inhibitor in human dilated cardiomyopathy. Circulation.
113:238–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toivonen R, Koskenvuo J, Merentie M,
Söderström M, Ylä-Herttuala S and Savontaus M: Intracardiac
injection of a capsid-modified Ad5/35 results in decreased he-art
toxicity when compared to standard Ad5. Virol J. 9:2962012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YJ, Yang SH, Li MH, Iqbal J,
Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL and Chen SL:
Berberine attenuates adverse left ventricular remodeling and
cardiac dysfunction after acute myocardial infarction in rats: Role
of autophagy. Clin Exp Pharmacol Physiol. 41:995–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Severs NJ, Dupont E, Thomas N, Kaba R,
Rothery S, Jain R, Sharpey K and Fry CH: Alterations in cardiac
connexin expression in cardiomyopathies. Adv Cardiol. 42:228–242.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko
YS and Matsushita T: Gap junction alterations in human cardiac
disease. Cardiovasc Res. 62:368–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Bowles NE, Scherer SE, Taylor MD,
Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B,
Brandon LI, et al: Desmosomal dysfunction due to mutations in
desmoplakin causes arrhythmogenic right ventricular
dysplasia/cardiomyopathy. Circ Res. 99:646–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XO, Huang W and Zhou LR: Construction
of lentiviral vector of RNA interference of ADAM10 gene and its
inhibitive role on the ADAMs cleavage of N-cadherin in cardiac
myocyte. Guo Ji Mian Yi Xue Za Zhi. 36:221–225. 2013.(In
Chinese).
|
|
27
|
Jakobsson L, Franco CA, Bentley K, Collins
RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G,
Medvinsky A, et al: Endothelial cells dynamically compete for the
tip cell position during angiogenic sprouting. Nat Cell Biol.
12:943–532. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Zhang W, Kennard S, Caldwell RB and
Lilly B: Notch3 is critical for proper angiogenesis and mural cell
investment. Circ Res. 107:860–870. 2010. View Article : Google Scholar : PubMed/NCBI
|