Introduction
Wogonin is a flavonoid-like chemical compound that
was identified in Eucommia ulmoides (1). Eucommia ulmoides extract is a
valuable Chinese herbal medicine which is considered a raw material
of gum as it is sweet, has a pungent taste and is non-toxic.
Furthermore, this compound may aid hepatic and renal function,
improve memory and relieve fatigue (2). Previous studies have demonstrated that
Eucommia ulmoides possesses estrogen-like effects (3,4). The
extract of Eucommia ulmoides is able to decrease the bone
loss in ovariectomized rats with diabetes mellitus through
increasing levels of estrogen (3).
Phytoestrogen properties of Eucommia ulmoides include:
Flavonoid components (wogonin and baicalein), lignan components
(pinoresinol and diglucoside) and iridoid glycosides (aucubin,
geniposidic acid and eucommiol), which have the material basis to
produce estrogen-like effects (5,6).
Phytoestrogen, a bisphenol structure chemical compound, elicits
estrogen-like effects without provoking estrogen-like side effects
such as increased risk of breast cancer, endometrial cancer and
ovarian cancers (7), and is an area
of interest for the treatment of skin disease as a substitute for
estrogen (8). A previous study
revealed that Eucommia ulmoides may inhibit melanocyte
proliferation, tyrosinase activity and melanin synthesis (9). Betulinic acid, a phytoestrogen
identified in Eucommia ulmoides, may inhibit the
proliferation of melanocyte and melanoma cells (10) and was revealed to induce cell
apoptosis and the expression of induced myeloid leukemia cell
differentiation protein.
The present study investigated the effect of wogonin
on the mechanism of melanin synthesis in A375 cells. The effect of
wogonin on melanogenesis may be associated with the regulation of
the ER-MAPK signaling pathway and TYR activity.
Materials and methods
Cells and reagents
Human A375 cells were supplied by the Cell Center of
Chinese Academy of Sciences (Beijing, China). Tissue and cell lysis
solution histiocyte lysate (WIP; Beyotime Institute of
Biotechnology, Haimen, China); MTT (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany); primer (Shanghai Shengong Biology Engineering
Technology Service, Ltd., Shanghai, China); Dulbecco's modified
Eagles medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA); goat anti-mouse immunoglobulin G (IgG) marked by
horseradish peroxidase (HRP; cat no. BST08k13B; Wuhan Boster
Biological Technology, Ltd., Wuhan, China); mouse anti-β-actin
monoclonal antibody (cat no. 140925; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing China); mouse anti-tyrosinase
monoclonal antibody (cat no. F0811; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA); and mouse anti-c-Jun N-terminal kinase (JNK)
monoclonal antibody (cat no. G0913) and TRIzol reagent (both Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Instruments
Nano-100 Micro spectrophotometer (Hangzhou Allsheng
Instruments Co., Ltd., Hangzhou, China); TCL6G-C table-top
high-speed refrigerated centrifuge (Shanghai Anting Scientific
Instrument Factory, Shanghai, China); TProfessional Basic PCR
Amplification instrument (Biometra GmbH, Göttingen, Germany); MK3
enzyme microplate reader (Redian, Shanghai, China); DYCZ-40B
transfer electrophoresis (Beijing Liuyi Biotechnology Co., Ltd.,
Beijing, China); and SmartChemi500 one piece micro-ChemiDoc XRS gel
imaging system (Beijing Sage Creation Science Co., Ltd., Beijing,
China).
Drugs
Wogonin with a purity >98% (cat no.
MUST-13092303; Nanjing Zelang Medical Technology Co., Ltd.,
Nanjing, China); estradiol with a purity >98% (cat no. L750N46;
J&K Scientific, Ltd., Beijing, China); ICI182780 (cat no.
20A/129473; Tocris Bioscience, Bristol, UK); and U0126 (S110202;
Selleck Chemicals, Houston, TX, USA).
Preparation of drug solution
A total of 5.5 mg estradiol was dissolved in 4 ml
anhydrous alcohol and combined with DMEM to prepare a stock
solution at a concentration of 20 µmol/l, which was diluted to
1×10−3 µmol/l when used. Wogonin (5.69 mg) was dissolved
in DMEM to prepare a solution with an initial concentration of 200
µmol/l, diluted to 10 µmol/l when used. ICI182780 (5 mg) was
dissolved in 1% dimethyl sulphoxide (DMSO), and added to DMEM to
prepare a solution with an initial concentration of 200 µmol/l,
which was diluted to 1 µmol/l when used. U0126 (8.53 mg) was
dissolved in 1% DMSO and combined with DMEM to prepare a stock
solution at a concentration of 400 µmol/l, which was diluted to 10
µmol/l when used. All solutions were filtered and sterilized using
a 0.22-µm filter membrane, and stored at −20°C.
Cell grouping and culture
A375 cells were grown to 80% confluence and digested
with 0.25% Trypsin, centrifuged at 1,000 × g for 5 min at room
temperature, and resuspended. Subsequently, cells were transferred
onto 96-well plates (200 µl DMEM with ~4,500 cells/well) or 6-well
plates (2 ml DMEM with 24×104 cells/well) and incubated
at 37°C in an atmosphere containing 5% CO2 for 24 h. The
medium was discarded and cells were divided into different groups
according to the treatment administered, including: The control
group, cells in the presence of DMEM without treatment; estradiol
group, cells in the presence of estradiol (10−3 µmol/l);
wogonin group, cells in the presence of wogonin (10 µmol/l)
solution; wogonin + ICI182780 group, cells in the presence of
ICI182780 (1 µmol/l) and 10 µmol/l of wogonin; and wogonin + U0126
group, cells in the presence of U0126 (10 µmol/l) and 10 µmol/l of
wogonin. All groups were cultured for 48 h. There are 6 wells for
duplicate in 96-well plates, 4 wells for duplicate in 6-well
plates.
Cell activity
Cells were seeded at a density of 8×103
cells/well. MTT solution (20 µl) was added into each well of the
96-well plates and cultured for 4 h at 37°C. Following the
discarding of the solution, 150 µl of DMSO solution was added to
dissolve the crystals and incubated at 37°C using an incubator
shaker for 10 min. To detect the optical density (OD), plates were
read at a wavelength of 490 nm using a microplate reader. The same
volume of PBS was used as a blank control.
Melanin synthesis
Solution in 6-well plates was discarded and cells
were washed with phosphate-buffered saline (PBS) twice, digested
with 1 ml 0.25% pancreatin for 1 min, and 1 ml of DMEM was
subsequently added to terminate digestion. Cell suspension was
collected in 15 ml centrifuge tubes, and centrifuged at 1,000 × g
for 5 min at 25°C and the supernatant was discarded. A total of 100
µl of 1 mol/l NaOH was added and the solution was mixed and
incubated at 37°C for 1 h. Following this, 400 µl of double
distilled water was added and 100 µl of solution from each
centrifuge tube was transferred onto 96-well plates. To detect the
OD, plates were read at a wavelength of 490 nm using a microplate
reader.
TYR activity
Solution was discarded and cells in the 96-well
plates were washed with PBS twice, 100 µl Triton 1% was added in
each well, and cells were frozen at −80°C for 30 min before 50 µl
PBS (supplemented with 0.2% L-dopa; pH 6.8) was added and cells
were incubated at 37°C for 3 h. To detect the OD, plates were read
at a wavelength of 490 nm using a microplate reader.
Western blot analysis
To analyze the protein expression of TYR and JNK,
total protein of each group in 6-well plates were separated by
SDS-PAGE. When cells reached 80% confluence, they were incubated
with WIP cell lysis buffer for 30 min at 0°C and centrifuged at
30,000 × g for 15 min at 4°C to extract the total protein. A total
of 30 µg total protein was loaded into each lane and separated by
12% SDS-PAGE. The proteins were transferred onto a polyvinylidene
difluoride membrane, and blocked with 5% skim milk for 1 h at room
temperature to prevent non-specific binding. The membrane was
subsequently incubated with primary antibodies (all 1:300)
overnight at 4°C. The primary antibodies used were as follows:
Mouse anti-β-actin monoclonal antibody, mouse anti-tyrosinase
monoclonal antibody and mouse anti-c-Jun N-terminal kinase (JNK)
monoclonal antibody. The membrane was washed with TBST 3 times for
10 min and incubated with the corresponding HRP goat anti-mouse IgG
(1:10,000) for 1 h at room temperature. The membrane was washed
again and bands were visualized using enhanced chemiluminescence.
Images were captured and analyzed. The quantity of target protein
was indicated by the ratio of its gray value to an internal
reference (β-actin). Bands were quantified using lane 1D gel
imaging analysis software (SmartChemi 500; Beijing Innovation
Technology, Ltd., Beijing, China).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
In order to detect the expression levels of TYR,
TRP-1, TRP-2, extracellular signal-regulated kinase (ERK)1, ERK2
and JNK2 mRNA, total RNA was extracted from each group of cells in
6-well plates using TRIzol reagent. The concentration and purity of
the total RNA extracted from A375 cells were measured using the
Nano-100 micro-spectrophotometer. A total of 3 µg of RNA was
reverse transcribed into cDNA using the AMV First-Strand cDNA
Synthesis kit (Sangon Biotech Co., Ltd., Shanghai, China). Total
RNA was combined with 1 µl oligo(dT) primer and made up to 11 µl
with RNase-free water and centrifuged at 5,000 × g for 3–5 sec at
25°C. The centrifuge tube was placed in a water bath at 70°C for 5
min followed by an ice bath for 30 sec. Primer sequences were
designed and provided by Sangon Biotech Co., Ltd. and are indicated
in Table I, along with the β-actin
internal reference. A final reaction volume of 25 µl was used for
PCR amplification, comprising 16.1 µl ddH2O, 2.5 µl 10X
PCR buffer, 2.5 µl dNTP, 1.3 µl MgCl2,1.5 µl cDNA, 0.5
µl forward primer, 0.5 µl reverse primer and 0.1 µl Taq DNA
polymerase. cDNA amplification conditions included: Initial
denaturation at 94°C for 2 min; denaturation for 30 sec at 94°C;
annealing for 40 sec (TYR at 60°C, TRP-1 at 58°C, TYP-2 at 58°C,
ERK-1 at 58°C, ERK-2 at 55°C, JNK-2 at 55°C, and β-actin at 55°C);
and extension for 40 sec at 70°C. A total of 35 PCR cycles were
used for the amplification of the samples. A total of 5 µl of PCR
amplification products were combined with 1 µl DNA loading buffer
(6X) and the PCR products were fractionated using 1.5% agarose gel
electrophoresis containing 0.5 µg/ml ethidium bromide at a constant
voltage of 120 V for 30 min. Results were detected using the gel
imaging system, and analyzed using gel-pro analysis software
(SmartChemi 500; Beijing Innovation Technology, Ltd.). mRNA levels
of each group were expressed according to the relative value
(TYR/β-actin, TRP-1/β-actin, TRP-2/β-actin, ERK-1/β-actin,
ERK-2/β-actin, and JNK-2/β-actin).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Upstream primer | Downstream
primer | Amplified fragment
(bp) |
|---|
| TYR |
5′TCACGGCTCTGTTGAATGTCT3′ |
5′CTGAAGTTGGGCGAGATGAT3′ | 300 |
| TRP-1 |
5′ACATCATTCCCTCACCAAAGAC3′ |
5′AGAAGTCCGAAAGCCAAGTAAA3′ | 303 |
| TRP-2 |
5′GTTCCTTTCTTCCCTCCAGTG3′ |
5′TTCCTTTATTGTCAGCGTCAG3′ | 300 |
| ERK1 |
5′GGGAGGTGGAGATGGTGAAG3′ |
5′AGCAGGTTGGAGGGCTTTAGAT3′ | 441 |
| ERK2 |
5′ACCCACACAAGAGGATTGAAGT3 |
5′AAAAGCCACAACTACCAGAAAC3′ | 353 |
| JNK2 |
5′CCTTCTTTACCAGATGCTTTGTG3′ |
5′ATACGGTCAGTGCCTTGGAATA3′ | 303 |
| β-actin |
5′CGTGGACATCCGCAAAGAC3′ |
5′AAGAAAGGGTGTAACGCAACTA3′ | 302 |
Statistical analysis
All data were expressed as the mean ± standard
deviation. One-way analysis of variance was used to analyze data,
using the SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA).
A 2:2 comparison was made using Fisher's least significant
difference. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of wogonin on cell
activity
Effects of wogonin on A375 cell activity are
displayed in Table II. Results from
the wogonin group indicate that 10 µmol/l of wogonin had no
significant effect on A375 cell activity when compared with the
control group; therefore, 10 µmol/l of wogonin was used in
subsequent experiments.
 | Table II.Effects of wogonin on A375 cell
activity. |
Table II.
Effects of wogonin on A375 cell
activity.
| Group | Concentration
(µmol/l) | OD | Cell proliferation
rate (%) |
|---|
| Control group | – | 0.470±0.014 | 100.00 |
| Estradiol group |
10−3 |
0.570±0.023a | 121.70a |
| Wogonin group | 10 | 0.448±0.032 |
95.32 |
Effects of wogonin on melanin
synthesis of A375 cells
Wogonin was demonstrated to significantly inhibit
melanin synthesis, which was indicated by the significant decrease
exhibited in the wogonin group (45.99%) when compared with the
control group (100%; P<0.01; Table
III). Both ICI182780 and U0126 treatment was indicated to
reverse the melanin decline elicited by wogonin, as demonstrated by
the significant increase exhibited by the wogonin + ICI182780 and
wogonin + U0126 groups (77.37 and 62.04%, respectively) when
compared with the wogonin group (45.99%; P<0.01; Table III).
 | Table III.Effects of wogonin on the melanin
synthesis of A375 cells. |
Table III.
Effects of wogonin on the melanin
synthesis of A375 cells.
| Group (µmol/l) | OD | OD treatment group/OD
control group (%) |
|---|
| Control group
(−) | 0.138±0.009 | 100.00 |
| Estradiol group
(10−3) |
0.167±0.006a | 121.90a |
| Wogonin group
(10) |
0.063±0.013a |
45.99a |
| Wogonin group
(10) + ICI182780 (1) |
0.106±0.003a,b |
77.37a,b |
| Wogonin group
(10) + U0126 (10) |
0.085±0.005a,b |
62.04a,b |
Effects of wogonin on the TYR activity
of A375 cells
Wogonin significantly inhibited TYR activity, which
was indicated by the significant decrease exhibited by the wogonin
group (65.53%) when compared with the control group (100%;
P<0.01; Table IV). ICI182780 and
U0126 were demonstrated to reverse the decline of TYR activity
elicited by wogonin, as indicated by the significant increases
observed in the wogonin + ICI182780 group (83.53%) and the wogonin
+ U0126 group (77.65%) when compared with the wogonin group
(63.53%; P<0.01; Table IV).
 | Table IV.Effects of wogonin on the TYR activity
of A375 cells. |
Table IV.
Effects of wogonin on the TYR activity
of A375 cells.
| Group (µmol/l) | OD | OD treatment group/OD
control group (%) |
|---|
| Control group
(−) | 0.085±0.005 | 100.00 |
| Estradiol group
(10−3) |
0.106±0.004a | 124.71a |
| Wogonin group
(10) |
0.054±0.004a |
63.53a |
| Wogonin group
(10) + ICI182780 (1) |
0.071±0.011a,b |
83.53a,b |
| Wogonin group
(10) + U0126 (10) |
0.066±0.009a,b |
77.65a,b |
Effects of wogonin on expression of
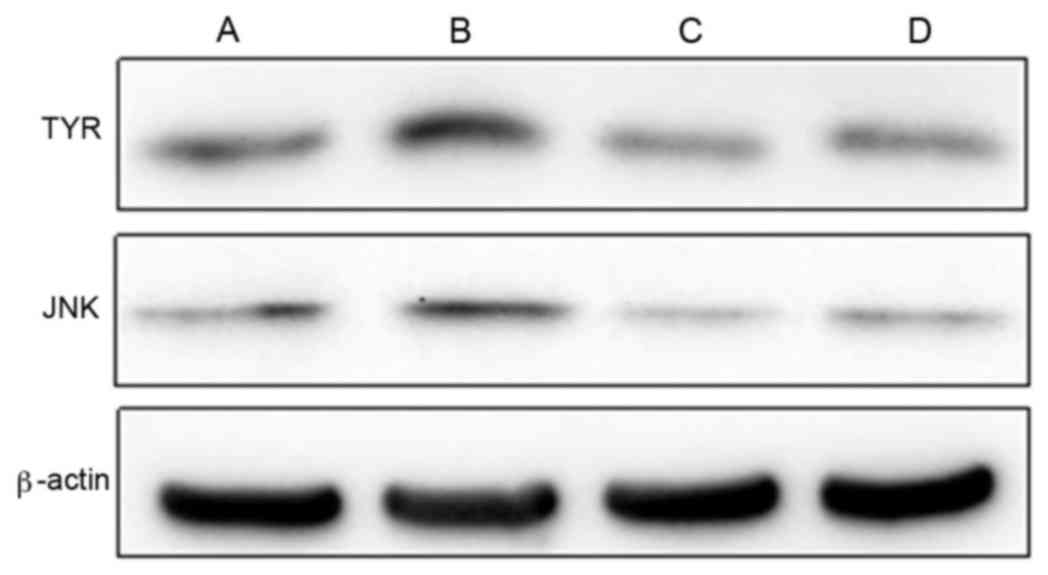
TYR and JNK protein
The wogonin group indicated that the presence of
wogonin significantly inhibited the expression levels of TYR and
JNK protein when compared with the control group (P<0.01;
Table V and Fig. 1). ICI182780 was indicated to reverse
the decline exhibited in the protein expression levels of TYR and
JNK initiated by wogonin, as suggested by the significant increase
displayed by the wogonin + ICI182780 group (TYR, 0.413±0.052 and
JNK, 0.339±0.035) when compared with the wogonin group (TYR,
0.302±0.013 and JNK, 0.214±0.023; P<0.01; Table V).
 | Table V.Expression levels of TYR and JNK
protein in different groups. |
Table V.
Expression levels of TYR and JNK
protein in different groups.
| Group (µmol/l) | TYR/β-actin | JNK/β-actin |
|---|
| Control group
(−) | 0.488±0.018 | 0.428±0.009 |
| Estradiol group
(10−3) |
0.645±0.020a |
0.520±0.012a |
| Wogonin group
(10) |
0.302±0.013a |
0.214±0.023a |
| Wogonin group
(10) + ICI182780 (1) |
0.413±0.052a,b |
0.339±0.035a,b |
Effects of wogonin and ICI182780 on
the mRNA expression levels of TYR, TRP-1, TRP-2, ERK1, ERK2 and
JNK2 in A375 cells
The significantly decreased levels of mRNA
expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 in the wogonin
group suggested that wogonin significantly inhibited the mRNA
expression levels of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 when
compared with the control group (P<0.01; Table VI and Fig. 2). ICI182780 was able to reverse the
decline of the mRNA expression levels of TYR, TRP-1, TRP-2, ERK1,
ERK2 and JNK2 instigated by wogonin, as indicated by the
significant increases in mRNA expression levels of TYR, TRP-1,
TRP-2, ERK1, ERK2 and JNK2 in the wogonin + ICI182780 group when
compared with the TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA
expression levels of the wogonin group (P<0.05; Table VI).
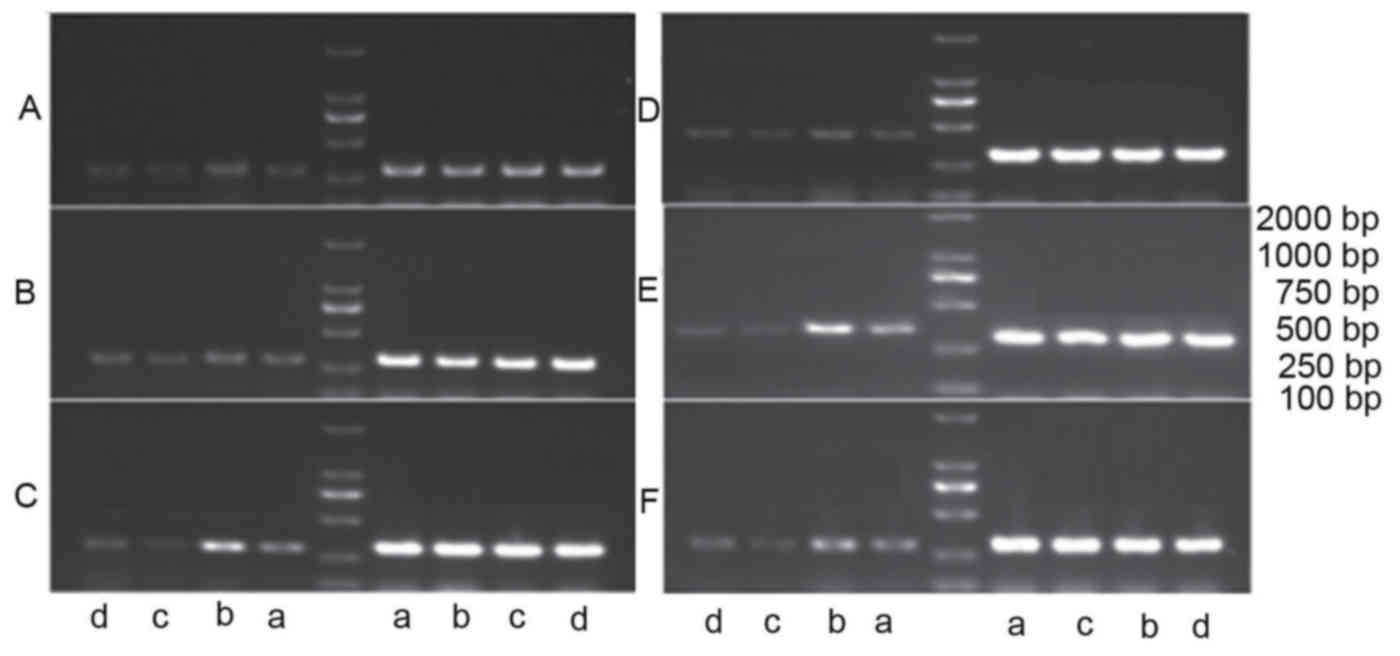 | Figure 2.mRNA expression levels of (A) TYR, (B)
TRP-1, (C) TRP-2, (D) ERK1, (E) ERK2 and (F) JNK2 were detected in
A375 cells by reverse transcription-quantitative polymerase chain
reaction. Groups were divided into (a) the control group, (b) the
estradiol group, (c) the wogonin group and (d) the wogonin +
ICI182780 group. β-actin bands are shown on the right of the
ladder. ICI182780, estrogen inhibitor; TYR, tyrosinase; TRP-1,
tyrosinase related protein 1; TRP-2, tyrosinase related protein 2;
ERK1, extracellular signal-regulated kinase 1; ERK2, extracellular
signal-regulated kinase 2; JNK2, c-Jun N-terminal kinase 2; control
group, cells in the presence of Dulbecco's modified Eagle medium
without treatment; estradiol group, cells in the presence of
estradiol (10−3 µmol/l); wogonin group, cells in the
presence of wogonin (10 µmol/l); wogonin + ICI182780 group, cells
in the presence of ICI182780 (1 µmol/l) and 10 µmol/l of
wogonin. |
 | Table VI.Polymerase chain reaction results of
the mRNA expression levels of TYR, TRP-1, TRP-2, ERK1, ERK2, JNK2
mRNA in different groups. |
Table VI.
Polymerase chain reaction results of
the mRNA expression levels of TYR, TRP-1, TRP-2, ERK1, ERK2, JNK2
mRNA in different groups.
| mRNA | Control group | Estradiol group
(10−3 µmol/l) | Wogonin group (10
µmol/l) | Wogonin group (10
µmol/l)+ ICI182780 group (10 µmol/l) |
|---|
| TYR/β-actin | 0.505±0.012 |
0.666±0.031a |
0.381±0.019a |
0.453±0.033a,b |
| TRP-1/β-actin | 0.441±0.024 |
0.515±0.005a |
0.298±0.012a |
0.389±0.018a,b |
| TRP-2/β-actin | 0.419±0.022 |
0.584±0.023a |
0.231±0.012a |
0.323±0.014a,c |
| ERK1/β-actin | 0.501±0.018 |
0.623±0.021a |
0.252±0.032a |
0.453±0.016a,c |
| ERK2/β-actin | 0.486±0.019 |
0.633±0.030a |
0.292±0.031a |
0.399±0.022a,c |
| JNK2/β-actin | 0.408±0.014 |
0.513±0.028a |
0.252±0.021a |
0.297±0.021a,c |
Effects of wogonin and U0126 on mRNA
expression levels of TYR, TRP-1 and TRP-2 in A375 cells
Results in the wogonin group indicated that wogonin
significantly inhibited the mRNA expression levels of TYR, TRP-1
and TRP-2 when compared with the control group (P<0.01; Table VII and Fig. 3). The presence of U0126 resulted in
the reversal of declined mRNA expression levels of TYR, TRP-1 and
TRP-2 elicited by wogonin, which was suggested by the significant
increases in mRNA expression levels of TYR (0.511±0.030), TRP-1
(0.340±0.039) and TRP-2 (0.299±0.023) exhibited in the wogonin +
U0126 group when compared with the mRNA expression levels of TYR
(0.405±0.018), TRP-1 (0.290±0.023) and TRP-2 (0.235±0.010) in the
wogonin group (P<0.05; Table
VII).
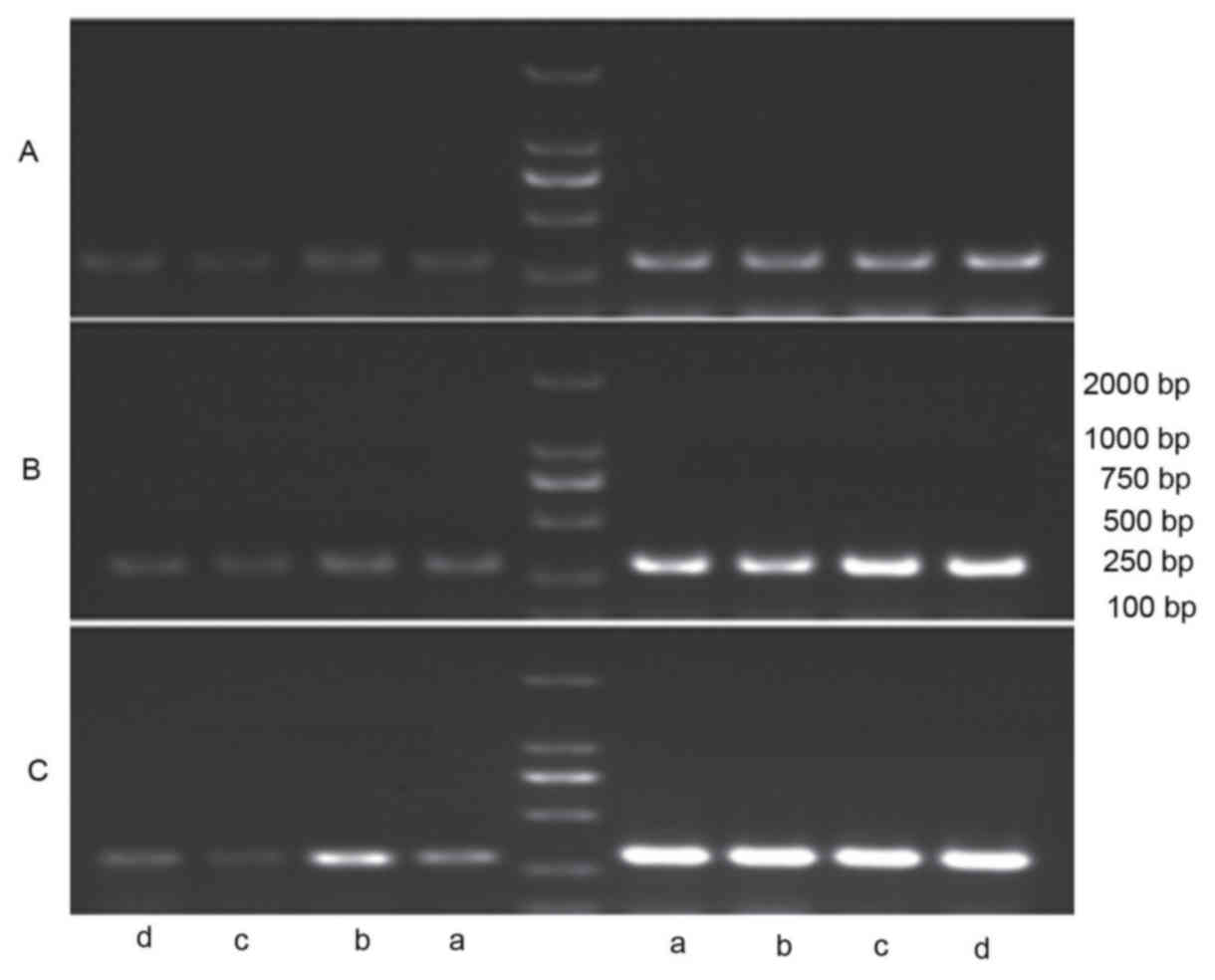 | Figure 3.mRNA expression levels of (A) TYR, (B)
TRP-1 and (C) TRP-2 in A375 cells were detected by reverse
transcription-quantitative polymerase chain reaction in (a) the
control group, (b) the estradiol group, (c) the wogonin group and
(d) the wogonin + ICI182780 group. β-actin bands are shown on the
right of the ladder ICI182780, estrogen inhibitor; TYR, tyrosinase;
TRP-1, tyrosinase related protein 1; TRP-2, tyrosinase related
protein 2; ERK1, extracellular signal-regulated kinase 1; ERK2,
extracellular signal-regulated kinase 2; JNK2, c-Jun N-terminal
kinase 2; control group, cells in the presence of Dulbecco's
modified Eagle medium without treatment; estradiol group, cells in
the presence of estradiol (10−3 µmol/l); wogonin group,
cells in the presence of wogonin (10 µmol/l); wogonin + ICI182780
group, cells in the presence of ICI182780 (1 µmol/l) and 10 µmol/l
of wogonin. |
 | Table VII.Polymerase chain reaction results of
the mRNA expression levels of TYR, TRP-1, TRP-2 mRNA in different
groups. |
Table VII.
Polymerase chain reaction results of
the mRNA expression levels of TYR, TRP-1, TRP-2 mRNA in different
groups.
| mRNA | Control group | Estradiol group
(10−3 µmol/l) | Wogonin group (10
µmol/l) | Wogonin group (10
µmol/l)+ICI182780 group (10 µmol/l) |
|---|
| TYR/β-actin | 0.576±0.018 |
0.654±0.040a |
0.405±0.018a |
0.511±0.030a,b |
| TRP-1/β-actin | 0.462±0.013 |
0.578±0.012a |
0.290±0.023a |
0.340±0.039a,b |
| TRP-2/β-actin | 0.426±0.033 |
0.591±0.014a |
0.235±0.010a |
0.299±0.023a,c |
Discussion
In humans, melanin is the primary determinant of
skin color (11), with a greater
production of melanin resulting in darker skin pigmentation
(12). Melanogenesis is a
complicated process that occurs within the melanosome of
melanocytes and consists of a series of oxidation reactions
catalyzed by various enzymes that are regulated by the tyrosinase
gene, including TYR, TRP-1 and TRP-2 (13–15).
Estrogen is able to bind to the estrogen receptor present in
melanocytes and consequently activate the ER-mediated
mitogen-activated protein kinase (MAPK) signaling pathway. MAPK is
a type of kinase that is specific to serine or threonine and
predominantly interacts with three members: JNK, ERK and p38 MAPK
(16).
Various studies have demonstrated that the MAPK
signaling pathway is involved in the regulation of melanogenesis
and has an important role in the regulation of the activity and
function of melanocytes (17). Park
et al (18) indicated that
the activated ERK pathway may inhibit melanin synthesis through
decreasing the expression of the microphthalmia-associated
transcription factor gene, and an alternative study suggested the
p38 MAPK pathway may promote melanin synthesis through increasing
the expression of the tyrosinase gene (19). Furthermore the JNK pathway may be
implicated in the inhibition of melanin synthesis (20). A previous study revealed that
phytoestrogen extracted from Eucommia ulmoides may inhibit
melanocyte proliferation, melanin synthesis and tyrosinase activity
(9); however, the regulation of
these mechanisms have not yet been fully clarified.
In the present study, safe doses of wogonin were
indicated to significantly inhibit melanin synthesis and tyrosinase
activity in A375 cells. Furthermore, this action was reversed by
the presence of the estrogen receptor inhibitor, ICI182780, and the
ERK inhibitor, U0126. The present findings indicate that wogonin
may inhibit melanin synthesis by decreasing tyrosinase activity via
the ER-ERK pathway. Western blot analysis revealed that wogonin
significantly inhibited the protein expression levels of TYR and
JNK in A375 cells, which was reversed by ICI182780. Decreasing the
protein expression levels of TYR and JNK may therefore be
associated with the regulation of melanin synthesis. RT-PCR showed
that wogonin significantly inhibited mRNA expression levels of TYR,
TRP-1, TRP-2, ERK1, ERK2 and JNK2, which may be involved in the
regulation of melanin synthesis. To conclude, the present study
revealed that wogonin may inhibit the synthesis of melanin in A375
cells by inhibiting the expression of TYR, TRP-1, TRP-2 and ERK1,
ERK2 and JNK2.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81173645),
Heilongjiang Province postdoctoral Research Starting Capital (grant
no. LBH-Q13162) and the Heilongjiang University of Chinese Medicine
Excellent Innovation Talents (grant no. 2012RCD22).
References
|
1
|
Akinyi M, Gao XM, Li YH, Wang BY, Liu EW,
Chai LJ, JawoBah A and Fan GW: Vascular relaxation induced by
Eucommiae ulmoides Oliv. and its compounds Oroxylin A and
wogonin: Implications on their cytoprotection action. Int J Clin
Exp Med. 7:3164–3180. 2014.PubMed/NCBI
|
|
2
|
Ye WF: Advance in studies on chemical
ingredient and pharmacological activities of Eucommiae
ulmoides Oliv. leaves and their utility. Lin Chan Hua Gong Tong
Xun. 38:40–44. 2004.(In Chinese).
|
|
3
|
Ong VY and Tan BK: Novel phytoandrogens
and lipidic augmenters from Eucommia ulmoides. BMC
Complement Altern Med. 7:32007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin ZY: Studies of Eucommia
ulmoides Oliv phytoestrogen (unpublished thesis). Tianjin
University. 2010.(In Chinese).
|
|
5
|
Xu Y, Zhang ZJ, Geng F, Su SB, White KN,
Bligh SW, Branford-White CJ and Wang ZT: Treatment with Qing'E, A
kidney-invigorating Chinese herbal formula, antagonizes the
estrogen decline in ovariectomized mice. Rejuvenation Res.
13:479–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Li MC, Yang J, Yang D, Su YF, Fan
GW, Zhu Y, Gao XM and Paoletti R: Estrogenic properties of six
compounds derived from Eucommia ulmoides Oliv. and their
differing biological activity through estrogen receptors alpha and
beta. Food Chem. 129:408–416. 2011. View Article : Google Scholar
|
|
7
|
Tian ZH: Phytoestrogens and human health.
Shanghai Yufang Yixue. 19:359–361. 2007.(In Chinese).
|
|
8
|
Shelly W, Draper MW, Krishnan V, Wong M
and Jaffe RB: Selective estrogen receptor modulators: An update on
recent clinical findings. Obstet Gynecol Surv. 63:163–181.
2008.PubMed/NCBI
|
|
9
|
Chen L, Zheng Y, Gao J and Zhang JM:
Effects of Yangyanqingewan on the tyrosinase activity and
melanogenesis in B-16 murine melanoma cells. Chin J Hosp Pharm.
22:151–153. 2002.
|
|
10
|
Selzer E, Pimentel E, Wacheck V, Schlegel
W, Pehambergera H, Jansen B and Kodym R: Effects of betulinic acid
alone and in combination with irradiation in human melanoma cells.
J Invest Dermatol. 114:935–940. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Gu H, Meihua G, Tu Y, Xu D and He
L: Pathogenesis research study in melasma: P-593. J Dermatol.
41:932014.
|
|
12
|
Punnonen K, Puntala A, Jansén CT and
Ahotupa M: UVB irradiation induces lipid peroxidation and reduces
antioxidant enzyme activities in human keratinocytes in vitro. Acta
Denn Venereol. 71:239–242. 1991.
|
|
13
|
Hashimoto Y, Ito Y, Kato T, Motokawa T,
Katagiri T and Itoh M: Expression profiles of melanogenesis-related
genes and proteins in acquired melanocytic nevus. J Cutan Pathol.
33:207–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu F, Yan D, Zhou X, Hu DN and Qu J:
Expression of melanin-related genes in cultured adult human retinal
pigment epithelium and uveal melanoma cells. Mol Vis. 13:2066–2072.
2007.PubMed/NCBI
|
|
15
|
Fang D, Kute T and Setaluri V: Regulation
of tyrosinase-related protein-2 (TYRP2) in human melanocytes:
Relationship to growth and morphology. Pigment Cell Res.
14:132–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinase. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang ZQ: The mechanism of active
components of two Chinese traditional medicines on melanogenesis.
Biochem Molecular Biol. 2009.
|
|
18
|
Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH,
Huh CH, Youn SW, Yoo ID and Park KC: Terrein: A new melanogenesis
inhibitor and its mechanism. Cell Mol Life Sci. 61:2878–2885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh SK, Sarkar C, Mallick S, Saha B,
Bera R and Bhadra R: Human placental lipid induces melanogenesis
through p38 MAPK in B16F10 mouse melanoma cells. Pigment Cell Res.
18:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bu J, Ma PC, Chen ZQ, Zhou WQ, Fu YJ, Li
LJ and Li CR: Inhibition of MITF and tyrosinase by
paeond-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am
J Chin Med. 36:245–263. 2008. View Article : Google Scholar : PubMed/NCBI
|