Introduction
Osteoarthritis (OA) is a chronic joint disease
characterized by articular cartilage degeneration and secondary
bone hyperplasia. Its early symptoms are joint pain, swelling and
deformity, which may affect patient quality of life as the disease
progresses (1,2). The overall prevalence of OA is 10–12%.
OA is more common in the elderly, ~60% of people with X-ray
osteoarthritis. Load-bearing and more active joints are more prone
to developing OA, especially the knee joint (3–7). The
most basic pathological change of knee osteoarthritis is the
degeneration and degeneration of articular cartilage (6). Changes in the morphology and metabolism
of chondrocytes and in the biochemical and structural composition
of the matrix are considered to be important induction factors of
OA (7). The most common method to
diagnose OA patients is radiographic examination using the
Kellgren-Lawrence grading system and magnetic resonance imaging
(8,9). Drugs, including non-steroidal
anti-inflammatory analgesics and steroids, are primarily used to
control the acute pain, swelling and other symptoms experienced by
patients with OA. However, there are currently no effective
treatments or drugs able to prevent or reverse disease progression
(10,11).
The Chinese teasel root is able to nourish the liver
and kidney, strengthen the bones and stimulates tocolysis. Saponins
are the primary effective components of the teasel root and 18
types of triterpenoid saponins have been isolated to date. It has
been demonstrated that the Chinese teasel root promotes the
proliferation and differentiation of osteoblasts, and is able to
regulate immune function, indicating that it may be used to treat
arthritis (12). A kind of saponin
in the medicinal herb Dipsacus asper wall has been used as an
antiosteoporosis drug (13).
However, its therapeutic mechanism of action remains unclear and
further studies are required to screen and separate active
components. It is important to determine the pharmacological effect
and mechanism of action of Chinese teasel root to identify the
active components. Thus the present study assessed the molecular
mechanism of treating OA with Dipsacus saponins extracted from the
Chinese teasel root by inhibiting the apoptosis of
chondrocytes.
Materials and methods
Experimental animals
A total of 30 specific-pathogen-free New Zealand
female rabbits with a mean weight of 3.0±0.5 kg (age, 6 months)
were obtained from Vital River Laboratories, Co., Ltd. (Beijing,
China). They were fed at 18–25°C, humidity 40–50%, in a natural
light cycle, with free access to food and water. These rabbits
underwent pre-feeding for 7 days with free access to food and water
to adapt to their environment. Ventilation of the feeding room was
good and rabbits were exposed to natural lighting. The rabbits were
randomly divided into a control group (n=10) and model group
(n=20). The OA model was established using Hulth's modeling method
(14). Animals were anesthetized
with 30 mg/kg intravenous injection of 3% sodium pentobarbital
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The rabbits were
in the supine position and a longitudinal incision in the inner
side of right hind knee joint was performed. The joint cavity was
exposed, the meniscus of the right hind leg was resected
completely, the tibial collateral ligament and anterior and
posterior ligaments were cut off and the wound was sutured layer by
layer and bound using a sterile dressing. Model rabbits were
treated with intramuscular injection of penicillin sodium
20,000,000 U/day for 3 days, whereas the control group received no
treatment. The success of the model establishment was determined by
pathological morphology (cystic degeneration and osteophyte
formation) and measurement of the levels of related inflammatory
cytokines [increased interleukin (IL)-1β, IL-6 and tumor necrosis
factor (TNF)-α]. Housing conditions and procedures involving
experimental animals were in accordance with the Guide for the Care
and Use of Laboratory Animals (15).
All experimental procedures were approved by the Care of
Experimental Animals Committee of Shandong Provincial Hospital
(Jinan, China).
Detection of IL-1β, IL-6 and TNF-α
using ELISA
Synovial fluid was obtained by intra-articular
injection of saline at 6 weeks after surgery. Levels of IL-1β, IL-6
and TNF-α in the synovial fluid were detected using ELISA kits
(cat. nos. ml112819, ml102828 and ml002859; Shanghai Meilian
Biological Technology Co., Ltd.), according to the manufacturer's
instructions. Optical density values at 450 nm were determined by
an ELISA detector (MR-96A, Mindray, Guangzhou, China).
Isolation of chondrocytes and
treatment with dipsacus saponins
Rabbits were anesthetized and sacrificed at 6 weeks
after surgery with artery air-embolism (~5 ml/kg) and rabbit femurs
were isolated under aseptic condition. Soft tissue and the
periosteum were removed, the femoral metaphysic was cut off and the
fracture end was washed with phosphate-buffered saline 3 times. The
femoral metaphysic was cut into bone pieces and cultured with 2 g/l
collagenase II (Gibco; Thermo Fisher Scientific, Inc.) at 37°C for
1.5 h. The digestive liquid was discarded and Dulbecco's Modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) was
added and cultured at 37°C with 5% CO2 for 7 days. A
cell viability curve was constructed using the MTT method. Purple
formazan was dissolved in dimethyl sulfoxide. Absorbance was
measured using a spectrophotometer at 570 nm.
Cells were treated with dipsacus saponins (cat. no.
33289–85-9; Shanghai Yuanye Biological Technology Co., Ltd.)
following three generations (37°C, 5% CO2) and were
divided into 0, 25, 50 and l00 µg/l dipsacus saponin groups. Cell
cycles were measured using flow cytometry following 72 h culture at
37°C in DMEM, using the Cell Cycle and Apoptosis Analysis kit (cat.
no. C1052; Beyotime Institute of Biotechnology, Shanghai, China)
and a flow cytometer. Results were analyzed using BD FACSComp
software (v5.1, BD Biosciences, Franklin Lakes, NJ, USA).
Chondrocytes in the OA model group were treated with sodium
nitroprusside (SNP; Sigma-Aldrich; Merck KGaA) to induce apoptosis.
Chondrocytes were cultured with DMEM containing l mmol/l SNP and
10% fetal bovine serum for 24 h and then treated with dipsacus
saponins for 24 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Harvested cells were washed with RNase free PBS.
Total RNA was extracted using an RNeasy Mini kit (cat. no. 74104;
Qiagen China Co., Ltd., Shanghai, China) according to the
manufacturer's protocol. RNA concentration and purity were detected
using a Qubit Fluorometer (Thermo Fisher Scientific, Inc.). A total
of 1 µg RNA was subjected to reverse transcription using a reverse
transcription kit (Promega Corporation, Madison, WI, USA). qPCR was
performed using a SYBR Green PCR Master mix (Qiagen, Inc.,
Valencia, CA, USA) and the primers used are presented in Table I. The quantification method used was
the 2−∆∆Cq method as described previously (16). GAPDH gene was used as an internal
control. The thermocycling conditions were as follows:
Pre-degeneration at 95°C for 10 min, followed by 40 cycles of
degeneration at 95°C for 10 sec, annealing at 56°C for 20 sec and
extension at 72°C for 33 sec.
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | GenBank accession
no. | Primer (5′-3′) | Length (bps) |
|---|
| Bcl-2 | DQ_529234.1 | For:
GAGCCATCTCAGTGTGTGGAG | 21 |
|
|
| Rev:
GCCAGCATTGCCATAAAAGAGTC | 23 |
| Bax | XM_002723696.2 | For:
CCCGAGAGGTCTTTTTCCGAG | 21 |
|
|
| Rev:
CCAGCCCATGATGGTTCTGAT | 21 |
| Caspase-3 | NM_001082117.1 | For:
CATGGAAGCGAATCAATGGACT | 22 |
|
|
| Rev:
CTGTACCAGACCGAGATGTCA | 21 |
| Caspase-9 | EF472887.1 | For:
CTTCGTTTCTGCGAACTAACAGG | 23 |
|
|
| Rev:
GCACCACTGGGGTAAGGTTT | 20 |
| GAPDH | NM_014364 | For:
TGTGGGCATCAATGGATTTGG | 21 |
|
|
| Rev:
ACACCATGTATTCCGGGTCAAT | 22 |
Western blotting
Cells were lysed with radioimmunoprecipitation assay
lysis buffer (BioVision, Inc., Milpitas, CA, USA). Total proteins
were extracted and and protein concentration was determined using
BCA. Proteins (50 µg per lane) were separated using 12% SDS-PAGE.
Proteins were then electrotransferred to a PVDF membrane. The PVDF
membrane was rinsed with TBS for 10–15 min, placed in TBS/T
blocking buffer containing 5% (w/v) skimmed milk powder and shaken
at room temperature for 1 h. It was incubated at room temperature
for 2 h following the addition of an appropriate dilution of
primary antibody (diluted with TBST containing 1% (w/v) skimmed
milk powder). Primary antibodies were as follows: β-actin
(dilution, 1:1,000; cat. no. 143128; United States Biological,
Salem, MA, USA), CDK4 (1:5,000; cat. no. AHZ0202; Thermo Fisher
Scientific, Inc.), p21 (1:5,000; cat. no. 701151; Thermo Fisher
Scientific, Inc.) and Cyclin D1 (1:5,000; cat. no. 710428; Thermo
Fisher Scientific, Inc.). The membrane was then rinsed with TBST
three times (5–10 min/wash) and then incubated at room temperature
for 1 h with horseradish peroxidase-labeled secondary antibody
(1:10,000; cat. no. 32260; Thermo Fisher Scientific, Inc.) diluted
with TBST containing 0.05% (w/v) skimmed milk powder. The membrane
was then rinsed three times with TBST (5–10 min/wash). Protein
bands were detected using an enhanced chemiluminescence kit (cat.
no. K820-500; BioVision, Inc.) and quantified as the ratio to
β-actin. Quantification was performed using ImageJ software (v1.37;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All results are presented as the mean ± standard
deviation and analyzed using SPSS 20.0 software (IBM Corp., Armonk,
NY, USA). One-way analysis of variance and Student's t test were
used to evaluate the differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
OA model
Rabbits in control group were normal and able to
move their joints freely. In model rats, restricted joint movement
was observed 6 weeks after the operation. The surface and edges of
joints from rabbits in the control group were smooth with a clear
and bright color and the synovial joint fluid was clear. By
contrast, the joint surface of rabbits in the model group was rough
with irregular edges and tiny cracks and the joint synovial fluid
was turbid (data not shown).
The results of ELISA measuring IL-1β, IL-6 and TNF-α
levels in the synovial fluid in all rabbits are presented in
Table II. The levels of IL-1β, IL-6
and TNF-α in the synovial fluid of rabbits in the model group were
significantly higher than those of control rabbits (P<0.05),
indicating that the OA model was successfully established.
 | Table II.IL-1β, IL-6, TNF-α levels in the
synovial fluid as detected by ELISA. |
Table II.
IL-1β, IL-6, TNF-α levels in the
synovial fluid as detected by ELISA.
| Group | IL-1β | IL-6 | TNF-α |
|---|
| Control (pg/ml) | 12.11±1.77 | 16.54±1.97 | 5.38±1.15 |
| Model (pg/ml) |
36.56±4.57a |
36.96±3.08b |
29.61±3.62a |
Dipsacus saponins promote the
viability of chondrocytes
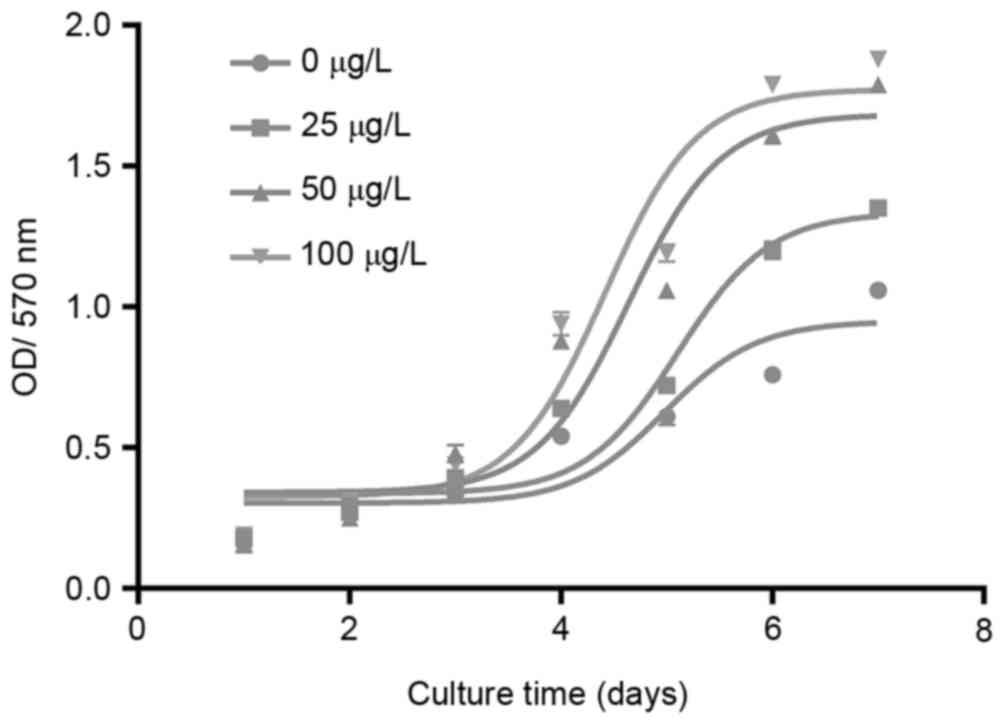
The cell viability curves of the different groups of
chondrocytes (treated with 0, 25, 50 and l00 µg/l dipsacus
saponins) are presented (Fig. 1).
This demonstrates that the viability of chondrocytes increased
following treatment with dipsacus saponins in a
concentration-dependent manner.
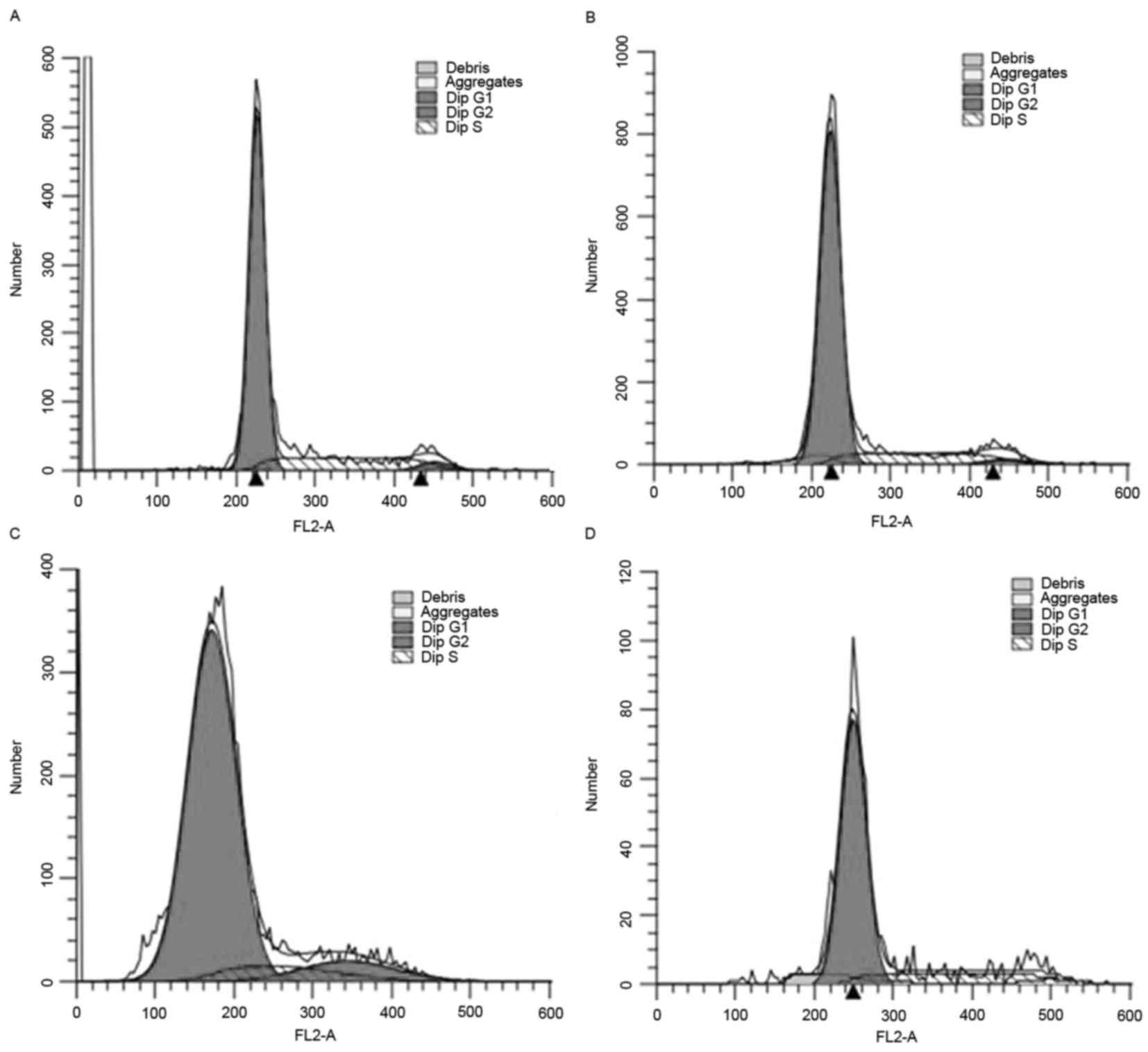
The results from flow cytometry demonstrated that
the number of chondrocytes in the G0/G1 phase decreased in the
groups treated with 50 and l00 µg/l dipsacus saponins, whereas the
number of chondrocytes in the G2/M phase increased in these groups
(Fig. 2).
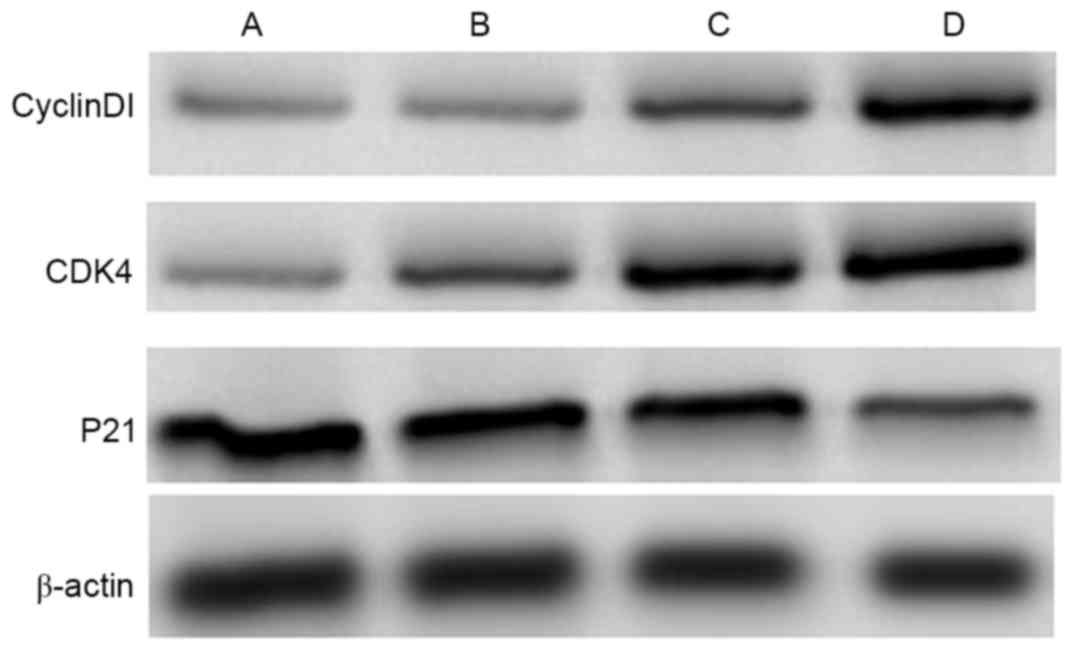
The results from western blotting are presented in
Fig. 3. Levels of Cyclin Dl and
cyclin-dependent kinase 4 (CDK4) expression increased in groups
treated with 50 and l00 µg/l dipsacus saponins and this increase
occurred in concentration-dependent manner. By contrast, levels of
p21 expression decreased in the groups treated with 50 and l00 µg/l
dipsacus saponins and this decrease occurred in a
concentration-dependent manner.
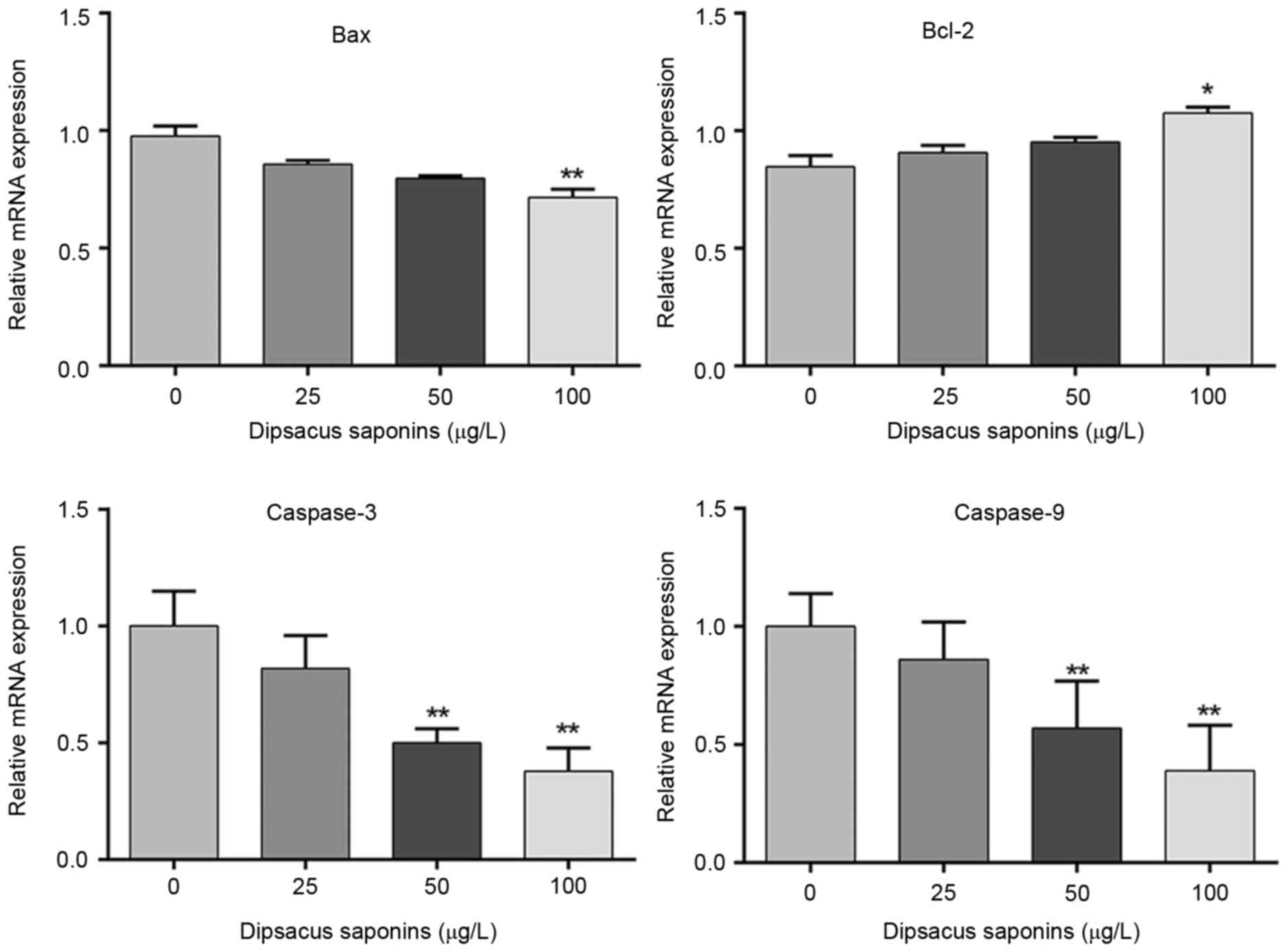
Dipsacus saponins inhibit the
apoptosis of chondrocytes
Exogenous SNP was added to cells to induce
apoptosis. Cells were then treated with 0, 25, 50 or l00 µg/l
dipsacus saponins. Levels of Bcl-2, Bax, caspase-3 and caspase-9
mRNA were measured by RT-qPCR. The results demonstrated that levels
of Bax, caspase-3 and caspase-9 mRNA decreased following treatment
with dipsacus saponins in a concentration-dependent manner. The
decreases in levels of Bax, caspase-3 and caspase-9 mRNA were
significant following treatment with 100 µg/l dipsacus saponins
(P<0.01). By contrast, levels of Bcl-2 mRNA significantly
increased in chondrocytes treated with l00 µg/l dipsacus saponins
(P<0.05; Fig. 4).
Discussion
Chondrocytes help maintain joint function and serve
an important role in the development of OA (17). The pathological changes that occur in
OA primarily manifest as cartilage lesions, which lead to
degenerative changes occurring in the cartilage cells and matrix
(18). The animal model of OA used
in the current study facilitated the study of OA pathogenesis. The
current study evaluated dipsacus saponins as a potential
therapeutic drug to treat OA and determined its effect on
chondrocytes.
The shape of chondrocytes is irregular at the early
stages of culture (19). The current
study determined that chondrocyte viability increased following
treatment with dipsacus saponins in a concentration-dependent
manner, suggesting that dipsacus saponins serve a role in promoting
cell viability. Alterations in the cell cycle affect the lifespan
of cells. Promoting the proliferation of chondrocytes is an
efficient treatment to delay the progression of cartilage
degradation (20). It was determined
that the proportion of cells undergoing DNA synthesis and in the
S-phase increased and the proportion of cells in the G0/G1 phase
decreased in the group treated with high-doses of dipsacus
saponins, promoting cell viability in this manner.
The cell cycle is regulated by numerous factors:
Cyclin A2 controls both S phase and G2/M transition (21), Cyclin E regulates the G1-S-phase
transition (22) and Cyclin B is
synthesized in late S and G2 phases and plays an important role in
G2-M phase transition (23). Cyclin
D1 is an important regulatory protein in the cell cycle that
promotes changes in the cell cycle by regulating receptors,
including the estrogen receptor and Sp1 (24). By contrast, p21 inhibits the growth
of cells and determines whether the cell divides or undergoes
apoptosis (25,26). The present study demonstrated that
levels of cyclin Dl and CDK4 expression significantly increased in
chondrocytes treated with 50 and l00 µg/l dipsacus saponins in a
concentration-dependent manner, whereas levels of p21 expression
significantly decreased in groups treated with 50 and l00 µg/l
dipsacus saponins and this decrease also occurred in a
concentration-dependent manner. These results suggest that dipsacus
saponins have a selective intervention role on chondrocytes and may
effectively promote the viability of chondrocytes by regulating the
cell cycle.
The regulation of cell apoptosis is an important
factor in the maintenance of cells and cell apoptosis serves an
important role in the regulation of the cellular pathology process
(27–29). Proteins belonging to the Bcl-2 family
have important regulatory effects on cell apoptosis and their
abnormal expression is closely associated with the occurrence and
development of many cancer types, including lung cancer (30). The present study determined that
levels of Bcl-2 expression increased in the group treated with l00
µg/l dipsacus saponins. Bax belongs to the Bcl-2 family and has an
inhibitory effect on Bcl-2. Additionally, it has been determined
that the ratio of Bax/Bcl-2 is a key factor in determining the
inhibition of apoptosis (31). Thus,
Bax is one of the most important genes in the promotion of
apoptosis. The present study demonstrated that levels of Bax
expression significantly decreased following treatment with
dipsacus saponins in concentration-dependent manner. SNP, which is
an in vitro nitric oxide donor, promotes the initiation of
apoptosis (32). The present study
demonstrated that dipsacus saponins may inhibit the apoptosis
induced by SNP and that the expression of caspases-3 and −9
decreased following treatment with dipsacus saponins in a
concentration-dependent manner. Therefore, dipsacus saponins may
inhibit the apoptosis of chondrocytes and preserve the function of
chondrocytes.
In conclusion, dipsacus saponins may inhibit
apoptosis signal transduction by up-regulating Bcl-2 and
down-regulating caspase-9, caspase-3 and Bax expression. This means
that dipsacus saponins may be used to effectively inhibit the
apoptosis of chondrocytes, maintain the function of chondrocytes
and suppress the loss of bone mass. Therefore, it may have
therapeutic potential for osteoarthritis.
References
|
1
|
Nelson AE, Smith MW, Golightly YM and
Jordan JM: ‘Generalized osteoarthritis’: A systematic review. Semin
Arthritis Rheum. 43:713–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laulan J, Marteau E and Bacle G: Wrist
osteoarthritis. Orthop Traumatol Surg Res. 101 (1 Suppl):S1–S9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mody GM and Brooks PM: Improving
musculoskeletal health: Global issues. Best Pract Res Clin
Rheumatol. 26:237–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grotle M, Hagen KB, Natvig B, Dahl FA and
Kvien TK: Prevalence and burden of osteoarthritis: Results from a
population survey in Norway. J Rheumatol. 35:677–684.
2008.PubMed/NCBI
|
|
6
|
Rahman MM, Kopec JA, Goldsmith CH, Anis AH
and Cibere J: Validation of administrative osteoarthritis diagnosis
using a clinical and radiological population-based cohort. Int J
Rheumatol. 2016:64753182016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doherty M, Watt I and Dieppe P: Influence
of primary generalised osteoarthritis on development of secondary
osteoarthritis. Lancet. 2:8–11. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi D, Roemer FW and Guermazi A:
Osteoarthritis year 2011 in review: Imaging in OA-a radiologists'
perspective. Osteoarthritis Cartilage. 20:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egloff C, Hügle T and Valderrabano V:
Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly.
142:w135832012.PubMed/NCBI
|
|
11
|
Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim
BJ, Min BH and Chun JS: Hypoxia-inducible factor-2alpha is a
catabolic regulator of osteoarthritic cartilage destruction. Nat
Med. 16:687–693. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mortarino M, Franceschi A, Mancianti F,
Bazzocchi C, Genchi C and Bandi C: Quantitative PCR in the
diagnosis of Leishmania. Parassitologia. 46:163–167. 2004.(In
Italian). PubMed/NCBI
|
|
13
|
Niu Y, Li Y, Huang H, Kong X, Zhang R, Liu
L, Sun Y, Wang T and Mei Q: Asperosaponin VI, a saponin component
from Dipsacus asper wall, induces osteoblast differentiation
through bone morphogenetic protein-2/p38 and extracellular
signal-regulated kinase 1/2 pathway. Phytother Res. 25:1700–1706.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogart JN, Barrach HJ and Chichester CO:
Articular collagen degradation in the Hulth-Telhag model of
osteoarthritis. Osteoarthritis Cartilage. 7:539–547. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals: Guide for the Care and Use
of Laboratory Animals. 8th. The national academies press; NW
Washington DC: 2010
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CG, Thuillier D, Chin EN and Alliston
T: Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix
metalloproteinase 13 expression to maintain articular cartilage and
prevent osteoarthritis. Arthritis Rheum. 64:3278–3289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mobasheri A, Kalamegam G, Musumeci G and
Batt ME: Chondrocyte and mesenchymal stem cell-based therapies for
cartilage repair in osteoarthritis and related orthopaedic
conditions. Maturitas. 78:188–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu XF, Zhou ZH and Zou J: MicroRNA-181
inhibits proliferation and promotes apoptosis of chondrocytes in
osteoarthritis by targeting PTEN. Biochem Cell Biol. 95:437–444.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Chen J, Liang W, Li H, Liu F, Weng
X, Lin P, Chen W, Zheng C, Xu H, et al: Bushen Zhuangjin Decoction
promotes chondrocyte proliferation by stimulating cell cycle
progression. Exp Ther Med. 9:839–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Peng L and Seto E: Histone
Deacetylase 10 Regulates the Cell Cycle G2/M Phase Transition via a
Novel Let-7-HMGA2-Cyclin A2 Pathway. Mol Cell Biol. 35:3547–3565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gavet O and Pines J: Progressive
activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell.
18:533–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duman-Seheel M, Weng L, Xin S and Du W:
Hedgehog regulates cell growth and proliferation by inducing cyclin
D and cyclin E. Nature. 417:299–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biyja V, Pachemik J, Vondrácek J, Soucek
K, Cajánek L, Horvath V, Holubcová Z, Dvorák P and Hampl A: Lineage
specific composition of cyclin D-CDK4/CDK6-p27 complexes reveals
distinct functions of CDK4, CDK6 and individual D-type cyclins in
differentiating cells of embryonic origin. Cell Prolif. 41:875–893.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baici A, Lang A, Hörler D and Knöpfel M:
Cathepsin B as a marker of the dedifferentiated chondroeytes
phenotype. Ann Rheum Dis. 47:684–691. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou WS, Li Z, Büttner FH, Bartnik E and
Brömme D: Cleavage site specificity of cathepsin K toward cartilage
proteoglycans and protease complrx formation. Biol Chem.
384:891–897. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fasshauer M, Klein J, Neumann S, Eszlinger
M and Paschke R: Isoproterenol inhibits resistin gene expression
through a G(S) protein-coupled pathway in 3T3-L1 adipocytes. FEBS
Lett. 500:60–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao XD, He YY, Gao J, Zhao C, Zhang LL,
Tian JY and Chen HL: High expression of Bcl-2 protein predicts
favorable outcome in non-small cell lung cancer: Evidence From A
Systematic Review And Meta-Analysis. Asian Pac J Cancer Prev.
15:8861–8869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Del Principe MI, Dal Bo M, Bittolo T,
Buccisano F, Rossi FM, Zucchetto A, Rossi D, Bomben R, Maurillo L,
Cefalo M, et al: Clinical significance of BAX/BCL-2 ratio in
chronic lymphocytic leukemia. Haematologica. 101:77–85. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Q, Mei X, Han G, Ling P, Guo B, Guo
Y, Shao H, Wang G, Cui Z, Bai Y and Xu F: Xanthan gum protects
rabbit articular chondrocytes against sodium nitroprusside-induced
apoptosis in vitro. Carbohydr Polym. 131:363–369. 2015. View Article : Google Scholar : PubMed/NCBI
|