Introduction
In recent years, heart failure (HF) has become a
global health problem and is a serious threat to human health
(1). Chronic heart failure (CHF) is
a relatively stable state of HF, and is a clinical syndrome with a
complex pathophysiology. CHF is used to describe any functional or
structural disorder that affects the heart and prevents the
ventricles from pumping blood efficiently and/or the heart chambers
filling completely (2,3). According to current treatment
guidelines, the available treatments for patients with CHF include
angiotensin receptor inhibitors or angiotensin-converting enzyme
inhibitors, diuretics, β-blockers, aldosterone receptor
antagonists, vasodilating agents and digitalis (4). However, the use of these drugs is
limited by their serious side effects (5,6). In
addition, despite significant advances in the diagnosis and
treatment of patients with CHF, their prognoses remains poor, with
an unacceptably high risk of mortality (7). Therefore, there is an urgent
requirement for the identification of novel therapeutic agents that
effectively treat patients with CHF and exhibit fewer side
effects.
Xanthium strumarium L. (X.
strumarium), from the Asteraceae family, is a medicinal
plant distributed widely in North America, Brazil, China, Malaysia,
and regions of India with warm climates (8). The X. strumarium fruits (also
known as ‘Cangerzi’ in Chinese) are traditionally used to treat
rhinitis, rheumatism, tuberculosis, cancer, ulcers and malaria
(9). A previous report demonstrated
that X. strumarium contains a number of chemical
constituents, including flavones, saponins, affeyolquinic acids,
caffeic acids, and sesquiterpene lactones (10). Caffeoylxanthiazonoside (CYT;
7-hydroxymethyl-8,8-dimethyl-4,8-dihydrobenzol), a thiazinedione
heterocyclic compound isolated from the fruits of X.
strumarium, has been reported to possess significant
anti-allergy, anti-inflammatory and analgesic properties (10,11).
However, to the best of the author's knowledge, no studies
published to date have investigated the cardioprotective effects of
CYT. Therefore, in the present study, a rat model of CHF was
generated to investigate the cardioprotective effects of CYT
isolated from the fruits of X. strumarium. The results
suggest that this compound may be beneficial for the clinical
treatment of patients with CHF in the future.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM)-F12, fetal
bovine serum (FBS) and radioimmunoprecipitation assay (RIPA) lysis
buffer were purchased from Invitrogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Creatine kinase (CK) (cat. no. A032) and
lactate dehydrogenase (LDH) (cat. no. A020-2) assay kits, and
pentobarbital were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). Tumor necrosis factor (TNF)-α (cat. no.
RTA00), interleukin (IL)-6 (cat. no. R6000B) and IL-1β (cat. no.
RLB00) ELISA kits were purchased from R&D Systems China Co.,
Ltd. (Shanghai, China). The bicinchoninic acid assay (BCA) kit and
primary antibodies against nuclear factor-κB (NF-κB) p65 (cat. no.
AN371; dilution, 1:1,000), IκB (cat. no. AI096; dilution, 1:1,000)
were obtained from Beyotime Institute of Biotechnology (Haimen,
China). The primary antibody against histone H1 (cat. no, ab203337;
dilution, 1:1,000) and GAPDH (cat. no. ab9485; dilution, 1:1,000)
were purchased from Abcam (Shanghai, China). All additional
chemicals and reagents were of analytical grade.
Isolation, purification and
identification of CYT
X. strumarium fruits were purchased from the
Tongrentang Chinese herbal medicine store (Taiyuan, China), and
their identity was verified by professor Xiaoming Guo affiliated to
the Department of Traditional Chinese Medicine of the Shanxi
Cardiovascular Hospital (Taiyuan, China). A voucher specimen was
deposited in the herbarium of the Shanxi Cardiovascular Hospital
(specimen no. SP1507-07a). The dried fruits of X. strumarium
were powdered and extracted with 75% EtOH by refluxing three times
for 2 h each time (solid: liquid ratio, 1:10 w/v). The extracts
were then filtered, and the solvent (75% EtOH) was evaporated at
50°C under reduced pressure in a vacuum using a vacuum rotary
evaporator (Model, CA-1115D; EYELA; Tokyo Rikakikai Co., Ltd.,
Tokyo, Japan). The residue was successively extracted with
petroleum ether, ethyl acetate and n-butanol. Based on a previous
report (11), the n-butanol fraction
was selected and eluted through silica gel (100–200 mesh) with
ethyl acetate and MeOH (at ratios of 20:1, 15:1, 12:1, 10:1, 8:1,
5:1, 2:1 and 1:2), and a series of sub-fractions (I–IV) were
obtained based on the Thin-layer chromatography analysis. Fraction
IV was subjected again to a series of chromatographic separation
techniques, including silica gel columns (200–300 mesh) (Qingdao
Haiyang Chemical Co., Ltd., Qingdao, China) and Sephadex LH-20
media (GE Healthcare Life Sciences; Little Chalfont, UK) and eluted
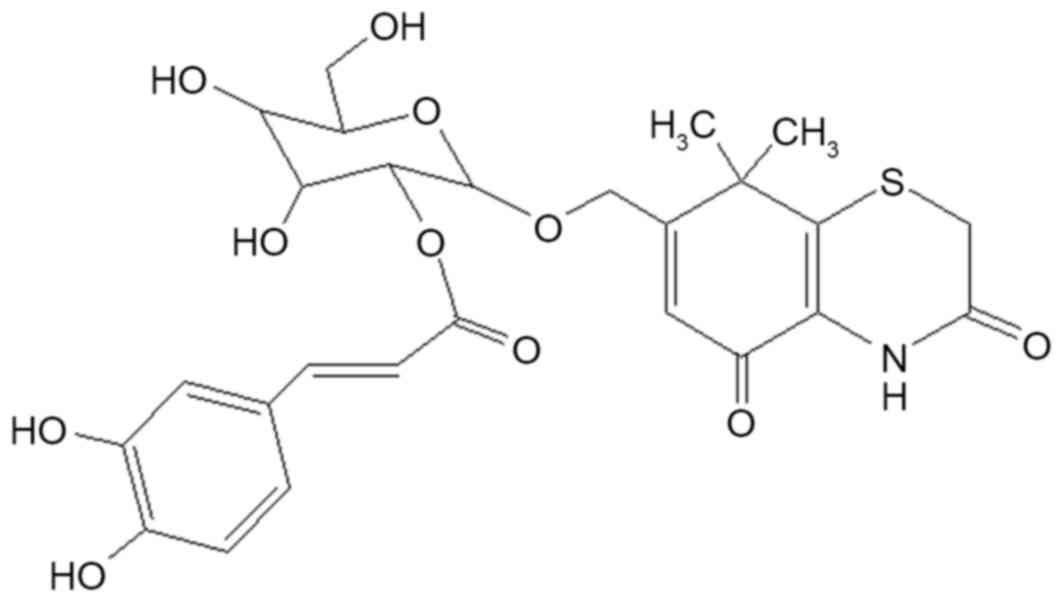
with pure MeOH to purify CYT. The molecular structure of CYT is
presented in Fig. 1. Purified CYT (5
mg for 1H, 20 mg for 13C) was identified by
1H-NMR and 13C-NMR recorded on Bruker
AVANCE-III (600 MHz for 1H and 150 MHz for
13C) spectrometer. The size of the NMR tube was 5×177.8
mm and the speed of rotation was 20 Hz. Data were acquired and
processed using the Bruker program TopSpin 2.1. The results were
confirmed by comparing with a previous study (12). An Agilent 1260 High-performance
liquid chromatography system (Agilent Technologies, Inc., Santa
Clara, CA, USA) was used to determine the purity of isolated CYT.
Sample separation was performed on a Diamonsil ODS column (250×4.6
mm) at 35°C. The mobile phase was consisted of acetonitrile (10%)
and 0.1% formic acid water solution (90%). The flow rate was 1.0
ml/min and the injection volume was 10.0 µl. The results
demonstrated that sample extracts were >98% pure.
Animals
A total of 80 male Sprague-Dawley (SD) rats (210±20
g; aged 6–7 weeks old) and 10 SD rats (8±1 g; aged1-3 days) were
purchased from the Shanghai Laboratory Animal Research Centre
(Shanghai, China). Throughout the experimental period, rats were
housed under controlled conditions at 21±2°C with 12-h light/dark
cycles, and access to a standard pellet diet (Nestlé Purina PetCare
Company, St. Louis, MO, USA) and water ad libitum. The rats
were maintained on this diet for 1 week prior to the initiation of
experimental procedures. All experimental protocols employed in the
present study were approved by the Animal Ethics Committee at
Shanxi Medical University (Taiyuan, China).
Generation of the CHF model
Rats were divided into the following 5 groups at
random (n=16 rats/group): Sham group, CHF group, and three CYT
treatment groups where CHF rats were administered with 10, 20 and
40 mg/kg/day CYT, respectively. CHF model rats were generated by
ligating the left anterior descending (LAD) coronary artery,
according to the methods described previously (13,14).
Briefly, rats were first anaesthetized by intraperitoneal injection
of pentobarbital (40 mg/kg). They were subsequently intubated and
ventilated with an automatic breathing machine (Shanghai Yuyan
Instruments, Co., Ltd., Shanghai, China) using the following
parameters: Respiratory rate, 80 cycles/min; tidal volume, 4–6 ml;
respiratory ratio, 1:1. The pericardium was then opened under
sterile conditions, the heart was exteriorized, and the LAD
coronary artery was ligated rapidly. Rats in the sham group
underwent the identical surgical procedure without ligation of the
LAD coronary artery. Rats were monitored for 24 h post-surgery, and
those that survived were selected for subsequent experiments (n=10
rats/group).
In the CYT-treated groups, rats were administered
with intraperitoneal injections of 10, 20 or 40 mg/kg/day CYT
continuously for 8 weeks, while rats in the sham and CHF model
groups were administered with an equal volume of saline. Following
8 weeks of treatment, the cardiac functions of rats were examined.
In addition, rats were anesthetized by intraperitoneal injection of
40 mg/kg pentobarbital, and blood samples were collected from the
abdominal aorta. Furthermore, heart tissues were collected for the
subsequent biochemical analyses.
Determination of cardiac function
A cardiac sonography was performed using a GE Vivid
i Portable Ultrasound System (GE Healthcare Life Sciences,
Shanghai, China) with a 12-MHz linear transducer (12 l), according
to the methods described previously (15). Rats were first anesthetized by
intraperitoneal injection of 40 mg/kg pentobarbital and fixed in a
supine position. Following removal of the chest fur, the detector
of the ultrasound system was placed on the left side of the chest.
The results were recorded using the workstation supplied by the
portable ultrasound system. This was used to measure the following
cardiac function indexes: Ejection fraction (EF), heart rate (HR),
fractional shortening (FS) and cardiac output (CO).
Determination of TNF-α, IL-6 and IL-β
levels in cardiac tissues
The heart tissues of rats were collected and
homogenized in RIPA lysis buffer (1:10, w/v), and centrifuged at
6,000 × g for 15 min at 4°C. The level of TNF-α, IL-6 and IL-β
cytokines in heart tissues was determined by using commercial ELISA
kits according to the manufacturer's instructions (R&D Systems
China Co., Ltd.). The absorbance was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Determination of serum LDH and CK
levels
Blood samples were collected from the abdominal
aorta, and the serum was separated from whole blood by high-speed
centrifugation at 6,000 × g for 15 min at 4°C. The level of
myocardial enzymes, LDH and CK, were measured using commercial
assay kits according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute). The absorbance was measured at
340 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Isolation of cardiac microvascular
endothelial cells (CMECs) and culture
CMECs were isolated from the heart tissues of 10 SD
rats (8±1 g; aged 1–3 days) using the methods described previously
(16). The rats were sacrificed, and
then the hearts of the rats were separated and sterilized with 70%
EtOH. CMECs were subsequently isolated from heart tissues by
incubating tissues in 0.2% type II collagenase and 0.08% trypsin in
Hank's balanced salt solution for 35 min at 37°C. The solution was
filtered and centrifuged at 1,000 × g for 10 min at 4°C. to remove
myocytes. The CMECs were then resuspended in DMEM-F12 medium
containing 20% FBS, and incubated in culture flasks for 2 h at
37°C. The cells were subsequently washed carefully with phosphate
buffered saline three times (30 sec each time) to remove any
non-adherent cells and debris. Isolated CMECs formed confluent
monolayers at 5–7 days of culture.
Determination of TNF-α, IL-1β and IL-6
levels in CMECs
CMECs (1×105 cells/ml) were seeded in
96-well plates. The cells in experimental groups were treated with
20, 40 or 80 µg/ml CYT for 24 h at 37°C in the presence of 100
ng/ml lipopolysaccharide (LPS). The cells in the control group were
only cultured in DMEM-F12 medium containing 20% FBS, whereas the
model group cells were cultured in DMEM with LPS stimulation (100
ng/ml). Cell culture supernatants were collected and centrifuged at
6,000 × g for 15 min at 4°C. The levels of TNF-α, IL-1β and IL-6 in
the cell culture supernatants were subsequently measured using the
same commercial assay kits as mentioned above according to the
manufacturer's instructions (R&D SystemsChina Co., Ltd.,
Shanghai, China). The absorbance was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Western blotting
CMECs (1×105 cells/ml) were seeded in
24-well plates. The experimental groups were treated with 20, 40 or
80 µg/ml CYT and 100 ng/ml LPS. The control group was only cultured
in DMEM, whereas the model group was cultured in DMEM with LPS
stimulation (100 ng/ml). Following incubation at 37°C for 24 h,
nuclear and cytoplasmic proteins were extracted from CMECs by using
the nuclear and cytoplasmic protein extraction kits (cat. no.
P0027; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The protein concentration was
determined using a BCA protein assay kit. For each sample, 30 µg
total proteins were separated using 10% SDS-PAGE and blotted onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk at room
temperature. They were subsequently incubated with the
corresponding primary antibodies including NF-κB p65 (dilution
1:1,000), IκB (dilution 1:1,000), histone H1 (dilution 1:1,000) and
GAPDH (dilution 1:1,000) as mentioned above for 24 h at 4°C,
followed by incubation with horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (cat. no. A0216; 1:1,000 dilution;
Beyotime Institute of Biotechnology) for 1 h at room temperature.
Protein bands were subsequently visualized using the Beyo-ECL Plus
reagent (Beyotime Institute of Biotechnology). Target protein
expression levels were normalized to that of GAPDH, and the
fold-change in expression was determined.
Statistical analysis
The results are expressed as the mean ± standard
deviation (n=3), and statistical analyses were performed using SPSS
software (version, 17.0; SPSS, Inc., Chicago, IL, USA). The results
were analyzed by one-way analysis of variance and Dunnett multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of CYT on the heart-body weight
index
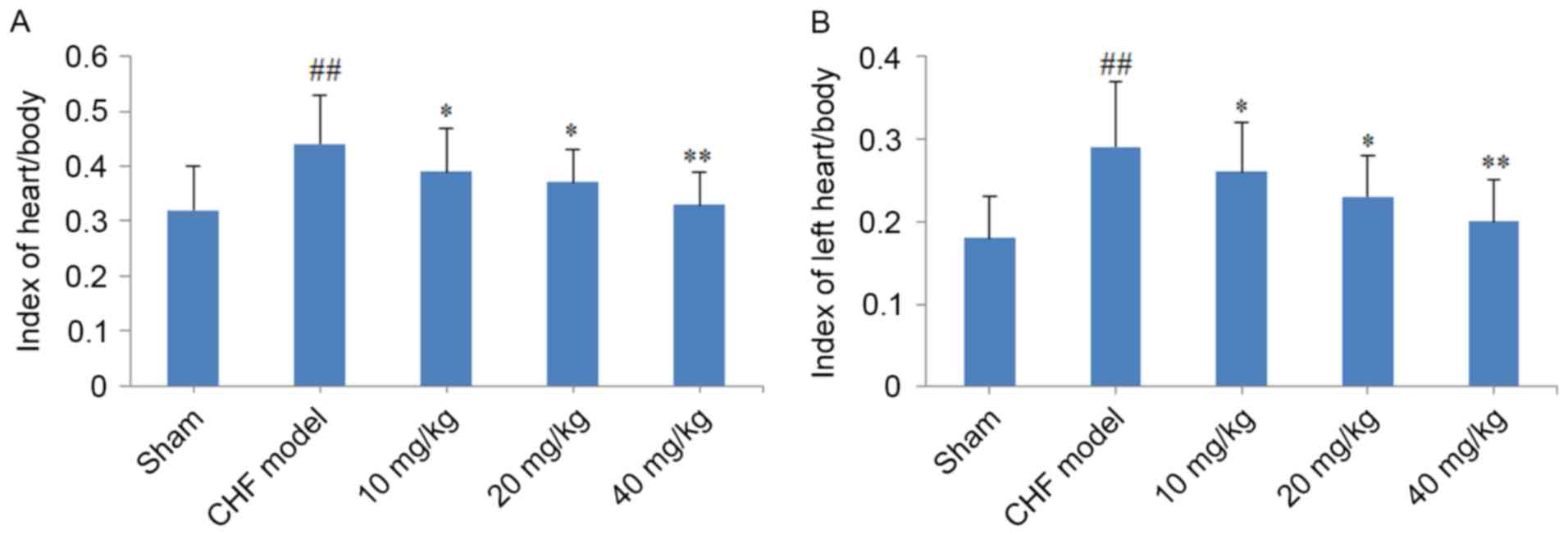
As demonstrated in Fig.
2, a significant increase in the heart/body weight and left
heart/body weight indexes (P<0.01) were observed in the CHF
model group when compared with the sham group. Notably, cardiac
hypertrophy was reversed following treatment of CHF model rats with
CYT for 8 weeks, as indicated by the significant reduction in the
heart/body weight and left heart/body weight indexes in the 10, 20
and 40 mg/kg CYT-treated groups when compared with the CHF model
group (P<0.05, P<0.05 and P<0.01, respectively; Fig. 2).
Effect of CYT on cardiac function in
CHF model rats
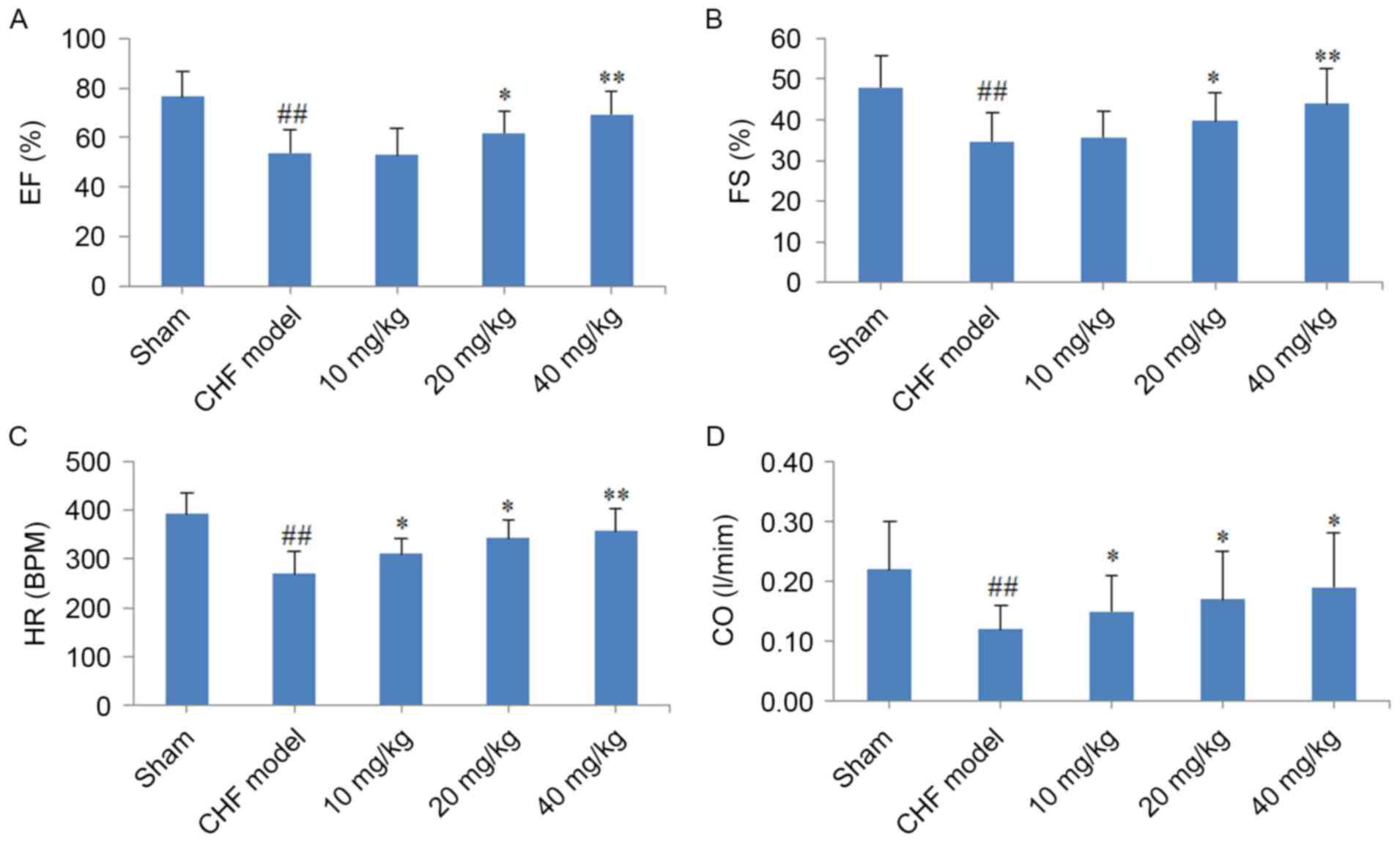
When compared with rats in the sham group, the FS,
EF, CO and HR cardiac function indexes were significantly decreased
in CHF rats (P<0.01; Fig. 3). By
contrast, treatment of CHF model rats with 20 or 40 mg/kg CYT for 8
weeks was associated with a significant increase in the EF and FS
indexes when compared with untreated CHF model rats (20 mg/kg,
P<0.05; 40 mg/kg, P<0.01; Fig. 3A
and B). Notably, HR was significantly increased following
treatment of CHF model rats with 10, 20 and 40 mg/kg CYT
(P<0.05, P<0.05 and P<0.01, respectively; Fig. 3C). In addition, the CO index was
significantly increased following treatment with increasing
concentrations of CYT (P<0.05; Fig.
3D).
Effect of CYT on the level of TNF-α,
IL-1β and IL-6 in cardiac tissues from CHF model rats
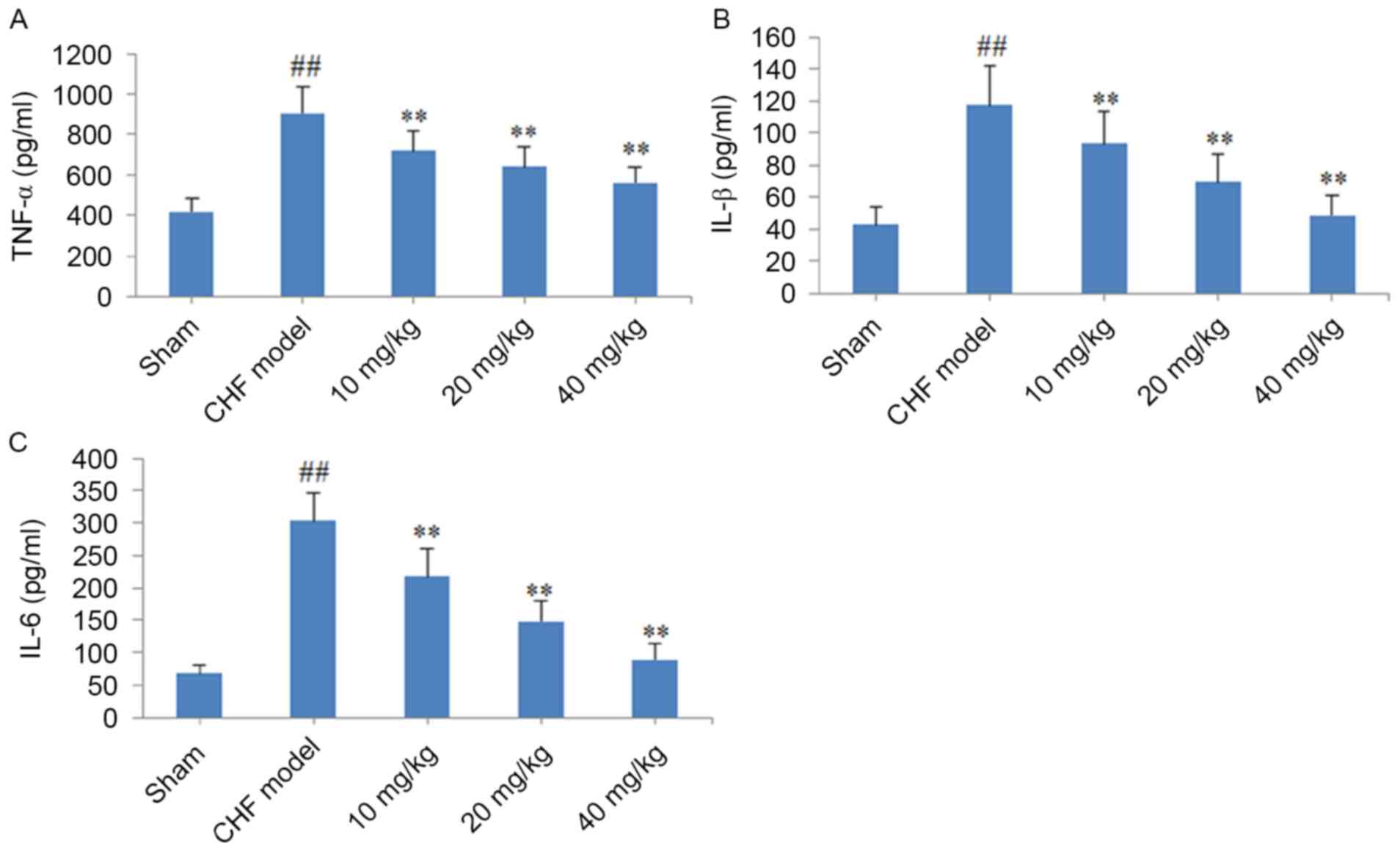
As demonstrated in Fig.
4, the production of TNF-α, IL-1β and IL-6 in cardiac tissues
was significantly increased in CHF model rats when compared with
sham group rats (P<0.01). By contrast, oral administration of
10, 20 or 40 mg/kg CYT for 8 weeks was associated with a
significant and dose-dependent decrease in the production of these
pro-inflammatory cytokines when compared with rats in the CHF model
group (P<0.01; Fig. 4).
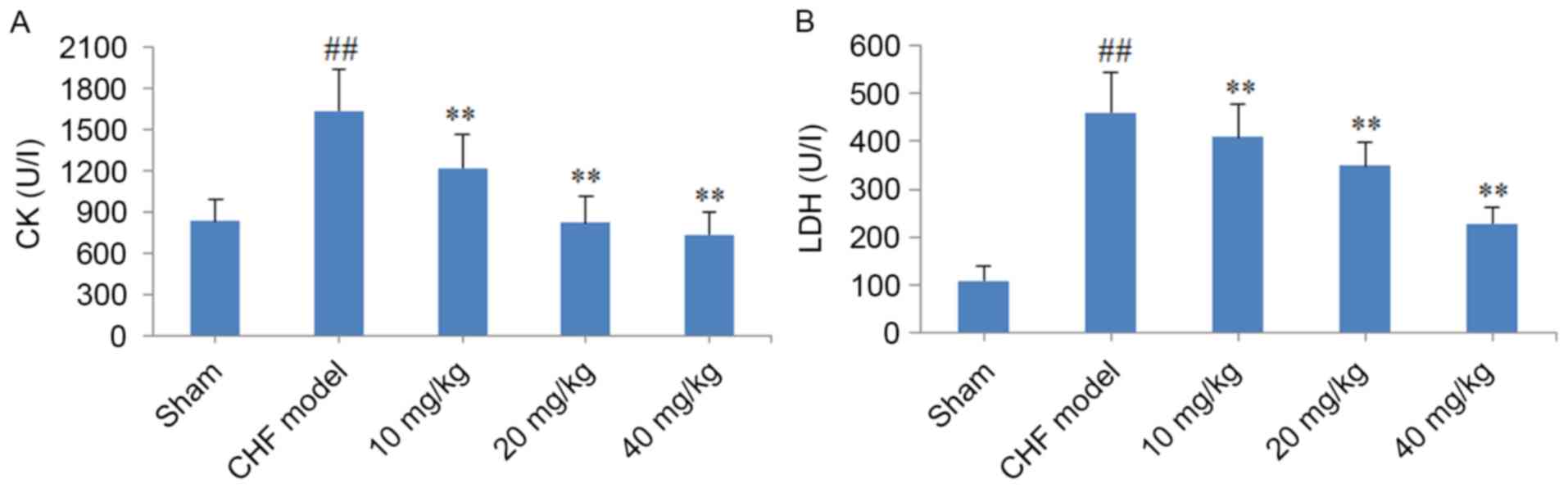
Effect of CYT on LDH and CK levels in
CHF model rats
As demonstrated in Fig.
5, serum LDH and CK levels in rats from the CHF model group
were significantly elevated when compared with those in the sham
group (P<0.01). By contrast, treatment of CHF model rats with
10, 20 and 40 mg/kg CYT for 8 weeks was associated with a
significant and dose-dependent reduction in serum CK and LDH levels
when compared with untreated CHF model rats (P<0.01; Fig. 5).
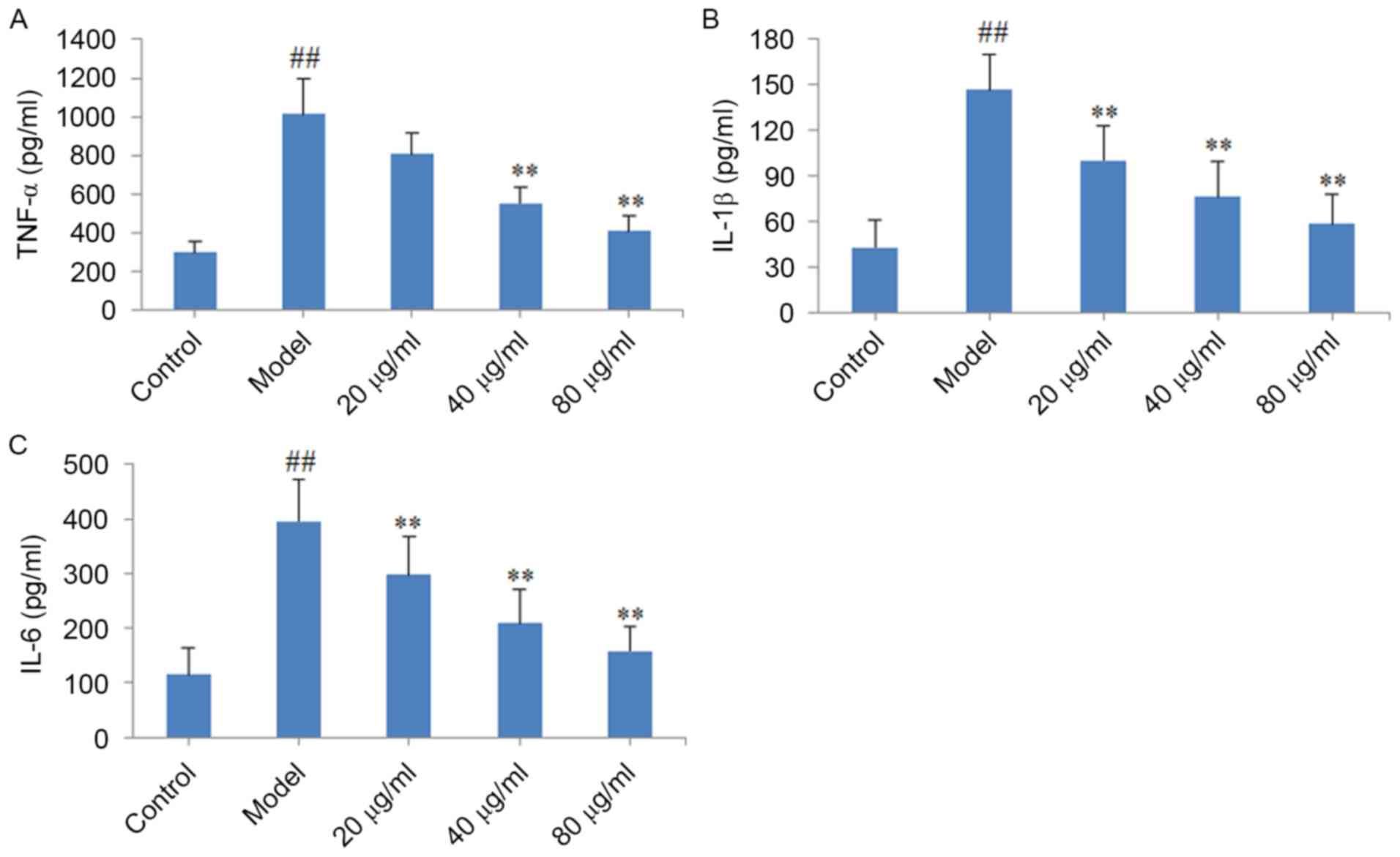
Effect of CYT on TNF-α, IL-1β and IL-6
levels in CMECs
When compared with the control group, the level of
TNF-α, IL-1β and IL-6 pro-inflammatory cytokines in the model group
were significantly elevated (P<0.01; Fig. 6). Consistent with the observed
expression of these factors in the heart tissues from CYT-treated
CHF model rats, treatment of CMECs with 40 or 80 µg/ml CYT
significantly decreased the level of TNF-α (P<0.01), and
treatment with 20, 40 or 80 µg/ml CYT significantly decreased the
levels of IL-1β and IL-6 (P<0.01) when compared with the model
group.
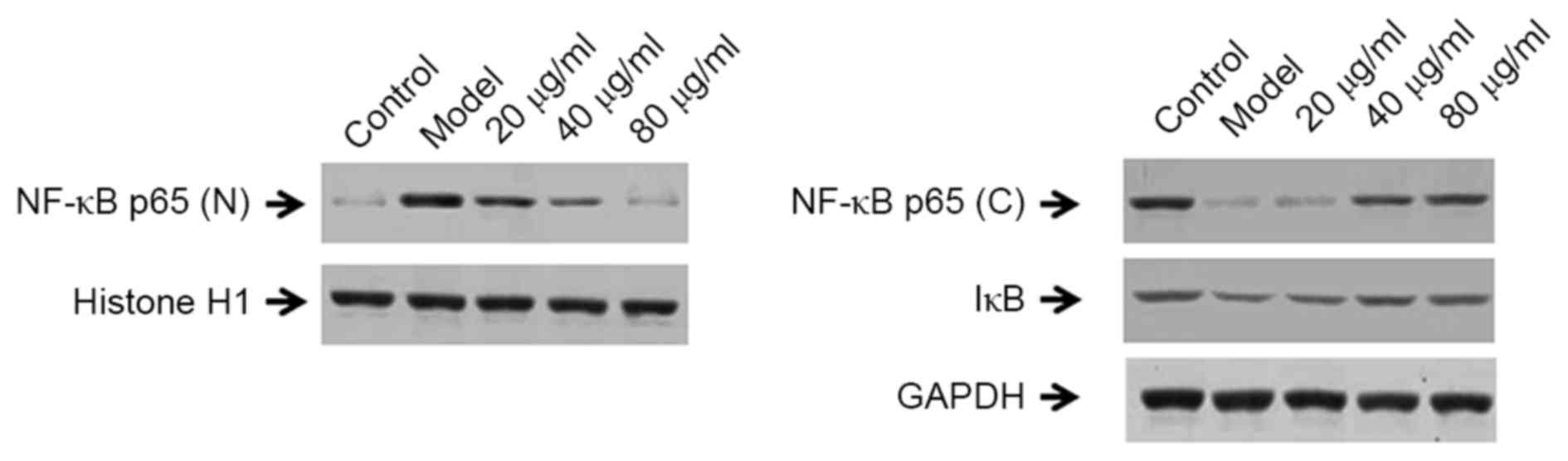
Effect of CYT on the expression of
NF-κB signaling pathway protein factors in CMECs
As demonstrated in Fig.
7, the protein expression levels of nuclear NF-κB p65 in
LPS-induced CMECs were markedly elevated when compared with normal
cells, indicating increased inflammation. By contrast, cytosolic
NF-κB p65 and IκB protein levels were reduced in LPS-induced model
group cells when compared with untreated controls (Fig. 7). Notably, nuclear NF-κB p65
expression was significantly decreased following treatment of
LPS-stimulated CMECs with 20, 40 and 80 µg/ml CYT when compared
with the untreated model group. By contrast, the cytosolic levels
of NF-κB p65 and IκB protein were markedly increased following
treatment with 20, 40 or 80 µg/ml CYT when compared with the model
group (Fig. 7).
Discussion
The results presented in the current study suggest
that CYT may exert significant cardioprotective effects in
vivo and in vitro. In addition, the results indicate
that these effects may be mediated by inhibition of the release of
inflammatory mediators, myocardial enzymes and the expression of
NF-κB signaling pathway proteins.
Previous reports have demonstrated that CK and LDH
are sensitive markers of myocardial damage (17,18).
Notably, CK levels serve a vital role in the early diagnosis of
myocardial infarction and additional cardiac diseases, and it is
known to be a gold standard marker of cardiomyocyte injury
(19,20). In the present study, the serum levels
of CK and LDH were significantly increased following LAD surgery;
however, this increase was attenuated by treatment with CYT. In
addition, the results demonstrated that CYT administration
significantly decreased the whole heart/body weight and left
heart/body weight ratios, and significantly increased cardiac
function parameters, including FS, EF, CO and HR. This indicates
that CYT may exert notable cardioprotective effects in LAD
surgery-induced CHF model rats.
Previous epidemiological studies have reported that
inflammatory lesions serve a central role in ischemia/reperfusion
(I/R)-induced cardiac injury, and cytokines have been demonstrated
to be closely associated with the inflammatory response in the
progress of I/R-induced cardiac injury (21,22). It
has been demonstrated that I/R increases the relative levels of
various cytokines in the myocardium, including TNF-α, IL-1β and
IL-6 (18,23). Taking these observations into
consideration, the level of these cytokines in the cardiac tissues
of CYT-treated CHF model rats was determined in the present study.
The results indicated that the expression levels of these
pro-inflammatory cytokines were significantly increased in CHF
model rats when compared with those in the sham group. Notably,
treatment of CHF model rats with CYT effectively reversed this
increase in TNF-α, IL-1β and IL-6 expression. Therefore, the
authors of the present study hypothesize that the cardioprotective
effects of CYT may be partly mediated by inhibition of
pro-inflammatory mediators.
The cardioprotective effects of CYT were further
verified using an in vitro model of cardiac inflammation,
whereby CMECs were induced with LPS. The results demonstrated that
CYT treatment decreased the levels of TNF-α, IL-1β and IL-6 in
LPS-induced CMECs. An increasing number of studies have revealed
that the NF-κB signaling pathway serves an important role in
regulating inflammatory responses (16,24).
NF-κB is comprised of NF-κB p50 and NF-κB p65 subunits, which are
generally localized in the cytoplasm together with its inhibitor,
IκB. NF-κB is transported to the nucleus following dissociation
with IκB, where it exerts its function as a transcription factor
and induces an inflammatory response (25,26). The
results of the present study indicated that CYT repressed nuclear
NF-κB p65 expression, and increased cytoplasmic NF-κB p65 and IκB
expression. These results suggest that CYT may regulate the NF-κB
signaling pathway in CMECs.
In conclusion, the results of the current study
provide experimental evidence that CYT exerts cardioprotective
effects in a rat model of CHF induced by LAD surgery. The
mechanisms underlying the effects of CYT may involve suppression of
inflammatory mediators and members of the NF-κB signaling
pathway.
Acknowledgements
The present study was funded by a grant from the
Natural Science Foundation of China (no. 81500285) and the 331
Early Career Researcher Grant of Shanxi Medical University (no.
201409).
References
|
1
|
Shimokawa H, Miura M, Nochioka K and
Sakata Y: Heart failure as a general pandemic in Asia. Eur J Heart
Fail. 17:884–892. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heusch G, Libby P, Gersh B, Yellon D, Böhm
M, Lopaschuk G and Opie L: Cardiovascular remodelling in coronary
artery disease and heart failure. Lancet. 383:1933–1943. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owan TE, Hodge DO, Herges RM, Jacobsen SJ,
Roger VL and Redfield MM: Trends in prevalence and outcome of heart
failure with preserved ejection fraction. N Engl J Med.
355:251–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Zhang J, Huang J, Ma A, Yang J, Li
W, Wu Z, Yao C, Zhang Y, Yao W, et al: A multicenter, randomized,
double-blind, parallel-group, placebo-controlled study of the
effects of qili qiangxin capsules in patients with chronic heart
failure. J Am Coll Cardiol. 62:1065–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo N, Yang D, Wang X, Dai J, Wang M and
Lei Y: Metabonomic study of chronic heart failure and effects of
Chinese herbal decoction in rats. J Chromatogr A. 1362:89–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parang P, Singh B and Arora R: Metabolic
modulators for chronic cardiac ischemia. J Cardiovasc Pharmacol
Ther. 10:217–223. 2015. View Article : Google Scholar
|
|
7
|
Marcinkiewicz-Siemion M, Ciborowski M,
Kretowski A, Musial WJ and Kaminski KA: Metabolomics-a wide-open
door to personalized treatment in chronic heart failure? Int J
Cardiol. 219:156–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrer Piloto J, Cozzi R, Cornetta T,
Stano P, Fiore M, Degrassi F, De Salvia R, Remigio A, Francisco M,
Quiñones O, et al: Xanthium strumarium L. extracts produce DNA
damage mediated by cytotoxicity in in vitro assays but does not
induce micronucleus in mice. Biomed Res Int.
2014:5751972014.PubMed/NCBI
|
|
9
|
Chen F, Hao F, Li C, Gou J, Lu D, Gong F,
Tang H and Zhang Y: Identifying three ecological chemotypes of
Xanthium strumarium glandular trichomes using a combined NMR and
LC-MS method. PLoS One. 8:e766212013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han T, Li HL, Zhang QY, Han P, Zheng HC,
Rahman K and Qin LP: Bioactivity-guided fractionation for
anti-inflammatory and analgesic properties and constituents of
Xanthium strumarium L. Phytomedicine. 14:825–829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng W, Ming QL, Han P, Zhang QY, Jiang
YP, Zheng CJ, Han T and Qin LP: Anti-allergic rhinitis effect of
caffeoylxanthiazonoside isolated from fruiats of Xanthium
strumarium L. in rodent animals. Phytomedicine. 21:824–829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin L, Han T, Li H, Zhang Q and Zheng H: A
new thiazinedione from Xanthium strumarium. Fitoterapia.
77:245–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH
and Wang W: AT1 receptor mRNA antisense normalizes enhanced cardiac
sympathetic afferent reflex in rats with chronic heart failure. Am
J Physiol Heart Circ Physiol. 287:H1828–H1835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W
and Zhu GQ: AT1 receptor in paraventricular nucleus mediates the
enhanced cardiac sympathetic afferent reflex in rats with chronic
heart failure. Auton Neurosci. 121:56–63. 2015. View Article : Google Scholar
|
|
15
|
Xing L, Jiang M, Dong L, Gao J, Hou Y, Bai
G and Luo G: Cardioprotective effects of the YiQiFuMai injection
and isolated compounds on attenuating chronic heart failure via
NF-κB inactivation and cytokine suppression. J Ethnopharmacol.
148:239–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C, Wu K, Li SH and You Q: Protective
effect of curcumin against cardiac dysfunction in sepsis rats.
Pharm Biol. 51:482–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badole SL, Chaudhari SM, Jangam GB,
Kandhare AD and Bodhankar SL: Cardioprotective activity of Pongamia
pinnata in streptozotocin-nicotinamide induced diabetic rats.
Biomed Res Int. 2015:4032912015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu M, Zheng AB, Jin J, Cui Y, Zhang N, Che
ZP, Wang Y, Zhan J and Tu WJ: Cardioprotective effects of genistin
in rat myocardial ischemia-reperfusion injury studies by regulation
of P2X7/NF-κB pathway. Evid Based Complement Alternat Med.
2016:53812902016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia A, Xue Z, Li Y, Wang W, Xia J, Wei T,
Cao J and Zhou W: Cardioprotective effect of betulinic acid on
myocardial ischemia reperfusion injury in rats. Evid Based
Complement Alternat Med. 2014:5737452014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu T, Wei G, Xi M, Yan J, Wu X, Wang Y,
Zhu Y, Wang C and Wen A: Synergistic cardioprotective effects of
Danshensu and hydroxysafflor yellow A against myocardial
ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1
pathway. Int J Mol Med. 38:83–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saini HK, Xu YJ, Zhang M, Liu PP,
Kirshenbaum LA and Dhalla NS: Role of tumour necrosis factor-alpha
and other cytokines in ischemia-reperfusion-induced injury in the
heart. Exp Clin Cardiol. 10:213–222. 2015.
|
|
22
|
Yuan X, Niu HT, Wang PL, Lu J, Zhao H, Liu
SH, Zheng QS and Li CG: Cardioprotective effect of Licochalcone D
against myocardial ischemia/reperfusion injury in
langendorff-perfused rat hearts. PLoS One. 10:e01283752015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia I, Olleros ML, Quesniaux VF, Jacobs
M, Allie N, Nedospasov SA, Szymkowski DE and Ryffel B: Roles of
soluble and membrane TNF and related ligands in mycobacterial
infections: Effects of selective and non-selective TNF inhibitors
during infection. Adv Exp Med Biol. 691:187–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Y, Zhou M, Xing L, Jiang M, Bai G and
Luo G: Identification of NF-κB inhibitors in Qishenyiqi dropping
pills for myocardial infarction treatment based on
bioactivity-integrated UPLC-Q/TOF MS. Biomed Chromatogr.
29:1612–1618. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
He CL, Yi PF, Fan QJ, Shen HQ, Jiang XL,
Qin QQ, Song Z, Zhang C, Wu SC, Wei XB, et al: Xiang-Qi-Tang and
its active components exhibit anti-inflammatory and anticoagulant
properties by inhibiting MAPK and NF-κB signaling pathways in
LPS-treated rat cardiac microvascular endothelial cells.
Immunopharmacol Immunotoxicol. 35:215–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Q, Cao J, Fang L, Zhao H, Liu Z, Ran
J, Zheng X, Li X, Zhou Y, Ge D, et al: Geniposide suppresses
LPS-induced nitric oxide, PGE2 and inflammatory cytokine by
downregulating NF-κB, MAPK and AP-1 signaling pathways in
macrophages. Int Immunopharmacol. 20:298–306. 2014. View Article : Google Scholar : PubMed/NCBI
|