Introduction
With improvements in the availability of food and
changes in lifestyle, the incidence of diabetes has increased
significantly. According to an estimate in 2007 by the
International Diabetes Federation, the number of people with
diabetes in China was ~39.8 million, ranking second in the world
after India. Furthermore, there was an annual increase of 1.01
million diabetics, among which 95% had type 2 diabetes (1). Great progress has been made in the
diagnosis and prevention of diabetic nephropathy (DN), a common
complication of diabetes (2).
However, kidney damage in patients with diabetes is not necessarily
due to DN (2).
DN pathogenesis is very complex, and involves lipid
disorders, hemodynamic abnormalities, the release of inflammatory
mediators, cytokines, oxidative stress and apoptosis (2). Recently with development of
biotechnological techniques, the research into and awareness of
podocytes have gradually increased (3). Furthermore, podocyte injury in the
pathogenesis of DN has been recognized as the key factor causing DN
proteinuria and glomerular sclerosis, which may prompt the
development of new strategies for the prevention and treatment of
DN (4).
The phosphoinositide 3 kinase (PI3K)/protein kinase
B (also known as Akt) signaling pathway participates in cell
differentiation, proliferation, apoptosis and migration, and
excessive activation of this pathway leads to cell dysfunction
(5). As mentioned above, podocyte
injury has been recognized to play a role in DN (6); changes of marker protein expression are
the basis of changes in podocyte function, and the PI3K/Akt
signaling pathway may change the podocyte phenotype and induce the
podocyte injury that is involved in the progression of DN (7).
Glycogen synthase kinase 3 (GSK-3) is a
rate-limiting enzyme for inhibiting glycogen synthesis, and is
involved in the regulation of various signals, including insulin
cell signaling (8). The GSK-3β
subtype is primarily involved in this; therefore, research into the
correlation between GSK-3β and type 2 diabetes has been conducted
(9). Previous studies have shown a
correlation between high expression levels of GSK-3β and decreased
insulin sensitivity (8,10).
Emodin is an anthraquinone derivative, which may be
obtained from the root of Rheum palmatum L. (rhubarb). The
anti-inflammatory effect of emodin has been demonstrated in many
experiments using animal models (11). Additionally, its antibacterial
effects on Pseudomonas aeruginosa and Staphylococcus
aureus, methicillin-resistant Staphylococcus aureus have
also been reported (12). Emodin has
also been shown to inhibit the growth of Helicobacter pylori in
vitro in a dose-dependent manner (13). Further in vitro experiments
have demonstrated that emodin significantly inhibits the release of
cytokines by mononuclear cells under lipopolysaccharide (LPS)
stimulation, and also inhibits the endotoxin-induced secretion of
tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8 and
other inflammatory cytokines, thus affecting the immune activation
associated with them (14,15).
Materials and methods
Experimental animals
All experimental procedures were pre-approved by the
First Affiliated Hospital of PLA General Hospital (Beijing, China).
Female adult Wistar rats (8 weeks old, 200–230 g) were obtained
from Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) and housed at 21–23°C with a 12:12 h light-dark
cycle and 55–60% relative humidity. Furthermore, standard
pelletized food and water were provided ad libitum. Rats
were randomly divided into three groups: Control (n=6); DN model
(n=8) and emodin (n=8) groups. The DN model and emodin group rats
were administered a 60 mg/kg intraperitoneal injection of
streptozotocin (Sigma-Aldrich, Inc.; Merck KGaA, Darmstadt,
Germany). The emodin group rats were administered emodin 100 mg/kg
dose once every 3 days (Sigma-Aldrich, Inc.; Merck KGaA) for 3
weeks.
Normalized kidney weight, glucose
urine albumin and creatinine measurements
Following the 3 weeks of treatment with emodin,
kidneys were acquired and washed with phosphate-buffered saline
(PBS). Next, each kidney was weighed and the normalized kidney
weight was determined. Glucose (F006), Urine albumin (A028-1) and
creatinine (C011-2) were quantified with commercial kits (Nanjing
Jiancheng Biology Engineering Institute, Nanjing, China) and the
Cayman creatinine assay kit (Ann Arbor, MI, USA) was also used to
determine creatinine levels.
Histological analysis and
determination of tubulointerstitial injury index (TII) score
After the 3 weeks of treatment with emodin, kidneys
were acquired and washed with PBS. The tissue was then fixed in 4%
formaldehyde for 24 h and embedded in paraffin. The tissue was cut
into 8-µm sections and stained using hematoxylin and eosin (Fuzhou
Maixin Biotech. Co., Ltd., Fuzhou, China). The stained kidney
tissue was then observed using light microscopy (Nikon 80i; Nikon,
Tokyo, Japan). Sections were stained with Periodic acid-Schiff
assay for 1 h. TII scores was scored as the percentage of
tubulointerstitial injury area within the total area: 0, normal; 1,
<25%; 2, 25–50%; 3, >50%.
ELISA analysis of inflammatory
factors, oxidative stress and caspase-3
Blood was obtained through the inferior vena cava of
every rat and centrifuged at 12,000 × g for 10 min at 4°C. IL-6,
TNF-α, superoxide dismutase (SOD), malondialdehyde (MDA) and
caspase-3 levels were measured using ELISA kits (Nanjing Keygen
Biotech, Co., Ltd., Jiangsu, China).
Western blot analysis
After the 3 weeks of treatment with emodin, kidney
tissues were lysed with ice-cold radioimmunoprecipitation assay
buffer (Bio-Rad Laboratories, Inc.) A bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China) was
used to quantify the protein contents. Protein (30 µg) was loaded
onto a gel for 10% Tris-glycine-SDS-PAGE and electroblotted onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were then blocked with 5% non-fat milk in PBS 0.05%
Tween 20 solution. Sections were then incubated with specific
primary antibodies against intercellular adhesion molecule 1
(ICAM-; sc-7891; 1:500), Bax (sc-6236; 1:500), p-Akt (sc-7985-R;
1:200), Akt (sc-8312; 1:500), p-GSK-3β (sc-135653; 1:200), GSK-3β
(sc-7879; 1:500), caspase-3 (sc-98785; 1:500, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and GAPDH (sc-25778; 1:2,000;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Membranes were
then washed with a PBS 0.05% Tween 20 solution prior to incubation
with polyclonal goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (sc-2031; 1:5,000; Santa Cruz Biotechnology,
Inc.) for 1 h at 37°C. The membranes were then detected using a
chemiluminescence detection kit (Amersham Pharmacia Biotech; GE
Healthcare Life Sciences, Chalfont, UK). Protein expression was
quantified using Image J 1.32 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Values are presented as the mean ± standard
deviation. One-way analysis of variance was used to identify
differences among groups using Tukey's tests. P<0.05 was used to
indicate a statistically significant difference.
Results
Blood glucose levels, normalized
kidney weight, urinary albumin excretion and serum creatinine
levels of diabetic rats
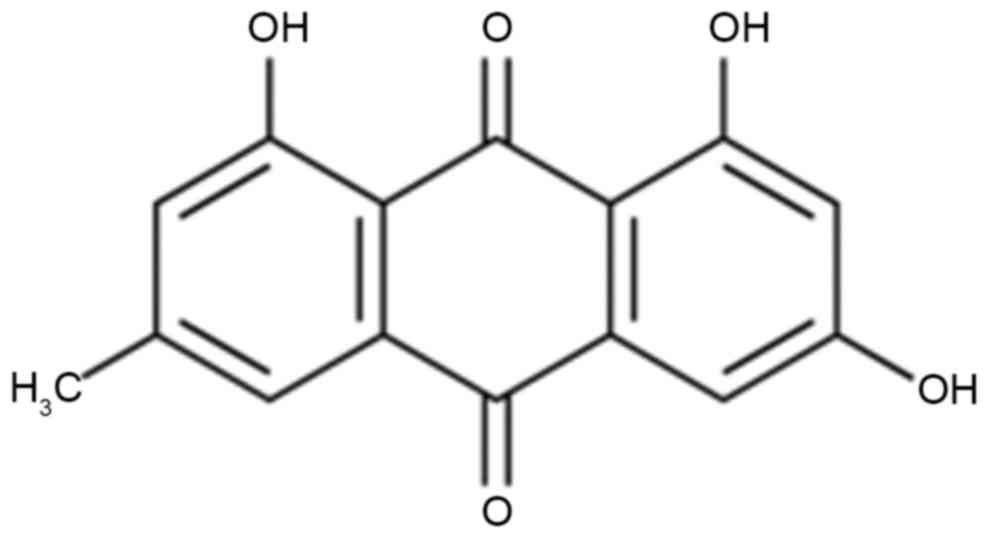
The chemical structure of emodin is shown in
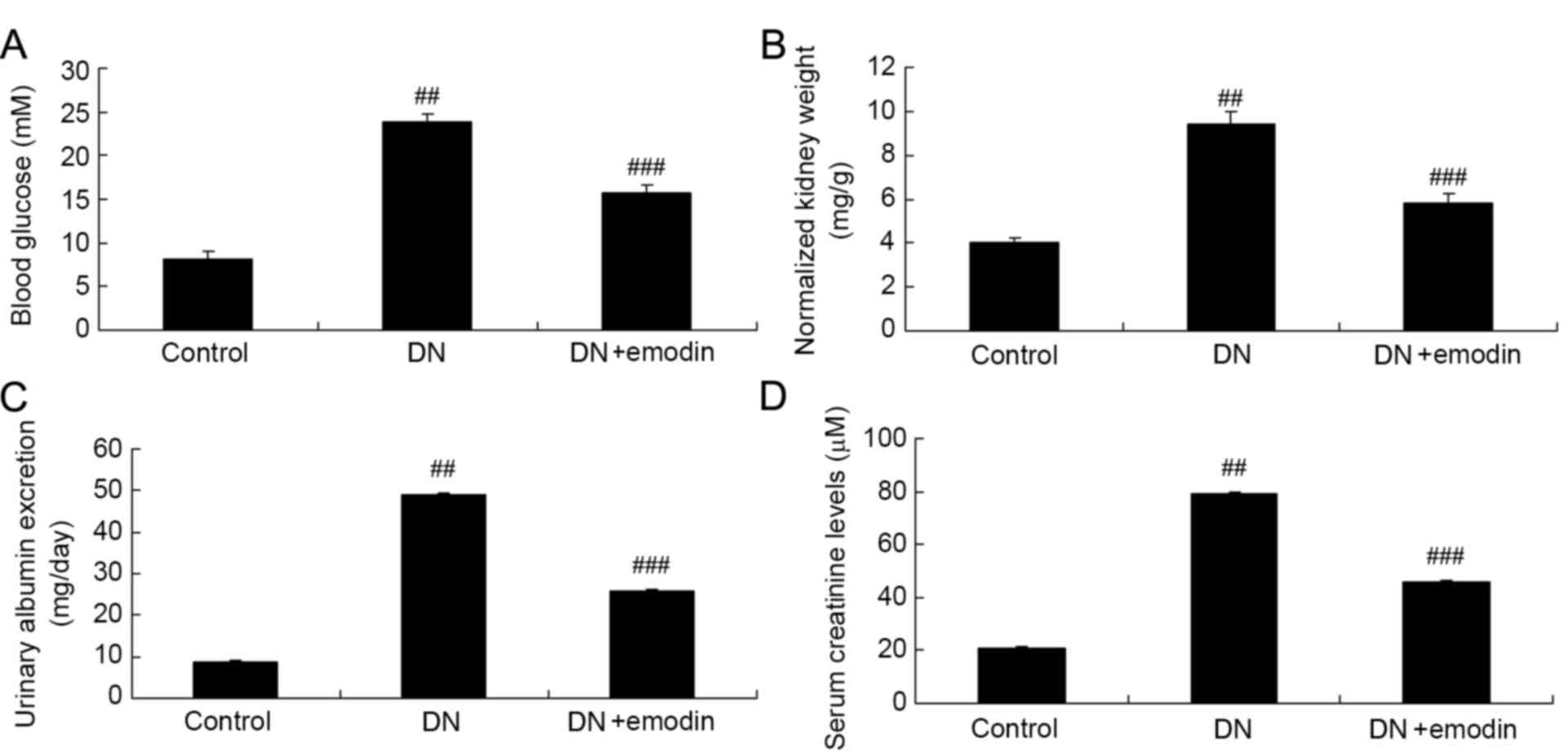
Fig. 1. As shown in Fig. 2, there was a significant increase in
the blood glucose level, normalized kidney weight, urinary albumin
excretion and serum creatinine level in the DN model group compared
with the control group. Following treatment with emodin, the
increases in blood glucose level, normalized kidney weight, urinary
albumin excretion and serum creatinine level were significantly
attenuated, compared with those in the DN model group (Fig. 2).
TII scores of diabetic rats
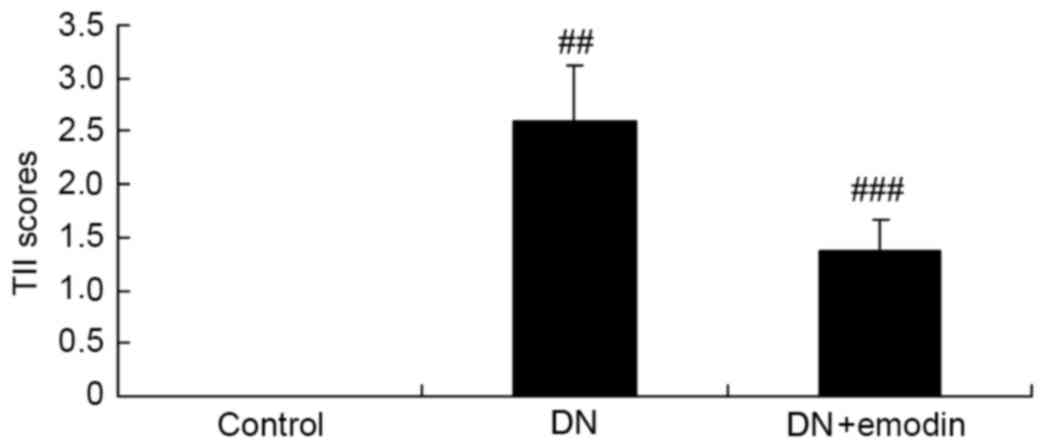
The TII scores of the DN model group were observed
to be significantly increased compared with those in the control
group (Fig. 3). Notably, treatment
with emodin significantly reduced the elevation of the TII scores
in the DN model rats (Fig. 3).
Inflammation factors of diabetic
rats
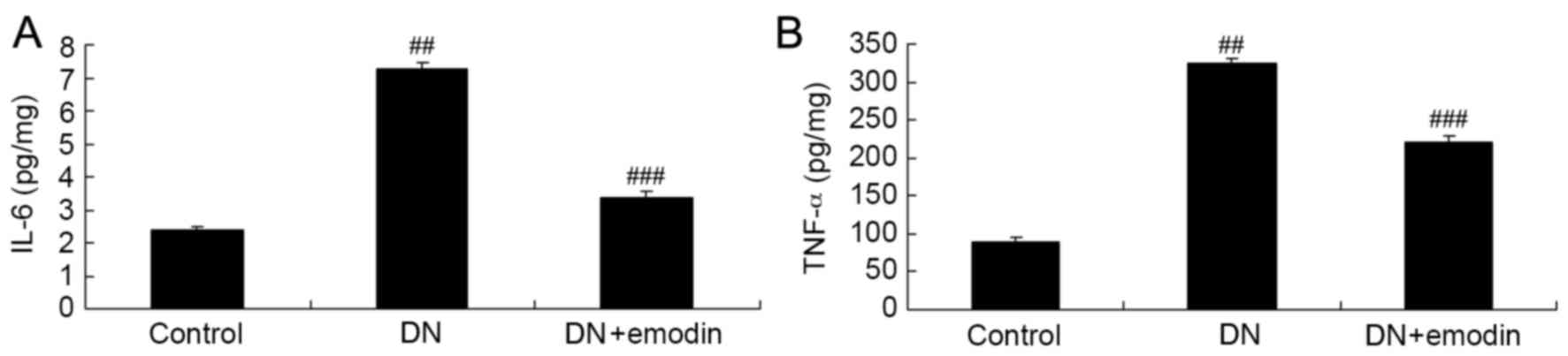
The potential anti-inflammatory effects of emodin in
rats with DN were investigated via the analysis of IL-6 and TNF-α
levels. The ELISA results presented in Fig. 4 revealed that the IL-6 and TNF-α
levels in the group were significantly increased compared with
those in the control group. Furthermore, comparison of the DN and
emodin groups indicated that emodin significantly decreased the
activation of IL-6 and TNF-α levels in rats with DN (Fig. 4).
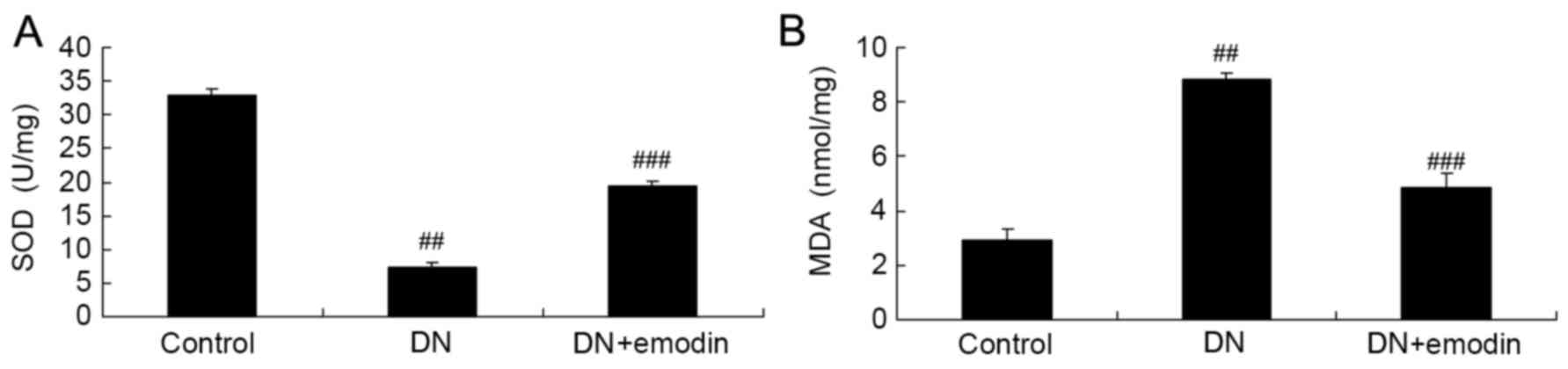
Oxidative stress of diabetic rats
SOD and MDA levels were analyzed in order to
determine whether emodin exhibited an anti-oxidative effect in rats
with DN. The results presented in Fig.
5 show that DN significantly reduced the SOD level and
increased the MDA level in diabetic rats, compared with the control
group. Furthermore, treatment with emodin significantly attenuated
the changes in SOD and MDA levels compared with those in the DN
model group (Fig. 5.
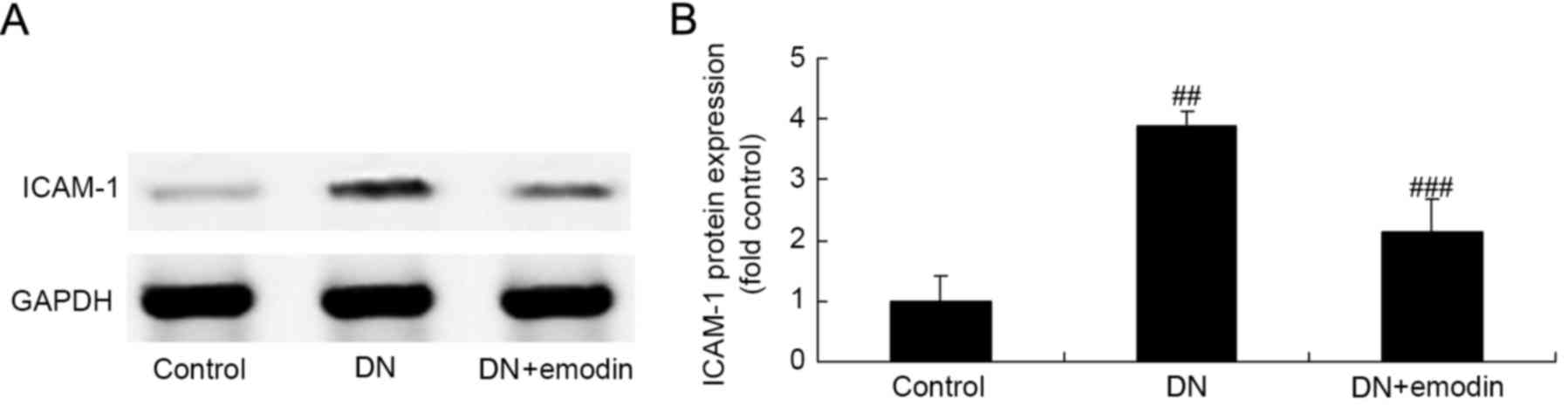
ICAM-1 protein expression of diabetic
rats
ICAM-1 expression was evaluated using western blot
analysis in order to investigate whether ICAM-1 pathways are
involved in the protective effect of emodin against DN. As shown in
Fig. 6, activation of ICAM-1
expression was observed in the DN model group, compared with the
control group. The increased ICAM-1 expression in the DN model was
significantly suppressed by treatment with emodin (Fig. 6).
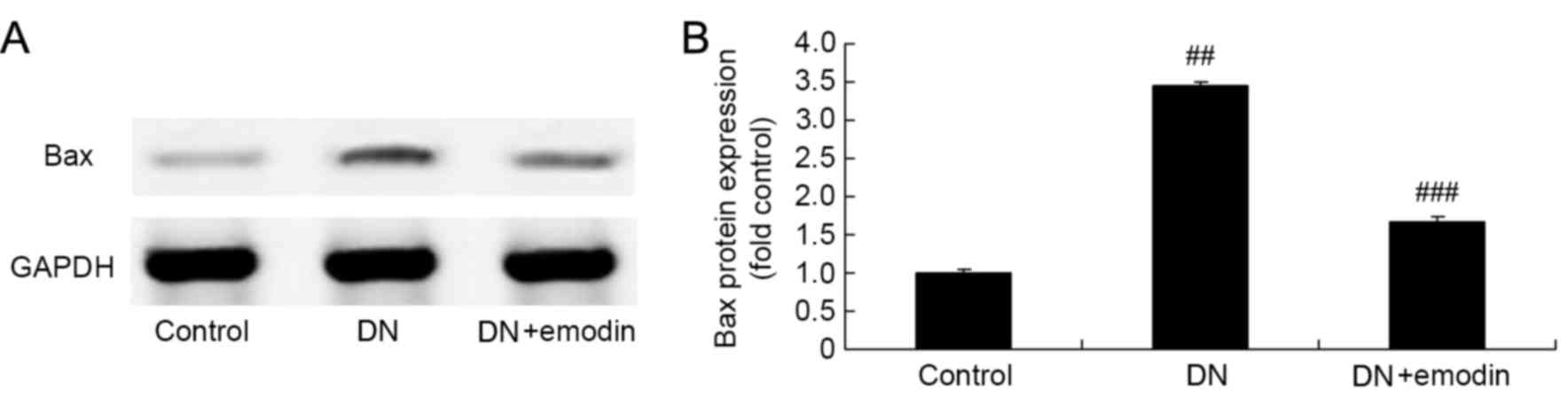
Bax protein expression of diabetic
rats
Bax expression in the kidneys of diabetic rats was
detected by western blot analysis (Fig.
7). DN significantly increased the expression of Bax in the DN
model group compared with the control group (Fig. 7). Treatment with emodin significantly
reduced the Bax protein expression compared with that in the DN
model group (Fig. 7).
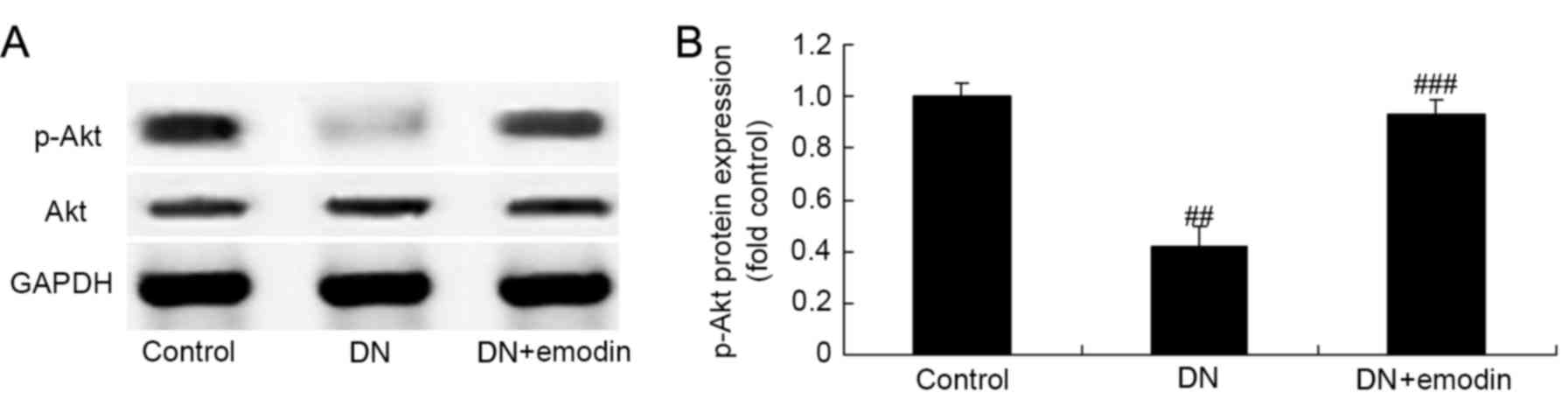
p-Akt protein expression of diabetic
rats
The effect of emodin on Akt pathways in the kidneys
of diabetic rats was also investigated. As shown in Fig. 8, there was a significant inhibition
of Akt pathways in the DN model group compared with the control
group, as demonstrated by a reduction in the phosphorylation level
of Akt. However, emodin significantly promoted the p-Akt protein
expression of rats with DN, when compared with the DN model group
(Fig. 8).
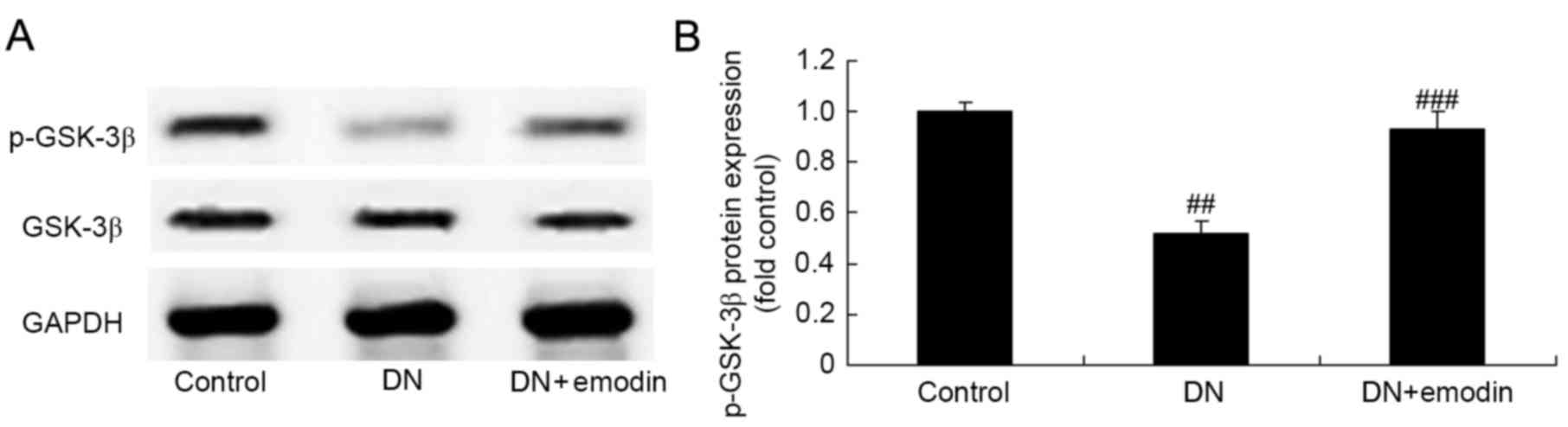
p-GSK-3β protein expression of
diabetic rats
p-GSK-3β protein expression was evaluated using
western blotting in order to examine whether there is an
association between GSK-3β pathways and the protective effect of
emodin on DN. The results shown in Fig.
9 indicate that DN significantly inhibited GSK-3β pathways and
p-GSK-3β protein expression in diabetic rats compared with the
control group, and treatment with emodin significantly activated
the protein expression of p-GSK-3β compared with that in the DN
model group (Fig. 9).
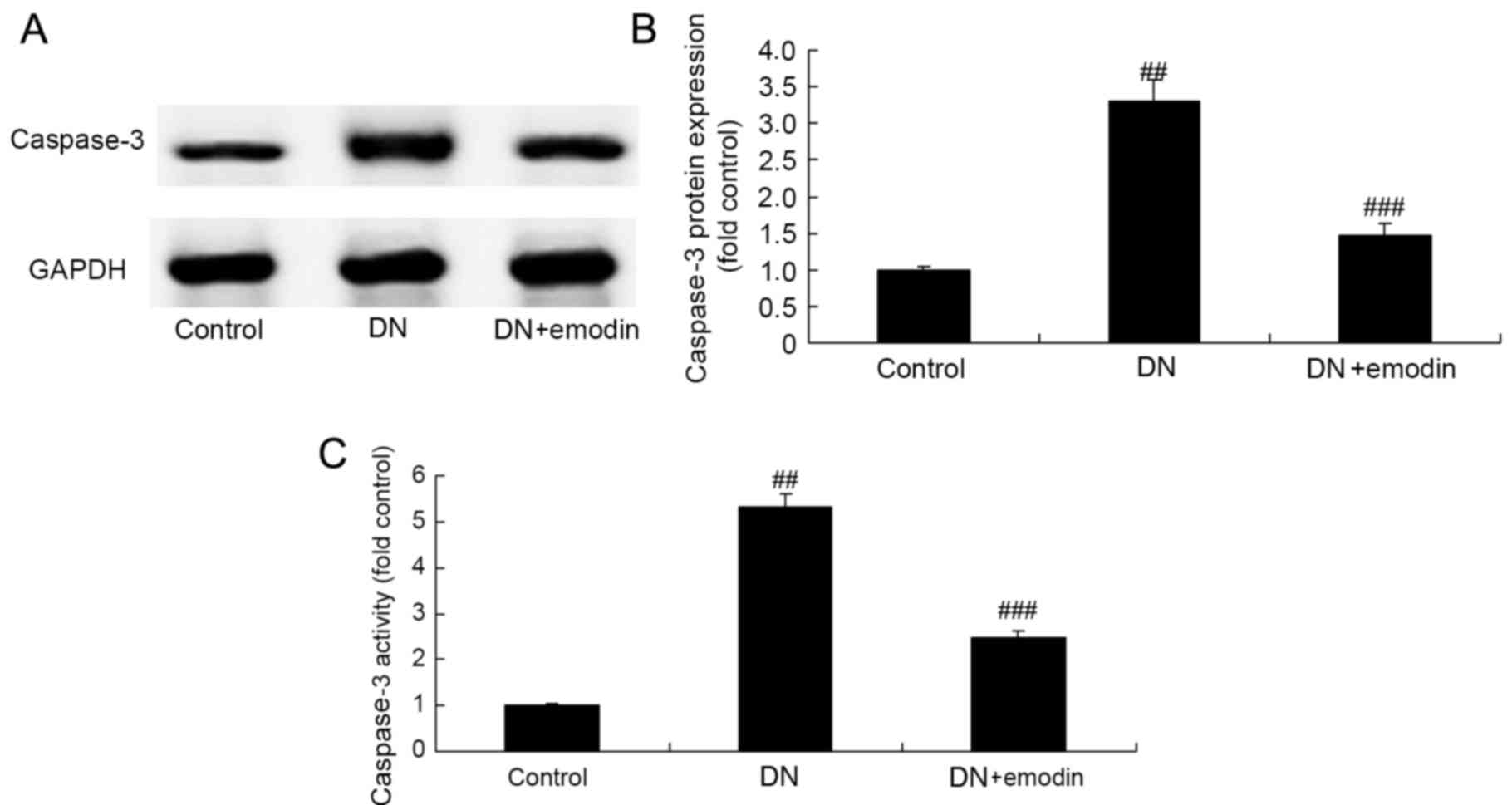
Caspase-3 expression of diabetic
rats
Caspase-3 expression in diabetic rats was measured
using ELISA and western blotting in order to elucidate the
protective effect of emodin on apoptosis in DN rats. Caspase-3
activity and protein expression in the DN model group was
significantly increased compared with that in the control group
(Fig. 10). However, these changes
to caspase-3 in the rats with DN were significantly inhibited by
treatment with emodin (Fig.
10).
Discussion
DN is a complication of diabetes, and the incidence
of nephropathy among diabetic patients is much higher than that in
non-diabetic patients (16). It is
classified as a serious microvascular disease, which can cause
kidney damage, and eventually lead to end-stage renal disease. The
basic pathological change in DN comprises glomerulosclerosis caused
by glomerular cell proliferation, the release of inflammatory
mediators and accumulation of extracellular matrix and other
factors (17). Proteinuria is the
clinical manifestation in the earliest stage. DN is not
traditionally considered a disease that is associated with the
immune system; however, a study has shown that activation of the
innate immune system and inflammatory mechanisms are important in
its pathogenesis (18). In patients
with DN-associated kidney inflammation, the secretion of adhesion
molecules, chemokine factors and cytokines are increased (19). Furthermore, a large number of immune
cells gather and infiltrate into the kidney tissue, resulting in a
further release of pro-inflammatory cytokines and growth factors,
thereby increasing the inflammatory response and progressively
exacerbating renal tissue damage and renal stromal fibrosis
(20).
Previous studies have shown that the interaction
between inflammation and DN includes complex molecular and pathway
networks, and confirm the important role of inflammatory pathways
in DN (17,21). The results of the present study
demonstrate the ability of emodin to significantly inhibit the
blood glucose level, reduce the normalized kidney weight, and
inhibit urinary albumin excretion and serum creatinine levels in
rats DN. Furthermore, it decreased TII scores, attenuated the
increased IL-6 and TNF-α levels, elevated SOD activity and
inhibited the MDA level in DN rats. Zeng et al (12) reported that emodin reduced uremic
toxins in rats with chronic kidney disease. Zhu et al
(14) demonstrated that emodin
suppressed the LPS-induced inflammation of RAW264.7 cells and Xue
et al (22) suggested that
emodin attenuated lung injury through the suppression of oxidative
damage in a mouse model.
ICAM-1 forms molecular bonds by the mutual contact
between intermediate cells or between the cell and matrix. The
majority of bonding sites comprise glycoproteins that are located
on the cell surface or the extracellular matrix. Furthermore,
ICAM-1 is expressed on a variety of cells, including endothelial
and epithelial cells, fibroblasts, activated T cells, monocytes,
macrophages and natural killer cells amongst others, which can
enhance the adhesion between monocyte-macrophage and endothelial
cells and cause inflammation (23).
A continuously increased expression of ICAM-1 can cause serious
damage to the structure and function of tissues and organs
(24). Furthermore, ICAM-1 molecules
are widely present in the body and are expressed in a variety of
hematopoietic and non-hematopoietic cells, which are distributed
mainly in various epithelial and endothelial cells, fibroblasts,
reticular cells, monocytes, macrophages and lymphocytes (25). In the present study, it was revealed
that emodin significantly suppressed ICAM-1 expression in DN model
rats. Consistent with this, Chen et al (15) previously reported that emodin
ameliorated LPS-induced corneal inflammation and ICAM-1 in
rats.
The PI3K/Akt signaling pathway is involved in cell
differentiation, proliferation, apoptosis and migration, and
excessive activation of this pathway leads to cell dysfunction
(26). A previous study indicated
that PI3K/Akt signaling pathway activation occurs in association
with DN, and high sugar or transforming growth factor-β1 levels can
cause changes to certain podocyte proteins such as ZO-1 and CD2AP
by activating this pathway (27).
Glomerular p-Akt expression in patients with DN has been observed
to be increased with aggravation of the disease, although in severe
lesions its expression is decreased and the expression of its
negative regulation gene PTEN is gradually decreased with the
increase of glomerular lesions; these results suggest that the
PI3K/Akt signaling pathway is important in the development of early
DN lesions (10,27). The present study found that emodin
significantly promoted p-Akt protein expression in the rats with
DN. Park et al (28)
previously demonstrated that emodin induces the neurite outgrowth
of Neuro2a cells through PI3K/Akt/GSK-3β signaling.
Among the factors associated with the insulin signal
transduction pathway, GSK-3β has received considerable attention
because of its multiple substrates and broad impact (29). For example, insulin receptor
substrate-1 (IRS-1) results in a reduction in insulin receptor
signaling following phosphorylation by GSK-3β (30). Cell experiments involving GSK-3β
overexpression demonstrated that GSK-3β phosphorylates the serine
and threonine residues of IRS-1, prevents tyrosine residues from
undergoing phosphorylation by the insulin receptor, and reduces the
level of IRS-1, thus weakening the insulin signaling pathway
(30). The phosphorylation of cyclin
D1 protein Thr268 by GSK-3β adjust the intracellular distribution
and turnover of cyclin D1 (30).
Furthermore, the axin and adenomatous polyposis genes may also be
phosphorylated by GSK-3β (30,31).
Proteins associated with multiple signal transduction systems are
regulated by GSK-3β phosphorylation, including transcription
factors (10); numerous
transcription factors are substrates of GSK-3β, and are directly
phosphorylated by GSK-3β. The present study showed that emodin
significantly increased the expression of p-GSK-3β and inhibited
caspase-3 activity and protein expression in rats with DN. A
previous study conducted by Wu et al (32) demonstrated that emodin protected
against diabetic cardiomyopathy through the KT/GSK-3β signaling
pathway.
In conclusion, the results of the present study
demonstrated that emodin significantly improved the average blood
glucose levels, normalized kidney weight, urinary albumin
excretion, serum creatinine levels and TII scores of diabetic rats.
Furthermore, the underlying mechanism may be associated with
anti-inflammatory and anti-oxidative activity, the suppression of
ICAM-1, Bax and caspase-3, and activation of the Akt/GSK-3β
signaling pathway. However, this requires further investigation in
future studies.
References
|
1
|
Saito I, Azuma K, Kakikawa T, Oshima N,
Hanson ME and Tershakovec AM: A randomized, double-blind,
placebo-controlled study of the effect of ezetimibe on glucose
metabolism in subjects with type 2 diabetes mellitus and
hypercholesterolemia. Lipids Health Dis. 14:402015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koloverou E, Panagiotakos DB, Pitsavos C,
Chrysohoou C, Georgousopoulou EN, Laskaris A and Stefanadis C:
ATTICA Study group: The evaluation of inflammatory and oxidative
stress biomarkers on coffee-diabetes association: Results from the
10-year follow-up of the ATTICA Study (2002–2012). Eur J Clin Nutr.
69:1220–1225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu HJ, Tzeng TF, Liou SS, Da Lin S, Wu MC
and Liu IM: Ruscogenin ameliorates diabetic nephropathy by
itsanti-inflammatory and anti-fibrotic effects in
streptozotocin-induced diabetic rat. BMC Complement Altern Med.
14:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyamoto S, Shikata K, Miyasaka K, Okada
S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka
HU, et al: Cholecystokinin plays a novel protective role in
diabetic kidney through anti-inflammatory actions on macrophage:
Anti-inflammatory effect of cholecystokinin. Diabetes. 61:897–907.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K,
et al: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribback S, Cigliano A, Kroeger N, Pilo MG,
Terracciano L, Burchardt M, Bannasch P, Calvisi DF and Dombrowski
F: PI3K/AKT/mTOR pathway plays a major pathogenetic role in
glycogen accumulation and tumor development in renal distal tubules
of rats and men. Oncotarget. 6:13036–13048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Lin MZ, Cheng D, Braet F, Pollock
CA and Chen XM: KCa3.1 mediates dysfunction of tubular autophagy in
diabetic kidneys via PI3k/Akt/mTOR signaling pathways. Sci Rep.
6:238842016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Mi S, Li Z, Hua F and Hu ZW:
Interleukin 17A inhibits autophagy through activation of PIK3CA to
interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial
cells. Autophagy. 9:730–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiao G, Le Y, Li J, Wang L and Shen F:
Glycogen synthase kinase-3β is associated with the prognosis of
hepatocellular carcinoma and may mediate the influence of type 2
diabetes mellitus on hepatocellular carcinoma. PLoS One.
9:e1056242014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1 and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Yang T, Zhou H, Du J, Zhu B and Sun
Z: Emodin Combined with nanosilver inhibited sepsis by
anti-inflammatory protection. Front Pharmacol. 7:5362017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng YQ, Dai Z, Lu F, Lu Z, Liu X, Chen C,
Qu P, Li D, Hua Z, Qu Y and Zou C: Emodin via colonic irrigation
modulates gut microbiota and reduces uremic toxins in rats with
chronic kidney disease. Oncotarget. 7:17468–17478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YQ, Huang GR, Wu MH, Tang HY, Huang
ZS, Zhou XH, Yu WQ, Su JW, Mo XQ, Chen BP, et al: Inhibitory
effects of emodin, baicalin, schizandrin and berberine on hefA
gene: Treatment of Helicobacter pylori-induced multidrug
resistance. World J Gastroenterol. 21:4225–4231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu T, Zhang W, Feng SJ and Yu HP: Emodin
suppresses LPS-induced inflammation in RAW264.7 cells through a
PPARγ-dependent pathway. Int Immunopharmacol. 34:16–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen GL, Zhang JJ, Kao X, Wei LW and Liu
ZY: Emodin ameliorates lipopolysaccharides-induced corneal
inflammation in rats. Int J Ophthalmol. 8:665–669. 2015.PubMed/NCBI
|
|
16
|
Li F, Lei T, Xie K, Wu X, Tang C, Jiang M,
Liu J, Luo E and Shen G: Effects of extremely low frequency pulsed
magnetic fields on diabetic nephropathy in streptozotocin-treated
rats. Biomed Eng Online. 15:82016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JJ, Zhang SX, Mott R, Chen Y, Knapp
RR, Cao W and Ma JX: Anti-inflammatory effects of pigment
epithelium-derived factor in diabetic nephropathy. Am J Physiol
Renal Physiol. 294:F1166–F1173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baban B, Liu JY and Mozaffari MS:
Endoplasmic reticulum stress response and inflammatory cytokines in
type 2 diabetic nephropathy: Role of indoleamine 2,3-dioxygenase
and programmed death-1. Exp Mol Pathol. 94:343–351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhattacharya S, Manna P, Gachhui R and Sil
PC: D-saccharic acid 1,4-lactone protects diabetic rat kidney by
ameliorating hyperglycemia-mediated oxidative stress and renal
inflammatory cytokines via NF-κB and PKC signaling. Toxicol Appl
Pharmacol. 267:16–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Yang T, Liu F, Li H, Guo Y, Yang
H, Xu J, Song J, Zhu Z and Liu D: Inflammatory factor-specific
sumoylation regulates NF-κB signalling in glomerular cells from
diabetic rats. Inflamm Res. 63:23–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue WH, Shi XQ, Liang SH, Zhou L, Liu KF
and Zhao J: Emodin attenuates cigarette smoke induced lung injury
in a mouse model via suppression of reactive oxygen species
production. J Biochem Mol Toxicol. 29:526–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jain SK, Croad JL, Velusamy T, Rains JL
and Bull R: Chromium dinicocysteinate supplementation can lower
blood glucose, CRP, MCP-1, ICAM-1, creatinine, apparently mediated
by elevated blood vitamin C and adiponectin and inhibition of
NFkappaB, Akt, and Glut-2 in livers of zucker diabetic fatty rats.
Mol Nutr Food Res. 54:1371–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su X, Chen X, Liu L, Chang X, Yu X and Sun
K: Intracellular adhesion molecule-1 K469E gene polymorphism and
risk of diabetic microvascular complications: A meta-analysis. PLoS
One. 8:e699402013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang LQ, Ni WJ, Cai M, Ding HH, Liu S and
Zhang ST: The renoprotective effects of berberine and its potential
impact on the expression of beta-arrestins and ICAM-1/VCAM-1 in
streptozocin induced-diabetic nephropathy rats. J Diabetes.
8:693–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XM, Yao M, Liu SX, Hao J, Liu QJ and
Gao F: Interplay between the Notch and PI3K/Akt pathways in high
glucose-induced podocyte apoptosis. Am J Physiol Renal Physiol.
306:F205–F213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim
CM, Choi YW and Lee YC: Emodin induces neurite outgrowth through
PI3K/Akt/GSK-3beta-mediated signaling pathways in Neuro2a cells.
Neurosci Lett. 588:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mariappan MM, Prasad S, D'Silva K, Cedillo
E, Sataranatarajan K, Barnes JL, Choudhury GG and Kasinath BS:
Activation of glycogen synthase kinase 3β ameliorates
diabetes-induced kidney injury. J Biol Chem. 289:35363–35375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang G, Tang X, Gao P, Guo F, Liu H, Zhao
Z, Chen Q, Jiang T, Zhang N and Li H: Sulforaphane attenuation of
experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2
signaling pathway. J Nutr Biochem. 26:596–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh LP, Jiang Y and Cheng DW: Proteomic
identification of 14-3-3zeta as an adapter for IGF-1 and
Akt/GSK-3beta signaling and survival of renal mesangial cells. Int
J Biol Sci. 3:27–39. 2007. View Article : Google Scholar
|
|
32
|
Wu Z, Chen Q, Ke D, Li G and Deng W:
Emodin protects against diabetic cardiomyopathy by regulating the
AKT/GSK-3beta signaling pathway in the rat model. Molecules.
19:14782–14793. 2014. View Article : Google Scholar : PubMed/NCBI
|