Introduction
Radiation therapy combined with chemotherapy is the
standard of care for several types of malignancies, including
carcinoma. These therapies directly target the bone marrow niche,
which are the predominant site of hematopoiesis and the
differentiation of blood cells (1,2).
Myelosuppression and hematopoietic dysfunction are the most common
clinical complications for patients receiving chemo- and
radiotherapy. These insults damage hematopoiesis by targeting
either rapidly proliferating hematopoietic stem cells (HSCs) or the
microenvironment or both (1,2). Therefore, it becomes pertinent to
promote the recovery of hematopoiesis from myelosuppression.
Different cytokines and growth factors have been studied and are
clinically in use to combat myelosuppression. The recovery of
hematopoiesis relies on the proliferation and differentiation of
undamaged HSCs and recovery of the microenvironment (3). Researchers are constantly in search of
therapeutic agents, which effectively relieve radiation damage and
restore the hematopoietic functions of bone marrow.
A broad range of bioactive peptides have already
been purified and characterized from scorpion venoms, with the
total number estimated to approach 100,000, among which only 1% is
fully known (4). These scorpion
venom polypeptides (SVPs) have been demonstrated to have a diverse
array of biological activities with high specificities to their
targeted sites, including anti-tumor, anti-epileptic, analgesic and
ion channel blocking properties (5–9). Our
group has focused on the SVP and SVPII from the Buthus
martensii scorpion venom (10–12). A
recent study by our group identified SVP-B5, a novel peptide from
the SVPII, which mitigates radiation-induced DNA damage and
improves the survival rate through the reactive oxygen
species-p16/p21 pathway (10). In
another study by our group, hematopoietic growth factor-like
effects of SVPII were reported (12). The present study demonstrated that
the principal component of SVPII, namely SVP-B5, promotes the
proliferation of irradiated hematopoietic cells. It therefore has
the potential to be considered as a therapeutic modality in
radiation therapy for the recovery of hematopoiesis; however, this
warrants further study.
Materials and methods
Purification of SVP-B5
A two-step chromatography method was used to purify
the SVP-B5 peptide from the crude scorpion venom (SVC; Zhengzhou
scorpion farm; Zhengzhou, China). In the first step, SVC was passed
through a Sephadex G-50 chromatography column (55×500 mm). The
elutions obtained were then further analyzed by a CM Sepharose FF
ion exchange chromatography column (55×1,000 mm) and phosphate
buffers (buffer A, 0.05 M
Na2HPO4-NaH2PO4, pH
6.4; buffer B: Buffer A supplemented with 0.3 M NaCl) using a
linear gradient at a flow rate of 2 ml/min. The fractions with the
peptide were concentrated and desalinated using a Vivaflow 50
membrane package and dried to a powder in a vacuum freeze-dryer.
The purity of each SVP component was further analyzed using
reverse-phase high-performance liquid chromatography (RP-HPLC;
Inertsil ODS-3 C18 chromatographic column, 4.6×250 mm). The
chromatographic conditions were as follows: Mobile phase A, 0.1%
trifluoroacetic acid in water; mobile phase B, 0.1% trifluoroacetic
acid in 70% acetonitrile in water at a flow rate of 0.5 ml/min. The
composite gradient of mobile phase B developed from 0 to 100% in 60
min.
Cell culture
The M-NFS-60 cell line (CRL-1838™) was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
was maintained in RPMI 1640 medium containing 10% fetal bovine
serum (FBS; both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin, 100 U/ml streptomycin,
5.958 g/l hydroxyethyl piperazineethanesulfonic acid, and 62 µg/l
recombinant human macrophage colony-stimulating factor (rhM-CSF;
cat. no. 300-25; PeproTech Inc., Rocky Hill, NJ, USA). The M-NFS-60
cell line was derived from a myelogenous leukemia induced with the
Cas-Br-MuLV wild mouse ecotropic retrovirus. The cells are
responsive to interleukin 3 (IL3) and macrophage (M)-CSF.FBMD-1
murine stromal cells at passage five were a kind gift from Dr
Daohong Zhou (Department of Pharmaceutical Sciences, University of
Arkansas for Medical Sciences, Fayetteville, AR, USA) and
maintained as described previously (13).
Isolation of BM-MNCs
Bone marrow mononuclear cells (BM-MNCs) were
isolated aseptically from 8–10 week-old male C57BL/6 mice (18–22 g,
n=24; Animal Center of Guangzhou Medical University, Guangzhou,
China) as described previously (12,13).
Mice were housed in specific-pathogen-free (SPF) conditions at
24–25°C, with ~70% humidity and a 14/10 h light/dark cycle, with
access to food and water ad libitum. The mice were
anesthetized with 2% isoflurane (Sigma Aldrich; Merck KGaA) in 98%
oxygen prior to sacrifice. Harvested BM-MNCs were irradiated with
X-rays (2 Gy at 300 MU/min). All animal experimental protocols were
approved by the Research Ethics Committee of Guangzhou Medical
University.
Cell proliferation assay
Cell proliferation was measured using a commercially
available kit (CCK-8; Dojindo, Kumamoto, Japan). In brief, cells
were seeded in a 96-well plate at a concentration of
5×103 cells/100 µl/well in complete media without M-CSF
and incubated with different concentrations of purified peptide
fractions for 24 or 48 h. A total of 10 µl CCK-8 reagent was
directly added to each well and the incubation was continued for an
additional 3 h. The absorbance was measured at 450 nm.
Cobblestone area forming cell (CAFC)
assay
The hematopoietic functions of hematopoietic stem
cells (HSCs) and progenitors were analyzed by the cobblestone
area-forming cell (CAFC) assay. The CAFC assay was performed as
described previously (13). In
brief, FBMD-1 feeder stromal cells were cultured in flat-bottomed
96-well plates at a density of 6×105 cells per well for
two weeks. BM-MNCs with different treatments (with/without SVP-B5,
1.0 µg/ml, with/without irradiation, 2 Gy) were then overlaid on
the feeder stromal cell layer. Cultures were fed weekly by changing
50% of the medium. The frequencies of CAFCs were determined at days
14 and 35. Wells were scored positive if at least one phase-dark
hematopoietic clone (containing 5 or more cells) was seen. The
frequency of CAFC was then calculated by using Poisson statistics
as described previously (13).
Colony-forming cell (CFC) assay
Irradiated bone marrow cells were cultured with
different concentrations of SVP-B5 (0.5 or 1.0 µg/ml) in MethoCult
M3534 medium (cat. no. 03534; StemCell Technologies, Vancouver, BC,
Canada) in 24-well plates at a cell density of 1×105/ml
for 7 days. After completion of incubation, colony-forming unit
granulocyte and macrophage (CFU-GM) colonies were counted under the
microscope. A mass consisting of >50 cells was defined as 1 CFU
(10).
Long-term bone marrow culture
(LTBMC)
LTBMC was performed as described previously
(13). A total of
2×106/ml irradiated BM-MNCs in Iscove's modified
Dulbecco's medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 20% FBS were seeded in a 24-well plate and maintained
for 14 days in the presence of purified scorpion venom peptide.
Half the volume of the media was replaced with fresh media every
week. The number of hemopoietic colonies (at least 1×106
cells per colony) was counted at 7 and 14 days.
Expression of IL-3 receptor (IL-3R)
and phosphorylation of Janus kinase 2 (JAK2) and STAT5
The effect of SVP-B5 on the expression of IL-3R was
determined in M-NFS-60 cells by immunofluorescence using a laser
scanning confocal microscope (Leica DM1 4000B; Leica Microsystems,
Wetzlar, Germany) as described previously (12). The M-NFS-60 cells were treated with
different concentrations of SVP-B5 for 24 and 48 h. Cells treated
with 10 ng/ml Recombinant Human IL-3 (cat. no. AF-200-03; PeproTech
Inc., Rocky Hill, NJ, USA) were used as a positive control.
Furthermore, for western blot analysis, and membrane proteins were
extracted with a ReadyPrep™ Protein Extraction kit (Membrane I)
(cat. no. 1632088; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
to determine the expression of IL-3R. Protein concentrations were
measured using Lowry's method. Total proteins (30 µg) extracted
from cultured cells were separated by 12% SDS-PAGE and transferred
to polyvinylidene difluoride membranes. Membranes were subsequently
blotted with antibodies against IL-3R (1:1,000; cat. no. sc-30007),
GAPDH (1:1,000; cat. no. sc-365062; both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), JAK2 (1:1,000; cat. no.
3230), p-JAK2 (1:1,000; cat. no. 3771), STAT5 (1:1,000; cat. no.
9363; Cell Signaling Technology, Inc.), and p-STAT5 (1:1,000; cat.
no. 9359; all from Cell Signaling Technology, Inc., Danvers, MA,
USA). Primary antibodies and appropriate horseradish
peroxidase-conjugated secondary antibodies (1:10,000; cat. nos.
sc-2004 and sc-2005; Santa Cruz Biotechnology, Inc.) were each
incubated for 1 h at room temperature. Bands were visualized using
enhanced chemiluminescence reagents (Santa Cruz Biotechnology,
Inc.) and exposed to X-ray films (Kodak, Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation. One-way analysis of variance was used for statistical
analysis, performed with SPSS software (version 15.0; SPSS, Inc.,
Chicago, IL, USA).
Results
Characterization of scorpion
venom
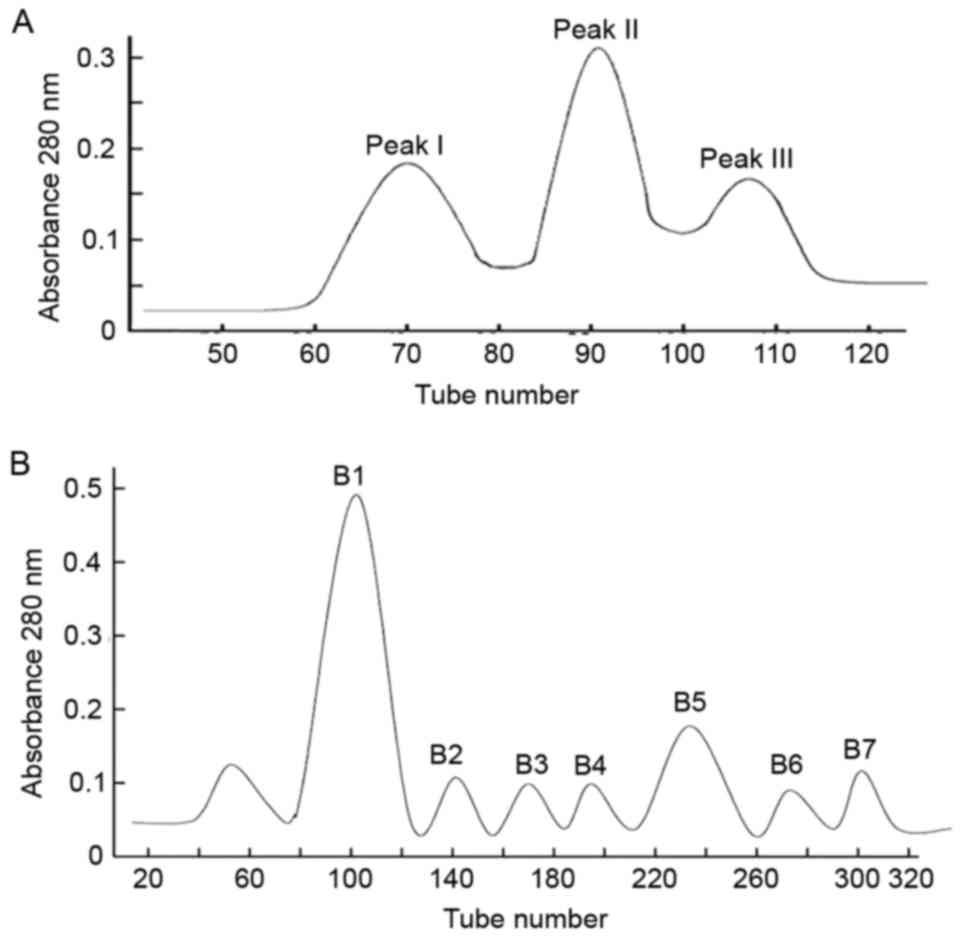
Three different peaks, SVP I–III, were obtained in
the first step (Fig. 1A).
Furthermore, seven peaks (B1, B2, B3, B4, B5, B6, and B7) were
obtained from SVP II using CM-Sepharose FF ion exchange column
chromatography (Fig. 1B). These
peaks were characterized using RP-HPLC (data not shown). Based on
the area normalization method, B5 showed the highest purity (95%;
retention time, 48.609 min) followed by B4 (retention time, 37.858
min). By contrast, components B3, B6 and B7 had numerous peaks and
were relatively less pure (data not shown).
Effects of SVP-B4 and SVP-B5 on
M-NFS-60 cell proliferation
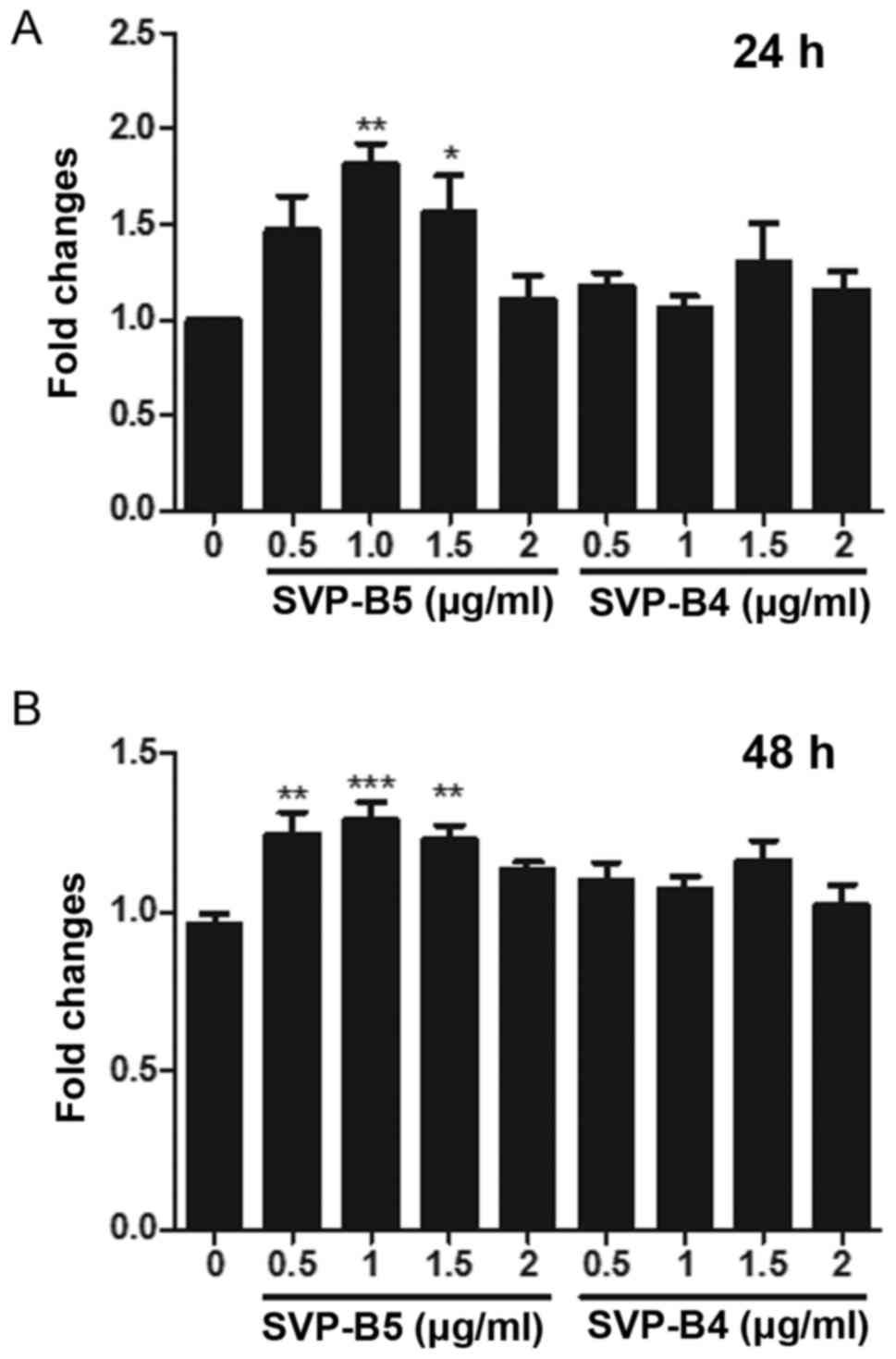
Next, the effect of SVP-B5 and SVP-B4 on the
proliferation of M-NFS-60 cells was determined. A significant
increase in the cell proliferation was observed when cells were
treated with SVPB5 for 24 and 48 h when compared with that in the
control group (Fig. 2A and B).
However, no significant difference in cell proliferation was
observed when the cells were treated with SVP-B4. As SVPB4 had no
significant effect on the proliferation of M-NFS-60 cells, this
peptide was not used in the further experiments, while SVP-B5 was
used at doses of 0.5 and 1 µg/ml.
Effect of SVPB5 on CAFC and
CFU-GM
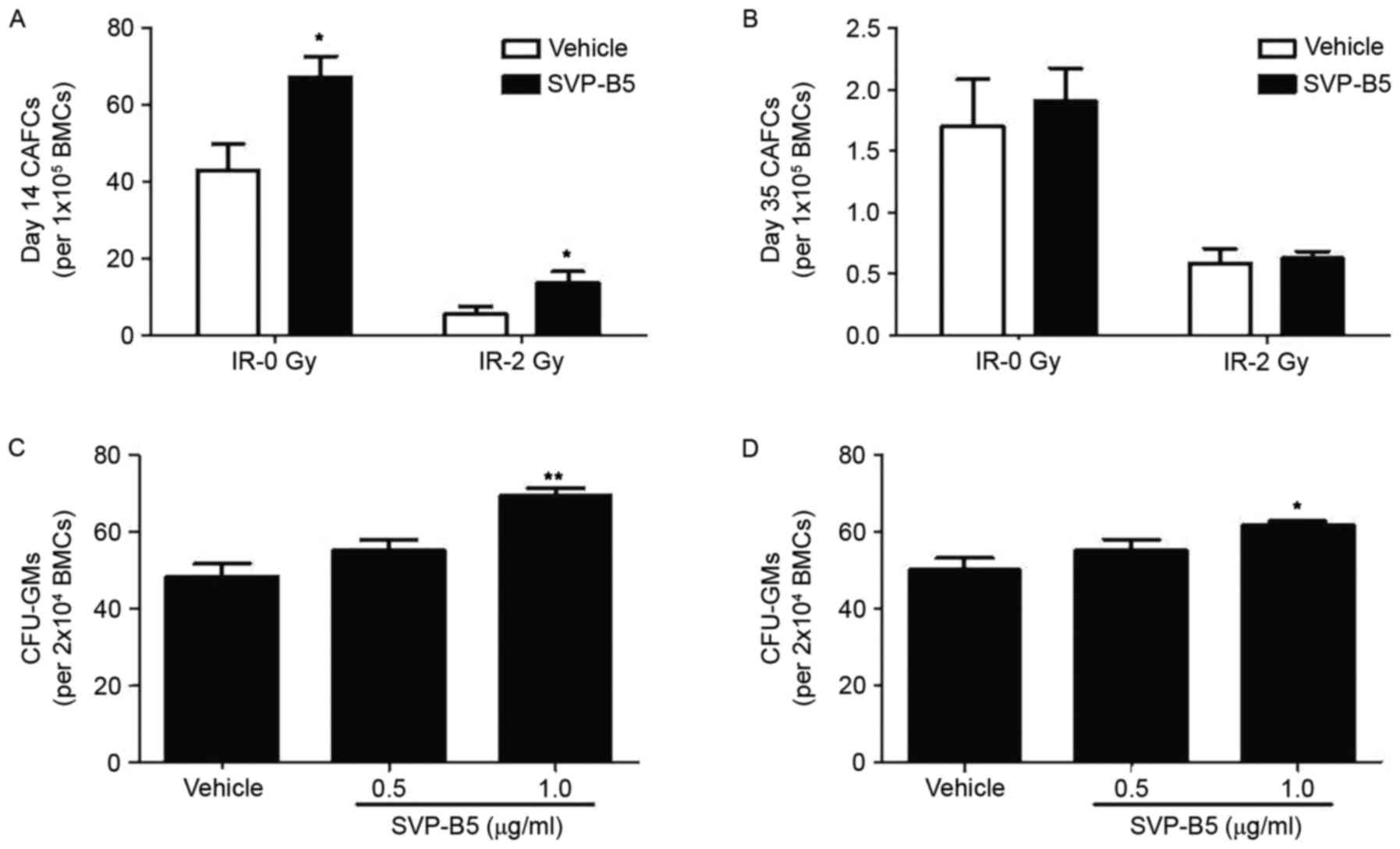
CAFC is a colony-forming assay widely applied to
assess the hematopoietic functions of HSCs. Irrespective of
irradiation, a significant increase in the CAFC in mouse bone
marrow cells was found when treated with 1.0 µg/ml SVP-B5 for 14
days compared with untreated cells (P<0.05; Fig. 3A). However, no statistically
significant difference was found in the number of CAFCs at day 35
(Fig. 3B). Furthermore, the
proportions of CFU-GMs in mouse bone marrow cells were determined.
Irradiated BM-MNCs were used in this experiment. Similar to the
CAFC results, a significant increase in CFU-GMs was observed in the
SVP-B5-treated group compared with that in the untreated group
(61.7±1.4 vs. 50±4.5 per 2×104 bone marrow monocytes;
P<0.05; Fig. 3C and D). These
results indicated that SVP-B5 supports hematopoietic cell
expansion.
Effect of SVPB5 on LTBMC
LTBMCs were established with bone marrow cells. A
significant increase in the number of hematopoietic colony-forming
cells was observed at day 14 when cells were cultured in the
presence of 0.5 µg/ml SVPB5 compared with that in the control
group. However, an increased dose of SVPB5 (1 µg/ml) did not cause
any significant change in the number of hematopoietic
colony-forming cells. Furthermore, 7 days of incubation was not
sufficient to cause any change in the number of colonies at any
dose of SVPB5 (Table I). These
results demonstrated that SVP-B5 treatment at a low dose had a high
stimulatory effect on the proliferative ability of these bone
marrow cells at 14 days. In this experiment, BM-MNCs were
irradiated.
 | Table I.Effect of SVP-B5 on irradiated
long-term bone marrow cell cultures. |
Table I.
Effect of SVP-B5 on irradiated
long-term bone marrow cell cultures.
|
| Number of
colonies |
|---|
|
|
|
|---|
| Group | 7 days | 14 days |
|---|
| IR− |
16.00±1.73 |
9.00±1.00 |
| IR+ |
1.83±1.04 |
5.50±2.00 |
| IR++SVPB5
(0.5 µg/ml) |
1.17±0.29 |
11.67±3.25a |
| IR++SVPB5
(1.0 µg/ml) |
1.50±0.87 |
6.00±3.46 |
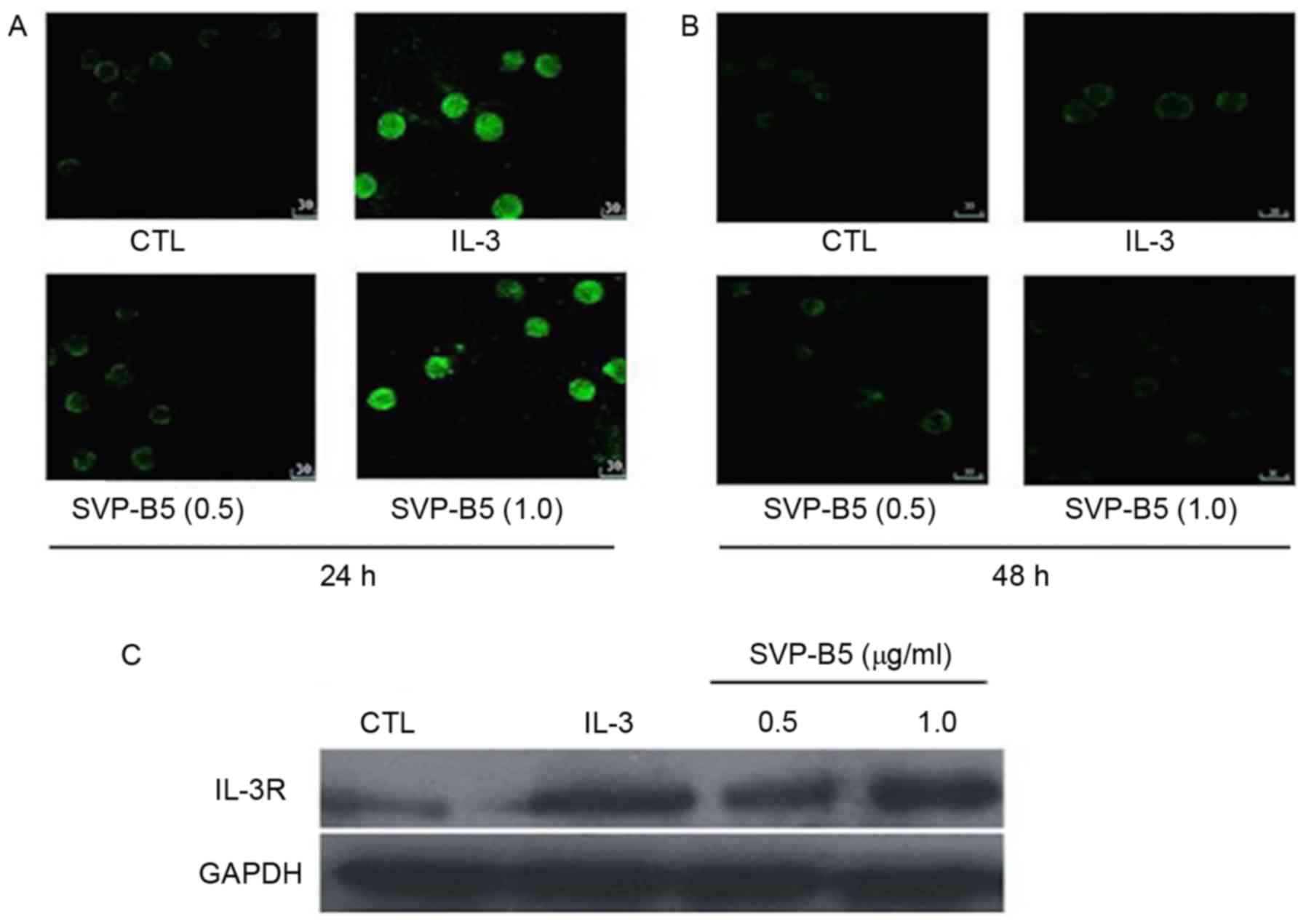
Effect of SVPB5 on IL-3R
expression
SVP-B5 was found to dose-dependently increase in the
expression of IL-3R in M-NFS-60 cells compared with that in the
controls at 24 and 48 h, as determined by immunohistochemistry
(Fig. 4A and B). Similar results
were observed when the expression of IL-3R was determined by
western blot analysis at 24 h (Fig.
4C).
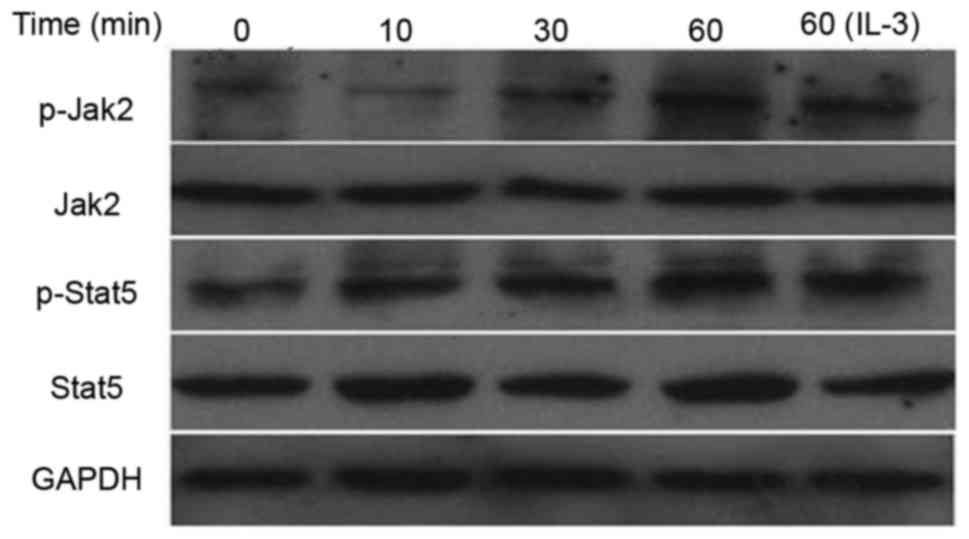
Effect of SVPB5 on the protein
expression and phosphorylation of JAK2 and STAT5
Next, the effect of SVP-B5 treatment on the
activation of the JAK2/STAT5 pathway was determined. It was
observed that SVP-B5 increased the expression of p-JAK2 and p-STAT5
in M-NFS-60 cells in a time-dependent manner. However, the
expression of total JAK2 and STAT5 remained unchanged with SVP-B5
treatment (Fig. 5).
Discussion
In search of novel therapeutic modalities, the use
of natural products as well as purification and characterization of
their active components have been pursued as a successful strategy
employed by modern medicinal researchers for numerous years. Our
laboratory is continuously working on characterizing components
from SVP and defining their effects on the post-radiation recovery
of hematopoietic cells. The novel principal component SVP-B5 was
isolated from the previously defined SVPII fraction (10). It was demonstrated that SVP-5B has
hyperproliferative effects on irradiated hematopoietic cells.
Treatment with SVP-B5 increased IL-3R expression and led to the
activation of the JAK/STAT5 pathway in M-NFS-60 cells.
Proliferation and differentiation of HSCs are the
major concern during radiation therapy in the recovery phase. SVPII
augments the proliferation of these cells in a
concentration-dependent manner (12). The results of the present study
demonstrated that the principal component in the SVPII, namely
SVP-B5, promotes the proliferation of the M-NFS-60 mouse-derived
myelocytic leukemia cell line. These results corroborate with the
findings of previous studies by our group and emphasize that
purified SVP-B5 is the peptide to which the properties of the crude
SVP II fraction of scorpion venom may be ascribed. Furthermore,
hematopoietic functions of HSCs were evaluated using different
methods, including CAFC, CFU and LTBMC assays. SVP-B5 promoted
colony formation of BM-MNCs and enhanced HSC recovery. These
observations demonstrated that SVP-B5 is the active principal
component within SVP II, which has the ability to promote the
proliferation of these cells, exerts growth factor-like properties,
and therefore warrants future study, whereas the other purified
peptide, SVPB4, failed to do so. These results clearly emphasized
the potential of SVPB5 as a hematopoietic growth factor.
IL-3 promotes pluripotent hematopoiesis by
stimulating the self-renewal of early pluripotent stem cells as
well as the proliferation and differentiation of marrow-derived
progenitor cells, resulting in the continued production and
survival of mature blood cells. IL-3 exerts its biological
activities by binding to its specific high-affinity receptor,
IL-3R, on hematopoietic and other cell types (14,15). In
the present study, SVP-B5 significantly increased the expression of
IL-3R in the M-NFS-60 mouse-derived myelocytic leukemia cell line.
It was speculated that the increment in cell proliferation and the
increased expression of IL-3R by SVP-B5 may be functionally linked;
however, this remains to be elucidated.
The JAK2/STAT5 pathway is an important signaling
pathway for a majority of cytokines in the regulation of HSC and
progenitor cell proliferation, self-renewal and differentiation. In
addition, the role of IL-3 and its cognate receptor IL-3R in
different hematopoietic expansions by the activation of JAK2/STAT5
is well documented in the literature (16). Considering the growth factor-like
properties and the enhanced expression of IL-3R post-treatment with
SVP-B5, the present study investigated whether SVP-B5 activates the
JAK2/STAT5 pathway. The results clearly demonstrated that SVP-B5
activated the JAK2/STAT5 pathway in the M-NFS-60 cells.
The sequence of the SVP-B5 peptide has been
previously discussed (10). Using
the Basic Local Alignment Search Tool protein database (https://blast.ncbi.nlm.nih.gov/Blast.cgi), it was
found that SVP-B5 shares >80% homology with the sequence of the
α-toxins family (data not shown). Importantly, SVP-B5 contains the
eight intrinsic cysteines and four pairs of disulfide bonds, which
is the characteristic feature of the α-toxins (17–19). The
α-toxins are known to induce a prolongation of the action potential
of nerves and muscles by fast inactivation of sodium channel
receptor affinity dependent upon membrane potential (20). This property of SVP-B5 and further
characterization should be explored in the near future.
The ability of natural toxins to bind specifically
to various cellular domains upholds new hope for anti-cancer drug
development. Latest developments in nanotechnology illustrate how
researchers are tuning drug candidates to target specific sites.
Recently, usage of scorpion venom encapsulated in nanoparticles
(NanoVenin) has been demonstrated to have the potential to treat
breast cancer (21). The assets of
purified SVP-B5 discussed in the present study put forward its
potential use as a novel drug candidate; however, further
investigation is required prior to commencing their clinical
application. As sufficient gaps exist in the literature regarding
the appropriate form of drug administration, the exact molecular
mechanism of its action, toxicological studies, and drug-drug
interactions, additional research work in this direction using a
rigorous system biology approach is required to address the
important issues highlighted above.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30670906) to Dr Weihua Dong.
The authors would like to thank the laboratory of Professor Daohong
Zhou (Division of Radiation Health, Department of Pharmaceutical
Sciences, University of Arkansas for Medical Sciences, Little Rock,
AR, USA) for their help with the CAFC and CFU-GM assays and
discussion of the results. We thank the SciencePen group
(Chandigarh, India) for their support in editing and proofreading
the article.
References
|
1
|
Greenberger JS and Epperly M: Bone
marrow-derived stem cells and radiation response. Semin Radiat
Oncol. 19:133–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirabayashi Y: Radiation-induced, cell
cycle-related gene expression in aging hematopoietic stem cells:
Enigma of their recovery. Ann N Y Acad Sci. 1310:69–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman CN, Stone HB, Moulder JE and
Pellmar TC: Medicine. Modulation of radiation injury. Science.
304:693–694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Possani LD, Becerril B, Delepierre M and
Tytgat J: Scorpion toxins specific for Na+-channels. Eur
J Biochem. 264:287–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Remijsen Q, Verdonck F and Willems J:
Parabutoporin, a cationic amphipathic peptide from scorpion venom:
Much more than an antibiotic. Toxicon. 55:180–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
du Plessis LH, Elgar D and du Plessis JL:
Southern African scorpion toxins: An overview. Toxicon. 51:1–9.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomes A, Bhattacharjee P, Mishra R, Biswas
AK, Dasgupta SC and Giri B: Anticancer potential of animal venoms
and toxins. Indian J Exp Biol. 48:93–103. 2010.PubMed/NCBI
|
|
8
|
Kozminsky-Atias A, Somech E and Zilberberg
N: Isolation of the first toxin from the scorpion Buthus
occitanus israelis showing preference for Shaker
potassium channels. FEBS Lett. 581:2478–2484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petricevich VL, Cruz Hernández A, Coronas
FI and Possani LD: Toxin gamma from Tityus serrulatus scorpion
venom plays an essential role in immunomodulation of macrophages.
Toxicon. 50:666–675. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Zhou M, Li T, Wang Y, Xing B, Kong
T and Dong W: Effects of scorpion venom peptide B5 on hematopoietic
recovery in irradiated mice and the primary mechanisms. Sci Rep.
5:153632015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong W, Wang L, Kong T and He Y: Scorpion
venom peptides accelerate hematopoietic recovery of
myelosuppression in irradiated mice. Am J Chin Med. 37:701–712.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu Y, Jiang L, Wang C, Wang Y, Li T, Xing
B, Zhou M, Kong T and Dong W: Scorpion venom peptide SPVII promotes
irradiated cells proliferation and increases the expression of the
IL-3 receptor. Cell Biosci. 3:282013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng A, Wang Y, Brown SA, Van Zant G and
Zhou D: Ionizing radiation and busulfan inhibit murine bone marrow
cell hematopoietic function via apoptosis-dependent and
-independent mechanisms. Exp Hematol. 31:1348–1356. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barhanpurkar AP, Gupta N, Srivastava RK,
Tomar GB, Naik SP, Joshi SR, Pote ST, Mishra GC and Wani MR: IL-3
promotes osteoblast differentiation and bone formation in human
mesenchymal stem cells. Biochem Biophys Res Commun. 418:669–675.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blalock WL, Weinstein-Oppenheimer C, Chang
F, Hoyle PE, Wang XY, Algate PA, Franklin RA, Oberhaus SM, Steelman
LS and McCubrey JA: Signal transduction, cell cycle regulatory and
anti-apoptotic pathways regulated by IL-3 in hematopoietic cells:
Possible sites for intervention with anti-neoplastic drugs.
Leukemia. 13:1109–1166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reddy EP, Korapati A, Chaturvedi P and
Rane S: IL-3 signaling and the role of Src kinases, JAKs and STATs:
A covert liaison unveiled. Oncogene. 19:2532–2547. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng XC, Li WX, Zhu SY, Peng F, Jiang DH,
Yang FH and Wu KL: Cloning and characterization of the cDNA
sequences of two venom peptides from Chinese scorpion Buthus
martensii Karsch (BmK). Toxicon. 38:893–899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng XC, Luo F and Li WX: Molecular
dissection of venom from Chinese scorpion Mesobuthus
martensii: Identification and characterization of four novel
disulfide-bridged venom peptides. Peptides. 27:1745–1754. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali SA, Wang B, Alam M, Beck A, Stoeva S,
Voelter W, Abbasi A and Duszenko M: Structure-activity relationship
of an alpha-toxin Bs-Tx28 from scorpion (Buthus sindicus)
venom suggests a new alpha-toxin subfamily. Arch Biochem Biophys.
445:81–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petricevich VL: Scorpion venom and the
inflammatory response. Mediators Inflamm. 2010:9032952010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Misra SK, Ye M, Kim S and Pan D: Highly
efficient anti-cancer therapy using scorpion ‘NanoVenin’. Chem
Commun (Camb). 50:13220–13223. 2014. View Article : Google Scholar : PubMed/NCBI
|