Introduction
Skin aging, which affects physical appearance and
leads to skin diseases, can be due to an interaction of multiple
factors, such as inflammation and trauma, nephropathy, hepatopathy
(1). At present, there are a number
of therapeutic options, including surgical and chemical peel
methods, to treat skin aging (1).
However, securer and more dependable potential treatments should be
investigated as current means have insignificant curative effects,
numerous adverse reactions and poor patient compliance (2).
Adipose-derived stem cells (ADSCs) are isolated and
purified from subcutaneous fatty tissues, possess a self-renewing
ability and multiple differentiation potentials and may secrete
biologically active factors to repair damaged organ tissues
including cardiac muscle, nerves, kidney and arthrodial cartilage
(3). Anti-aging functions of ADSCs
have been preliminarily supported by a previous study (4).
Fullerenol is a generic term for a hollow
spheroidal, ellipsoid-shaped, columnar order and tracheary molecule
formed completely by carbon (5). It
is also the fourth allotropic substance of carbon identified
following adamas, graphite and amorphous carbon (6). Fullerenol is a water-soluble fullerene
derivative, and has previously been demonstrated to be a powerful
antioxidant (6). For a number of
neurodegenerative diseases, glutamic acid receptors are excessively
activated, resulting in large production of nitric oxide free
radicals and reactive oxygen species (7). In primary cultured mouse neurons,
fullerenol possesses good oxidation resistance, which may inhibit
the apoptotic process of neuronal excitability caused by oxidative
stress (8). Every fullerenol may
absorb a number of oxygen free radicals to decrease the damage
effects of oxygen free radicals on nerve tissues (6). Furthermore, fullerenol may interdict
functions of glutamic acid receptors to further prevent neurons
from apoptosis (9). The aim of the
present study was to use a mouse model of skin aging induced by
D-galactose to analyze the anti-aging effects of fullerenol on skin
aging through derived stem cells.
Materials and methods
Isolation and culture of ADSCs
A total of 24 male green fluorescent
protein-expressing mice (6-week-old; 20–22 g) were obtained from
the Animal Center of Harbin Medical University (Harbin, China).
Mice were housed at 22–24°C and 55–60% humidity with a 12 h
light/dark cycle with free access to food and water. Mice were
sacrificed via decapitation under anesthesia (35 mg/kg
pentobarbital; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)
following sacrifice, the skin was incised and inguinal fat pad
adipose tissue samples were separated and washed with PBS, which
were sliced into sections (5–10 mm) and digested with 0.15% type I
collagenase for 30 min at room temperature. The supernatant was
separated from pellets by centrifugation at 1,500 × g and the
pellets were resuspended with phosphate-buffered saline (PBS). The
solution was then filtered with a 200 mm mesh into spin down
stromal-vascular fraction cell pellets. The retrieved cell fraction
was cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 mg/ml streptomycin (both from Sigma-Aldrich; Merck KGaA) at
37°C with 5% CO2 for 3 weeks. Animal experimental
protocols were approved by the Second Affiliated Hospital of Harbin
Medical University Heilongjiang Laboratory Animal Administration
Committee (Harbin, China) and experiments were in accordance with
the Institutional Guidelines for Animal Experimentation.
D-galactose-induced aging model and
animal experiments
A total of 30 male 6-week-old nude mice (20–22 g)
were provided by the Harbin Medical University Heilongjiang
Experimental Animal Center. Mice were housed and 22–24°C, 55–60%
humidity with access to food and water ad libitum. Mice were
randomly divided into three groups (n=10 in each group): Control,
model and fullerenol groups. The model and fullerenol groups of
mice were administered 1,000 mg/kg D-galactose (Sigma-Aldrich;
Merck KGaA) by subcutaneous injection. Following 2 weeks of
acclimation, the control and model groups were administered PBS by
subcutaneous injection. The fullerenol group mice were administered
a subcutaneous injection of 106 GFP-expressing ADSCs and
100 mg/kg fullerenol (Sigma-Aldrich; Merck KGaA), as previously
described (10). Following 3 weeks
of treatment, mice were sacrificed by decapitation under 30 mg/kg
of pentobarbital. Skin was immediately harvested washed and stored
at −80°C.
Differentiation of mice ADSC
ADSCs were divided into two groups: Control and
fullerenol. In the control group, ADSCs were induced with
β-glycerophosphate and 10−7 M dexamethasone; in the
fullerenol group, ADSCs were induced using 10 mM β-glycerophosphate
and 10−7 M dexamethasone in medium supplemented with 100
µM fullerenol for 3 weeks. All ADSCs were stained using Oil-Red O
staining, which identifies fat, bone and cartilage cells.
Differentiation of ADSCs was observed using a BX51 light microscope
(Olympus, Tokyo, Japan).
Differentiation of retention rate and
thickness of the dermal portion of skin
Skin tissue samples were fixed in 4%
paraformaldehyde at room temperature for 24 h, dehydrated and
paraffin-embedded. Then, hematoxylin and eosin was used to stain
skin tissue samples, which were extracted from the cell injection
site of the mice at the midline of the dorsum. Skin tissue samples
were cut into 6-mm sections and assessed under a BX51 light
microscope and imaged using a DP71 digital camera (both from
Olympus). The total collagen content was analyzed with the aniline
blue staining, which was observed using ImageJ software version 3.0
(National Institutes of Health, Bethesda, MD, USA).
Lactate dehydrogenase (LDH) and cell
viability assay using MTT
A total of 2–3×103 ADSC were seeded into
96-well plates and cultured with 0.1, 0.3, 1 or 3 µM fullerenol for
24 h at 37°C. Cytotoxicity was assessed using 1% Triton X-100 and a
detergent leading to a complete release of the LDH enzyme
(Sigma-Aldrich; Merck KGaA). To assess cell viability, 100 µl
sterile MTT dye (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) was
incubated with the ADSCs for 4 h at 37°C and 150 µl dimethyl
sulfoxide for 15 min. Cytotoxicity and cell viability were measured
at 490 nm using a microplate ELISA reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of Runt-related transcription
factor 2 (Runx2), alkaline phosphatase (ALP) and osteocalcin
(OCN)
Mice were sacrificed and skin tissue was immediately
collected and stored at −80°C. Total ribonucleic acid (RNA) was
isolated from skin tissue samples using the RNeasy® Mini
kit in accordance with the manufacturer's instructions (Qiagen,
Valencia, CA, USA). Using the Reverse Transcription System kit in
accordance with the manufacturer's instructions (Promega
Corporation, Madison, WI, USA), 1 µg total RNA was reverse
transcribed into cDNA. The gene expression of Runx2, ALP and OCN
were measured using Real-time PCR with the QuantiTect®
SYBR Green PCR master mix in accordance with the manufacturer's
instructions (Qiagen). The primer sequences and product sizes are
presented in Table I. GAPDH was used
as a reference gene. The PCR amplification was initiated at 94°C
for 3 min, followed by 40 cycles of 95°C for 35 sec, 60°C for 45
sec, 72°C for 30 sec and a final step at 72°C for 5 sec. The
experiment was repeated three times.
 | Table I.Primer sequences of Runx2, ALP and
OCN. |
Table I.
Primer sequences of Runx2, ALP and
OCN.
| Molecule | Sense | Antisense |
|---|
| Runx2 |
5′-AGATGATGACACTGCCACCTCTG-3′ |
5′-GGGATGAAATGCTTGGGAACTGC-3′ |
| ALP |
5′-ACCATTCCCACGTCTTCACATTTG-3′ |
5′-AGACATTCTCTCGTTCACCGCC-3′ |
| OCN |
5′-CAGCGAGGTAGTGAAGAGAC-3′ |
5′-TGAAAGCCGATGTGGTCAG-3′ |
| GAPDH |
5′-ATGGCATCAAGAAGGTGGTG-3′ |
5′-CATACCAGGAAAATGAGCTTG-3′ |
Western blot analysis
Skin tissue samples were homogenized in an ice-cold
lysis buffer and total protein levels were quantified using a BCA
protein assay kit (both from Beyotime Institute of Biotechnology,
Haimen, China). For each sample, 50 µg total protein was run on
10–12% SDS-PAGE and electro-transferred to nitrocellulose membranes
(Thermo Fisher Scientific, Inc.). The membranes were blocked using
Tris-buffered saline and Tween-20 (50 mM Tris, pH 7.6, 150 mM NaCl,
0.05% Tween-20) with 5% bovine serum albumin (Shanghai Sangong
Pharmaceutical Co., Ltd., Shanghai, China) for 1 h at room
temperature. The membranes were washed with TBST, and incubated
with anti-peroxisome proliferator-activated receptor-γ (PPAR-γ,
sc-9000; Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-
forkhead box protein O1 (FoxO1, ab52857; Abcam, Cambridge, UK) and
β-actin (Santa Cruz Biotechnology, Inc.) overnight at 4°C. The
membranes were incubated with anti-rabbit horseradish
peroxidase-conjugated secondary antibody (A0208, 1:2,000; Beyotime
Institute of Biotechnology) for 1 h at room temperature followed by
incubation at 37°C for 1 h with chemiluminescent substrate for
horseradish peroxidase antibody and enhancer solution (Thermo
Fisher Scientific, Inc.). Protein blank was analyzed by Quantity
One software 3.0 (Bio-Rad Laboratories, Inc.). Three replicates
were performed.
Statistical analysis
The results of the quantitative and morphometric
analyses were calculated as the mean ± standard error of the mean
by SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical analysis
was conducted using one-way analysis of variance followed by
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
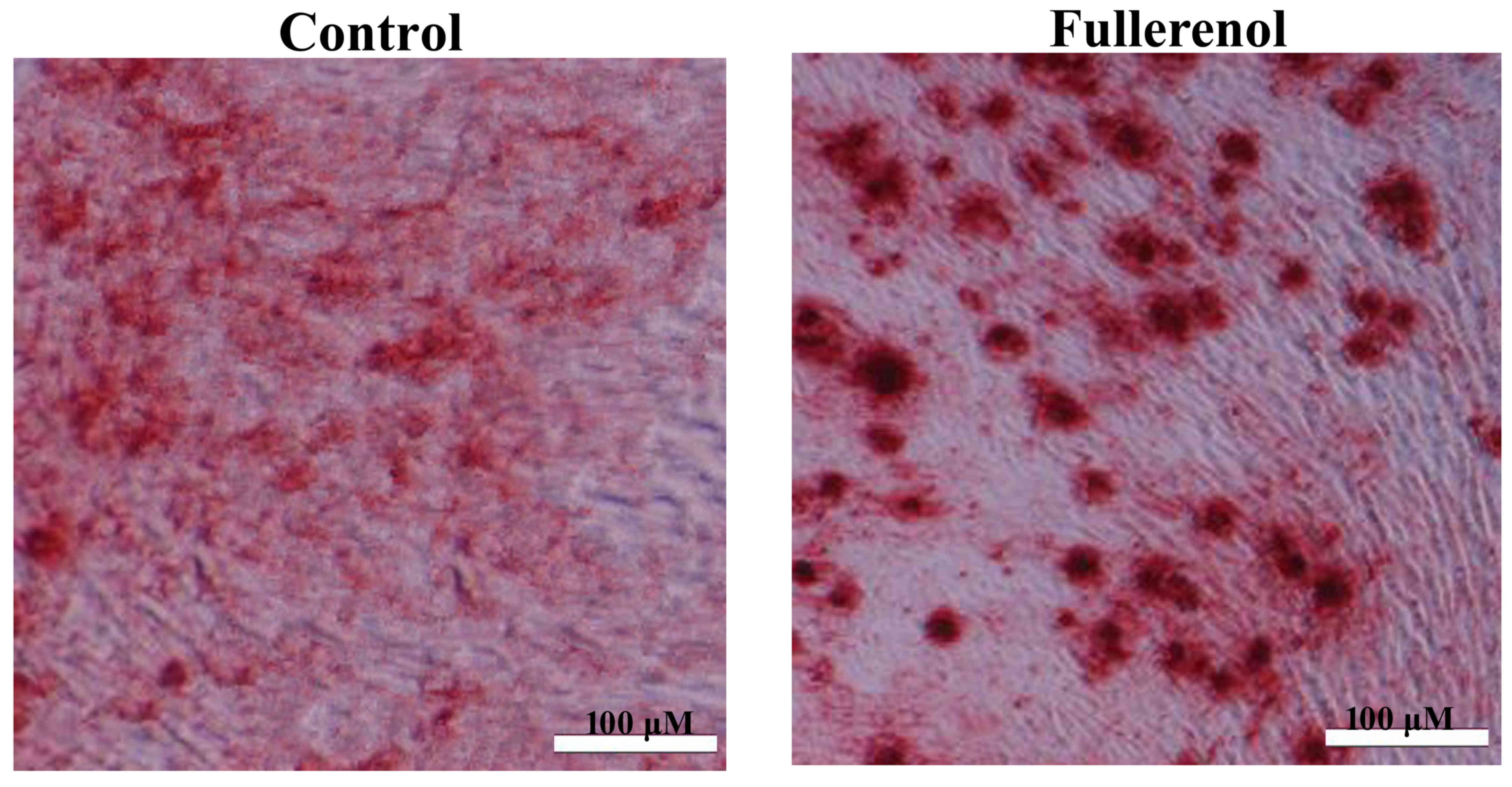
Effect of fullerenol on adipose
differentiation of ADSCs
ADSCs induced by fullerenol following 3 weeks were
observed using a microscope. There was a significant inhibition of
adipose differentiation of ADSC in model group, compared with the
fullerennol group (P<0.05; Fig.
1). As presented in Fig. 1,
fullerenol induced adipose differentiation of ADSC significantly
when compared with the control group (P<0.05).
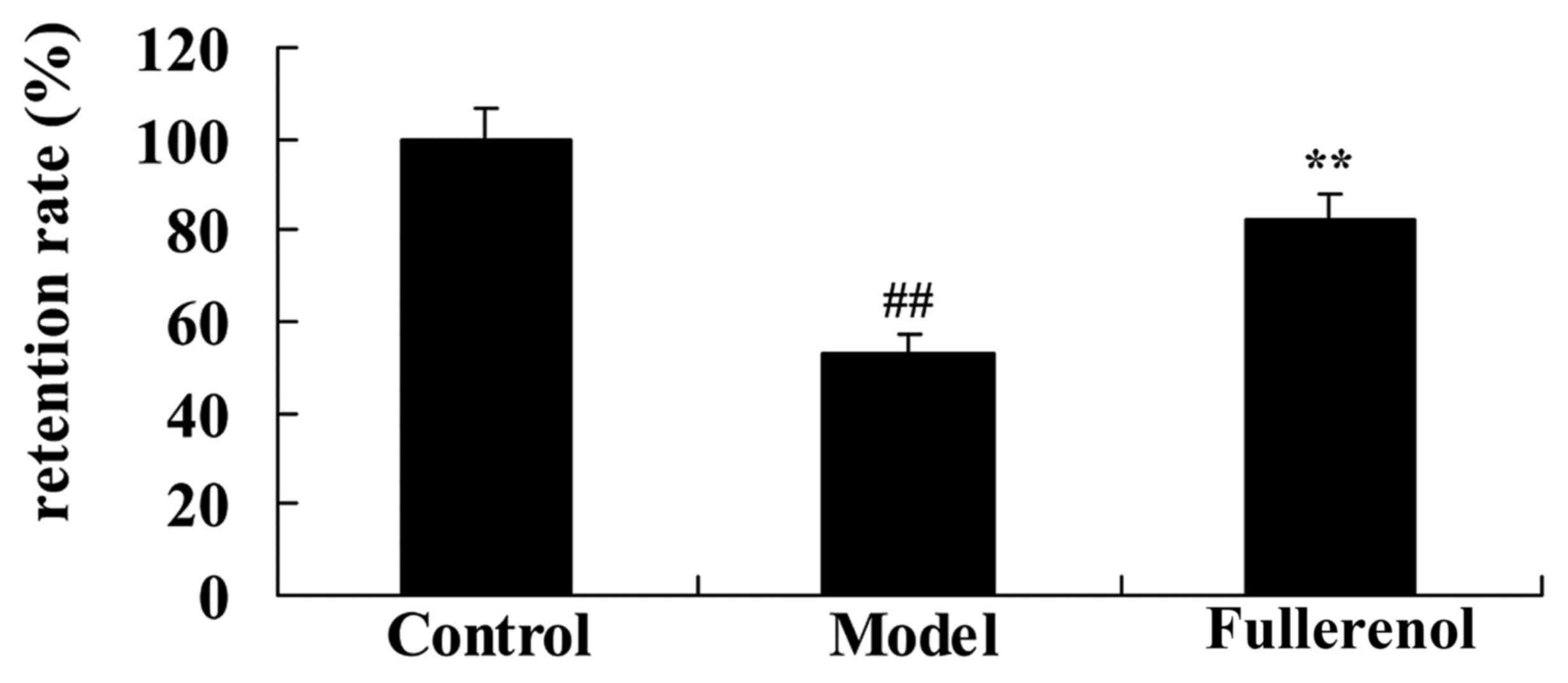
Effect of fullerenol on the retention
rate of D-galactose-induced mice
The retention rate of the D-galactose model group
was lower than that of control group. ADSCs and 100 mg/kg
fullerenol markedly increased the D-galactose-inhibition of
retention rate in mice (Fig. 2).
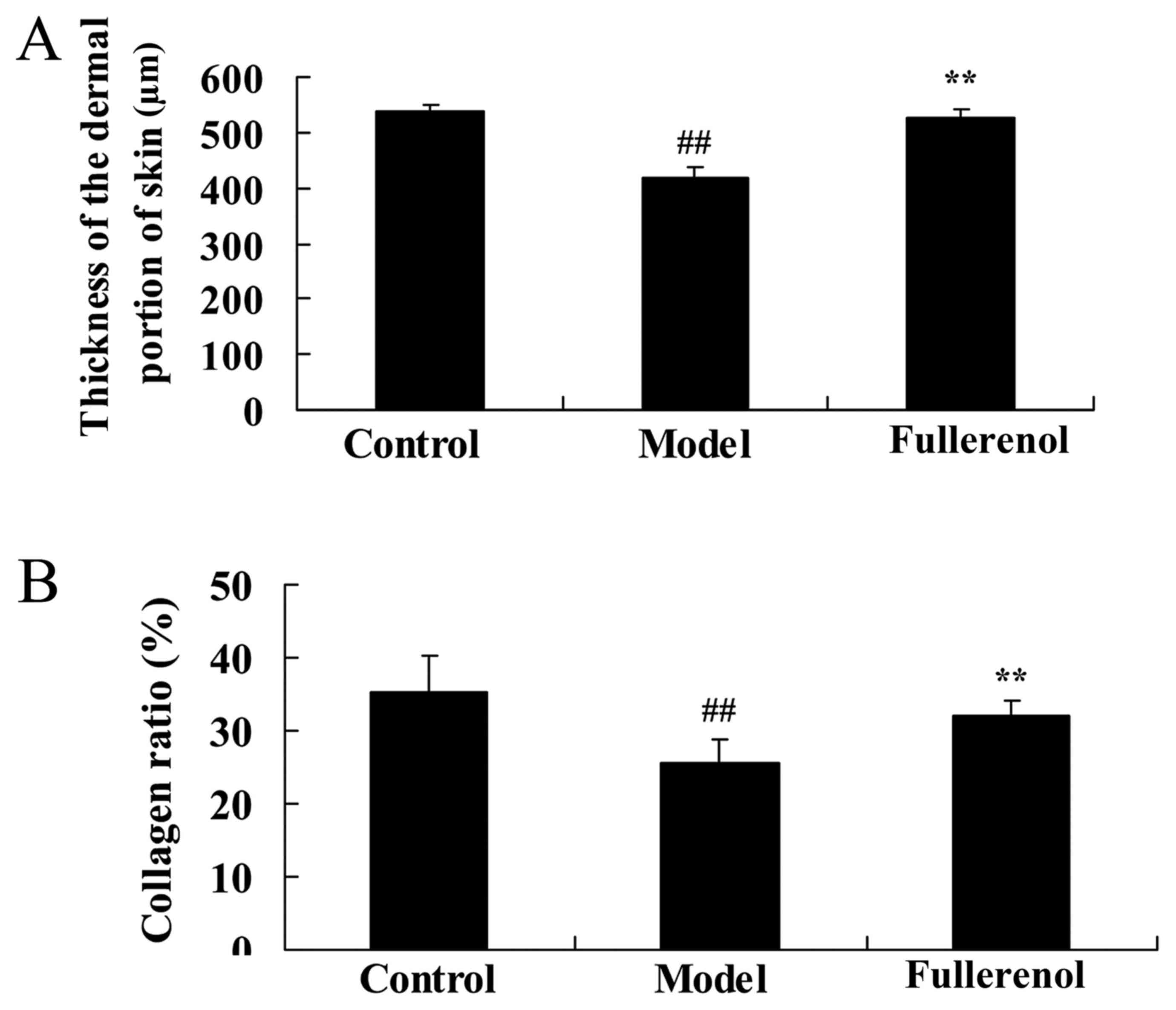
Effect of fullerenol on the thickness
of the dermal portion of skin and collagen ratio
Following fullerenol treatment, the thickness of the
dermal portion of skin and collagen ratio was significantly
decreased compared with the control group rats (P<0.05; Fig. 3). As presented in Fig. 3, treatment with fullerenol
significantly promoted D-galactose-inhibition of the thickness of
the dermal portion of skin and collagen ratio mice.
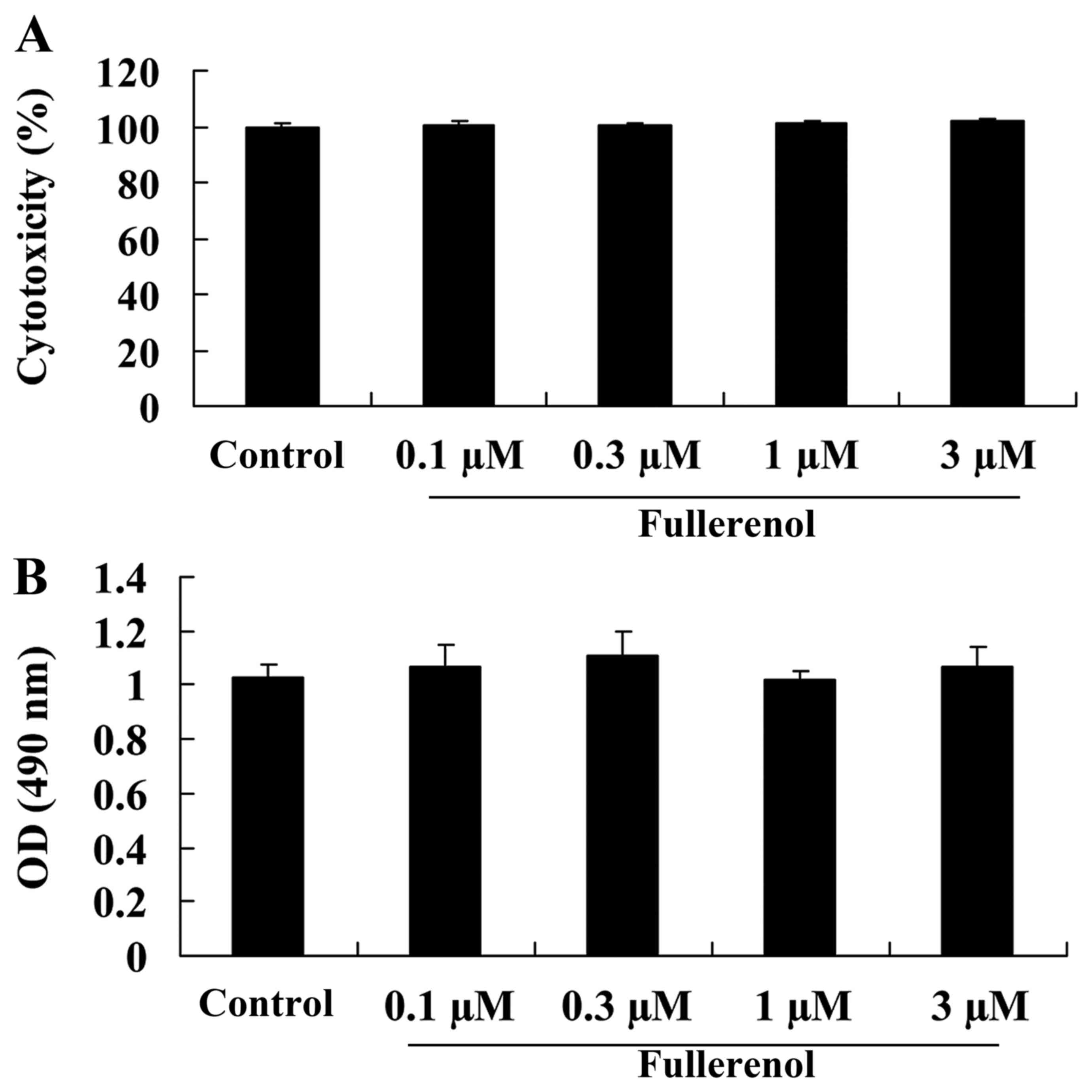
Effect of fullerenol on cytotoxicity
and cell viability of D-galactose-induced mice
The anti-aging effect of fullerenol on the
cytotoxicity (Fig. 4A) and cell
viability (Fig. 4B) of
D-galactose-induced mice was identified and Fig. 4 indicates the cytotoxicity and cell
viability rates for all groups treated with 0.1, 0.3, 1 or 3 µM
fullerenol. No significant difference was observed between groups
(P>0.05; Fig. 4).
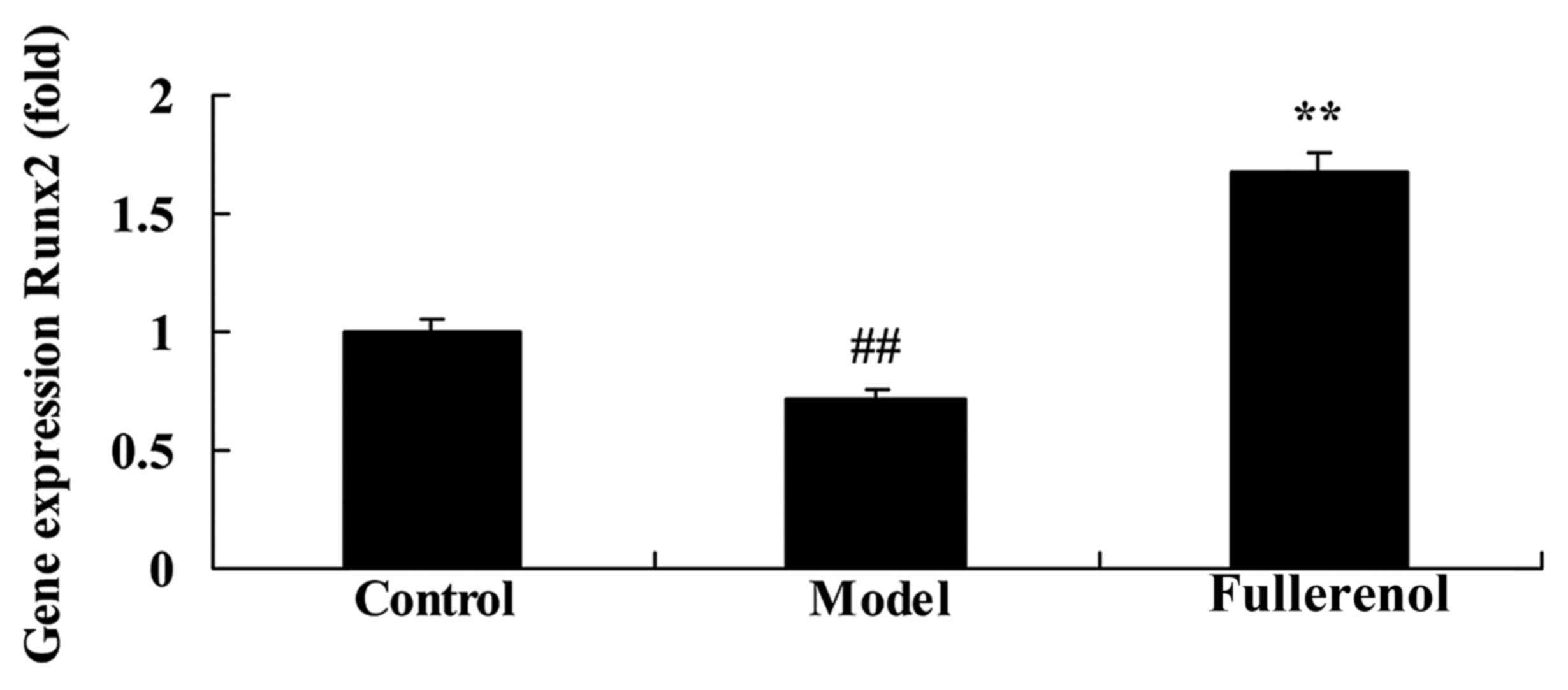
Effect of fullerenol on Runx2 of
D-galactose-induced mice
To investigate the anti-aging effect of fullerenol
on Runx2 of D-galactose-induced mice, the expression of the Runx2
gene was detected using RT-qPCR. As presented in Fig. 5, Runx2 gene expression in the
D-galactose-induced model group was significantly lower than that
of control mice (P<0.05). Treatment with fullerenol was
effective in significantly decreasing the expression of Runx2 in
D-galactose-induced mice (P<0.05; Fig. 5).
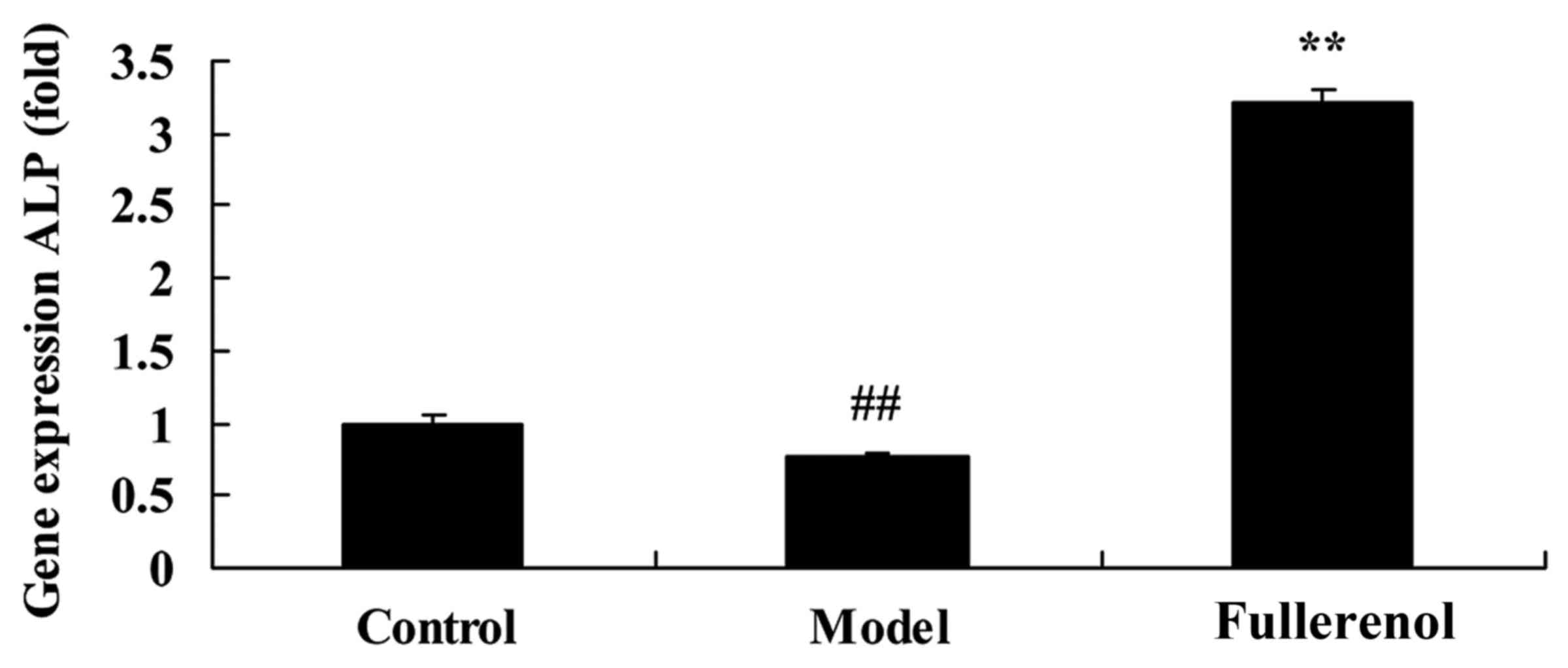
Effect of fullerenol on ALP of
D-galactose-induced mice
In order to confirm that fullerenol has a protective
anti-aging effect on skin, ALP gene expression in
D-galactose-induced mice was assessed. Gene expression of ALP in
D-galactose-induced mice was significantly lower than that of
control mice (P<0.05; Fig. 6).
Treatment with fullerenol significantly increased the expression of
the ALP gene in D-galactose-induced mice (P<0.05; Fig. 6).
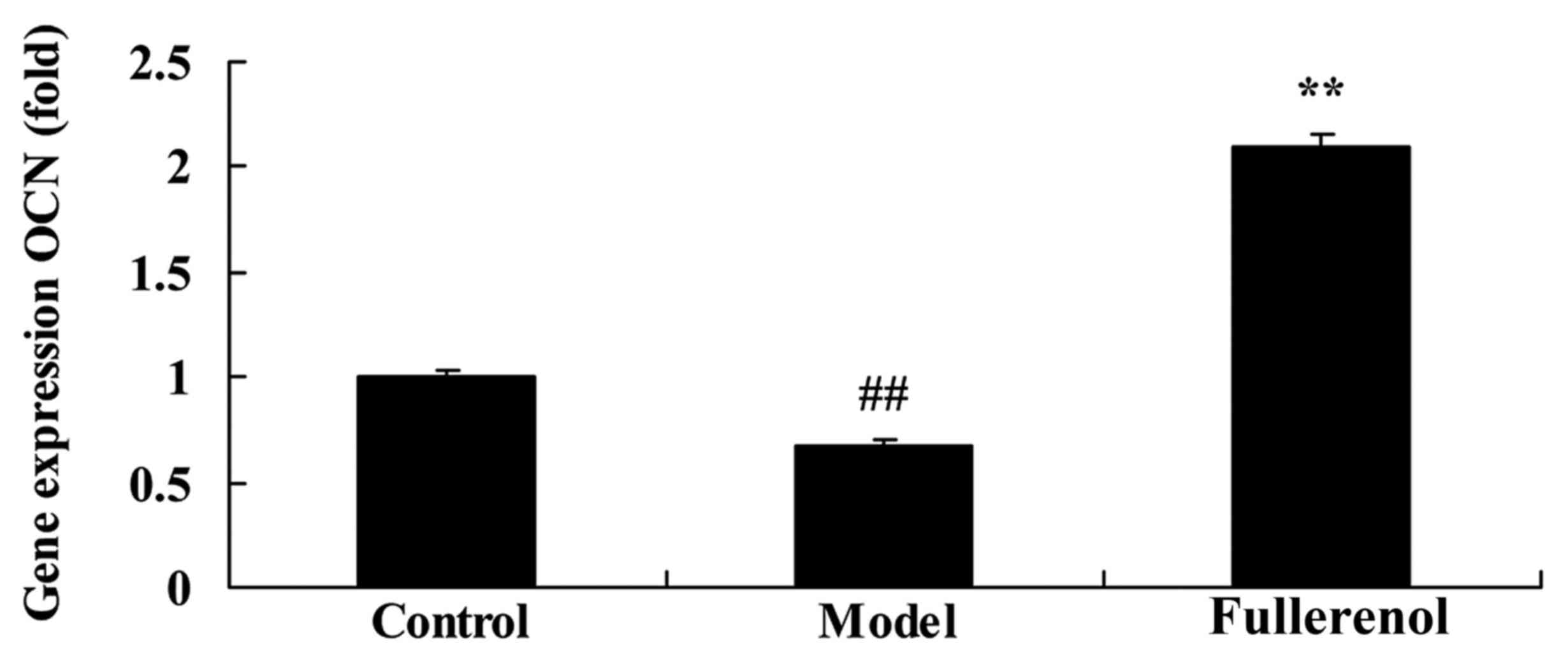
Effect of fullerenol on OCN of
D-galactose-induced mice
To confirm the anti-aging effect of fullerenol on
OCN in ADSCs, OCN gene expression was measured using RT-qPCR.
Compared with control mice, there was a significant decrease in OCN
gene expression of D-galactose-induced model mice (P<0.05;
Fig. 7). As presented in Fig. 7, fullerenol treatment significantly
inhibited the D-galactose-inhibition of OCN gene expression in
D-galactose-induced mice compared with that of D-galactose-induced
model group, leading to a significant increase in the expression of
OCN (P<0.05; Fig. 7).
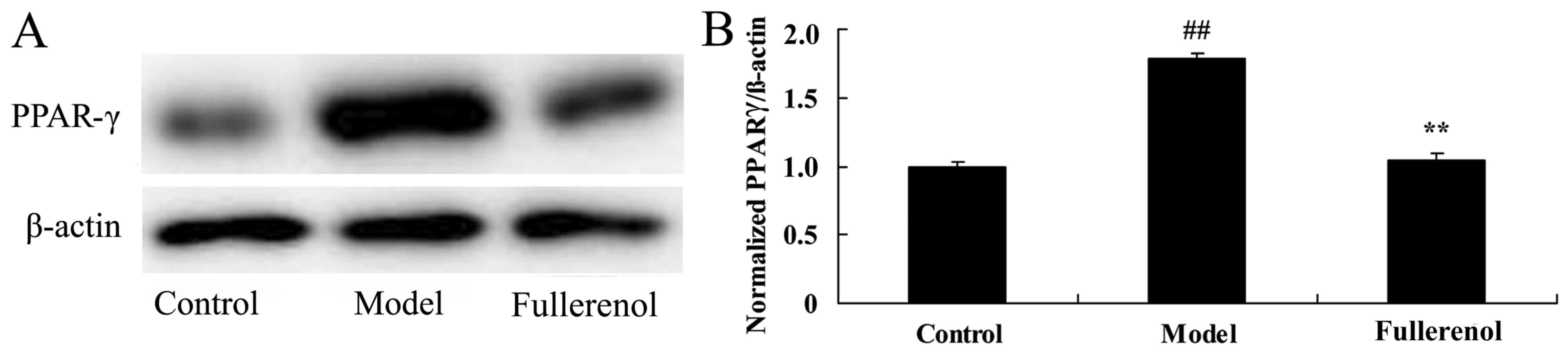
Effect of fullerenol on PPAR-γ of
D-galactose-induced mice
The anti-aging effect of fullerenol on PPAR-γ of
D-galactose-induced mice was investigated by assessing the protein
expression of PPAR-γ using western blot analysis. Western blot
analysis demonstrated that PPAR-γ protein expression in
D-galactose-induced mice model group was significantly increased
compared with that of the control group (P<0.05; Fig. 8). Pretreatment with fullerenol
significantly decreased the D-galactose-induced PPAR-γ protein
expression in mice, compared with mice in the D-galactose-induced
model group (P<0.05; Fig. 8).
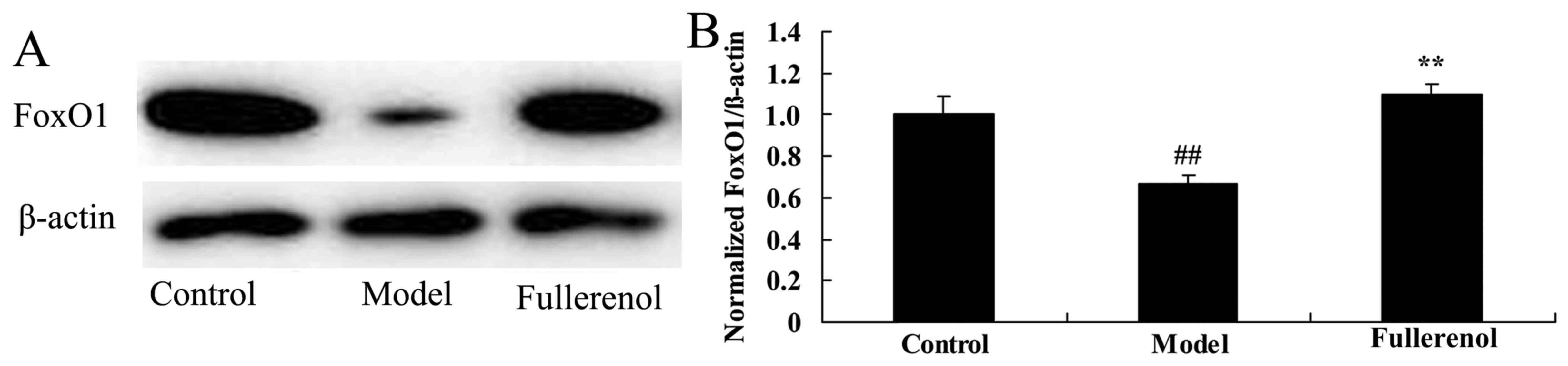
Effect of fullerenol on FoxO 1
expression in D-galactose-induced mice
To study the potential mechanistic pathways
underlying the promotion of fullerenol, FoxO1 protein expression
was assessed using western blot analysis. FoxO1 protein expression
in D-galactose-induced mice was significantly reduced in the model
group, compared with the control group (P<0.05; Fig. 9). However, treatment with fullerenol
significantly increased D-galactose-induced inhibition of FoxO1
protein expression in mice (P<0.05; Fig. 9).
Discussion
Skin aging is caused by endogenous and exogenous
factors and is characterized by thinner and laxer skin, dry and
rough skin, reduced elasticity, formation of wrinkles,
over-pigmentation and angiotelectasis (11). In vitro and animal experiments
have demonstrated the effects of ADSCs and associated cytokines on
anti-aging (3,11). However, clinical studies are limited
to case reports, thus the security and feasibility should be
further investigated through a large numbers of clinical trials
(3). In addition, in vitro
expansion of stem cells may cause aging, which affects curative
effects, including ameliorating inflammation and trauma (11). Under the prerequisite of anti-aging
functions of cytokines secreted by ADSCs, clinical application of
these cytokines is potentially more feasible (12). Consequently, following the
purification of cytokines in culture medium, externally applied
drugs are manufactured to increase the feasibility of ADSCs
application in skin care (3). In the
present study, treatment with fullerenol and ADSCs significantly
increased the D-galactose-inhibition of retention rate, thickness
of the dermal portion of skin and collagen ratio in
D-galactose-induced mice (P<0.05).
Runx2 is transcription factor in the structural
domain gene family, which serves an essential role in the
developmental process of bone and teeth (13). It is able to bind to specific binding
sites on DNA promoter regions to regulate the transcription of
target genes and participate in organ formation (14). It has been demonstrated that Runx2,
as a specific transcription factor regulating the differentiation
of osteoblasts, may directly induce the differentiation of
mesenchymal stem cells into osteoblasts and serves an important
role in adult bone tissue repair and reconstruction (5). Furthermore, the present study
identified that fullerenol treatment significantly increased the
Runx2 gene expression in D-galactose-induced mice (P<0.05).
ALP is a phenotypic marker for osteoblasts, which
may directly represent the activities and functional status of
osteoblast cells (15). During early
stages of osteogenesis, the activity of ALP is marked. With the
increase of calcium salt deposition during the middle and late
stages of osteogenic differentiation, activities of ALP gradually
decrease (16). OCN is a common
signal molecule that promotes osteogenesis and bone inducing
factors (17). OCN is a common
signal molecule to promote osteogenesis of bone inducing factors
(18). Therefore, the present study
identified that treatment with fullerenol significantly increased
the expression of ALP and OCN in D-galactose-induced mice
(P<0.05). Yang et al (18)
reported that fullerenol promotes osteogenesis of human ADSCs
through Runx2, ALP and OCN.
PPAR-γ is a subtype of peroxisome
proliferator-activated receptors. Following ligand-activation, it
participates in the regulation of cell differentiation,
proliferation and apoptosis (19).
Previous results have indicated that PPAR-γ is a key regulation
factor of adipose differentiation, which has a key regulatory role
on the direction of differentiation in bone marrow stem cells
(20). Both gegenbaur and fat cells
in the marrow cavity originate from mesenchymal stem cells, and
quantities are waxing and waning. Previous studies have
demonstrated that as PPAR-γ mediates lipid differentiation of MSCs,
it may directly affect the differentiation of gegenbaur cells
(21). Osteoclasts develop from
hematopoietic stem cells in bone marrow, and are a primary cause of
osteoporosis as they may enhance bone resorption (20). Furthermore, the present study
indicated that treatment with fullerenol significantly inhibited
the protein expression of PPAR-γ in D-galactose-induced mice
(P<0.05). Furthermore, Liu et al (7) suggested that fullerenol suppresses the
expression of PPAR-γ in bone marrow stromal cells.
As a sub-type of forkhead transcription factor,
FoxOs are fundamental redox sensitive transcription factors and key
downstream effectors in phosphoinositide 3-kinase/protein kinase B
pathway (22). They also controls
genes regulating cell changes, including cell proliferation,
survival and oxidative defense (23). It has been indicated that the
decrease of FoxOs in hematopoietic stem cells may cause a decrease
in antioxidase including reactive oxygen species, manganese
superoxide dismutase and scavenger enzymes, resulting in
dysfunction of mitochondria and cell apoptosis (24). Following a high dose of
H2O2 applied to bone marrow mesenchymal stem
cells, the expression of FoxO1, FoxO3 and FoxO4 mRNA significantly
decreases (25). The present study
used treatment with fullerenol and observed a significant increase
in the D-galactose-induced inhibition of FoxO1 protein expression
in mice. Yang et al (18)
reported that fullerenol promotes osteogenesis of human ADSCs
through promotion of FOXO4.
In conclusion, the results of the present study
provide preliminary evidence that the anti-aging effect of
fullerenol is able to enhance the D-galactose-inhibition of
retention rate, thickness of the dermal portion of skin and
collagen ratio in D-galactose-induced mice. This is completed by
ADSC-modulation of the PPAR-γ/FoxO1 pathway in D-galactose-induced
mice. The results of the present study indicate that fullerenol may
be a potential novel drug to treat skin aging. Future studies
should use clinical samples to confirm that these findings have
potential clinical applications.
References
|
1
|
Senbel AM, AbdelMoneim L and Omar AG:
Celecoxib modulates nitric oxide and reactive oxygen species in
kidney ischemia/reperfusion injury and rat aorta model of
hypoxia/reoxygenation. Vascul Pharmacol. 62:24–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weng TI, Wu HY, Chen BL and Liu SH:
Honokiol attenuates the severity of acute pancreatitis and
associated lung injury via acceleration of acinar cell apoptosis.
Shock. 37:478–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishikawa C, Arbiser JL and Mori N:
Honokiol induces cell cycle arrest and apoptosis via inhibition of
survival signals in adult T-cell leukemia. Biochim Biophys Acta.
1820:879–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark RA, Weragoda N, Paterson K,
Telianidis S and Williams G: A pilot investigation using global
positioning systems into the outdoor activity of people with severe
traumatic brain injury. J Neuroeng Rehabil. 11:372014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erden A, Idilman R, Erden I and Ozden A:
Veins around the esophagus and the stomach: Do their calibrations
provide a diagnostic clue for portal hypertensive gastropathy? Clin
Imaging. 33:22–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Injac R, Prijatelj M and Strukelj B:
Fullerenol nanoparticles: Toxicity and antioxidant activity.
Methods Mol Biol. 1028:75–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Q, Jin L, Shen FH, Balian G and Li XJ:
Fullerol nanoparticles suppress inflammatory response and
adipogenesis of vertebral bone marrow stromal cells-a potential
novel treatment for intervertebral disc degeneration. Spine J.
13:1571–1580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu JM, Lin LP, Jiang SL, Cui ML, Jiao L,
Zhang XY, Zhang LH, Zheng ZY, Lin X and Lin SQ:
Fullerol-fluorescein isothiocyanate-concanavalin agglutinin
phosphorescent sensor for the detection of alpha-fetoprotein and
forecast of human diseases. Spectrochim Acta A Mol Biomol
Spectrosc. 115:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Mahendra S, Lyon DY, Brunet L, Liga
MV, Li D and Alvarez PJ: Antimicrobial nanomaterials for water
disinfection and microbial control: Potential applications and
implications. Water Res. 42:4591–4602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kakutani H, Hino S, Ikeda K, Sumiyama K,
Uchiyama Y, Kuramochi A, Kawamura M and Tajiri H: Prediction of
recurrence of esophageal varices-special reference to a role for
endoscopic ultrasonography. Hepatol Res. 33:259–266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marmulla R and Harder J: Microbial
monoterpene transformations-a review. Front Microbiol. 5:3462014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YJ, Tsai KS, Chan DC, Lan KC, Chen
CF, Yang RS and Liu SH: Honokiol, a low molecular weight natural
product, prevents inflammatory response and cartilage matrix
degradation in human osteoarthritis chondrocytes. J Orthop Res.
32:573–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiang J, Shen YC, Wang YH, Hou YC, Chen
CC, Liao JF, Yu MC, Juan CW and Liou KT: Honokiol protects rats
against eccentric exercise-induced skeletal muscle damage by
inhibiting NF-kappaB induced oxidative stress and inflammation. Eur
J Pharmacol. 610:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang LY, Zhang B, Cui J, Liu YX, Wu CR
and Lin SJ: Sequential therapy for patients with cirrhosis
complicated by common bile duct stones and moderate to severe
gastroesophageal varices. Chin Med J (Engl). 125:4312–4314.
2012.PubMed/NCBI
|
|
15
|
Lo EA, Wilby KJ and Ensom MH: Use of
proton pump inhibitors in the management of gastroesophageal
varices: A systematic review. Ann Pharmacother. 49:207–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harrison-Felix C, Kreider SE,
Arango-Lasprilla JC, Brown AW, Dijkers MP, Hammond FM,
Kolakowsky-Hayner SA, Hirshson C, Whiteneck G and Zasler ND: Life
expectancy following rehabilitation: A NIDRR traumatic brain injury
model systems study. J Head Trauma Rehabil. 27:E69–E80. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zafonte RD, Bagiella E, Ansel BM, Novack
TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Eisenberg H,
Hart T, et al: Effect of citicoline on functional and cognitive
status among patients with traumatic brain injury: Citicoline Brain
Injury Treatment Trial (COBRIT). JAMA. 308:1993–2000. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Li CJ, Wan Y, Smith P, Shang G and
Cui Q: Antioxidative fullerol promotes osteogenesis of human
adipose-derived stem cells. Int J Nanomedicine. 9:4023–4031. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soontornniyomkij V, Choi C, Pomakian J and
Vinters HV: High-definition characterization of cerebral β-amyloid
angiopathy in Alzheimer's disease. Hum Pathol. 41:1601–1608. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roh JH, Huang Y, Bero AW, Kasten T,
Stewart FR, Bateman RJ and Holtzman DM: Disruption of the
sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with
Alzheimer's disease pathology. Sci Transl Med. 4:150ra1222012.doi:
10.1126/scitranslmed.3004291. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying
WH and Tian HL: SIRT2 inhibition exacerbates neuroinflammation and
blood-brain barrier disruption in experimental traumatic brain
injury by enhancing NF-κB p65 acetylation and activation. J
Neurochem. 136:581–593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turkstra LS, Quinn-Padron M, Johnson JE,
Workinger MS and Antoniotti N: In-person versus telehealth
assessment of discourse ability in adults with traumatic brain
injury. J Head Trauma Rehabil. 27:424–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stein TD, Montenigro PH, Alvarez VE, Xia
W, Crary JF, Tripodis Y, Daneshvar DH, Mez J, Solomon T, Meng G, et
al: Beta-amyloid deposition in chronic traumatic encephalopathy.
Acta Neuropathol. 130:21–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalinin S, Gavrilyuk V, Polak PE, Vasser
R, Zhao J, Heneka MT and Feinstein DL: Noradrenaline deficiency in
brain increases beta-amyloid plaque burden in an animal model of
Alzheimer's disease. Neurobiol Aging. 28:1206–1214. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zwan MD, Okamura N, Fodero-Tavoletti MT,
Furumoto S, Masters CL, Rowe CC and Villemagne VL: Voyage au bout
de la nuit: Aβ and tau imaging in dementias. Q J Nucl Med Mol
Imaging. 58:398–412. 2014.PubMed/NCBI
|