Introduction
Acute kidney injury (AKI) is associated with high
mortality and occurs in up to 35% of hospitalized patients
(1). In patients who receive general
surgery, the incidence of AKI has been reported to be ~1%, while it
may occur in up to 70% of critically ill patients, and if AKI is
part of the multiple organ dysfunction syndrome, the in-hospital
mortality is 50% (2,3). AKI is an independent risk factor for
mortality (4), and surviving
patients have an increased risk of developing chronic kidney
disease. AKI comprises multiple clinical conditions and outcomes
are influenced by underlying disease. In critically ill patients,
sepsis is the most common cause of AKI. Sepsis is a severe systemic
inflammatory response triggered by a bacterial, viral or fungal
infection and represents a major cause of shock or mortality
(5). Sepsis-induced AKI is
characterized by a rapid and often profound decline in the kidney's
function to filter blood and eliminate nitrogen waste products,
usually evolving over hours to days after the onset of sepsis
(6).
At present, no therapeutic measures are available to
prevent or treat sepsis-induced AKI due to the limited
understanding of the pathophysiological mechanisms. The involvement
of inflammation, microvascular dysfunction and adaptive responses
of tubular cells in the development of sepsis-induced AKI provides
novel therapeutic and diagnostic avenues. Among the numerous
pathophysiological processes underlying sepsis, inflammation has
prominent roles (7), which results
from the activation of nuclear factor-κB (NF-κB) through
intracellular signaling pathways (8). NF-κB has been demonstrated to mediate
the transcription of a large number of genes, the products of which
are known to have important roles in septic pathophysiology
(9). Mice with deficiency of these
NF-κB-dependent genes are resistant to the development of septic
shock and sepsis-associated mortality (10). Pro-inflammatory cytokines, including
interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) have been
demonstrated to be significantly higher in patients with sepsis
than in those without (7). Numerous
clinical trials have been performed to modulate the excessive
inflammatory response in sepsis-induced AKI cases, but to date, no
immunomodulatory drug has been demonstrated to decrease the
mortality of patients with severe sepsis-induced AKI (11). Therefore, there is a compelling need
to develop novel therapeutic options for patients with
sepsis-induced AKI.
Tenuigenin (TNG) is a major active component of the
Chinese herb Polygala tenuifolia root, also known as ‘Yuan
Zhi’ according to the Chinese Materia Medica, and is used to treat
patients with insomnia, neurosis and dementia (12). TNG has been reported to have various
biological and pharmacological activities, including anti-oxidant,
anti-inflammatory, anti-dementia and anti-aging effects (13,14).
Given that TNG, which is extensively applied in Traditional Chinese
Medicine, inhibits the inflammatory response, which has crucial
roles in the pathophysiological development of sepsis-induced AKI,
the present study sought to investigate the ability of TNG to
reduce it and assessed whether the underlying mechanism involves
the NF-κB pathway.
Materials and methods
Animals
Adult male Kunming (KM) mice, weighing 25–30 g and 6
weeks old, were obtained from the Experimental Animal Center of
Shandong Luye Pharmaceutical Co., Ltd. (Yantai, China). The animals
were kept under a 12-h light/dark cycle at a temperature of 22±2°C
and 55±5% relative humidity. All animal experiments were performed
according to the protocols approved by the Animal Care and Use
Ethics Committee of Laiyang Central Hospital (Yantai, China) and
were in strict accordance with US National Institutes of Health
Guide for the Care and Use of Laboratory Animals (15).
Experimental protocol
A total of 100 KM mice were randomly divided into
five groups: A Sham group, a cecal ligation and puncture (CLP)
model group, a high-dose TNG (H) group, a moderate-dose TNG (M)
group and a low-dose TNG (L) group. Mice underwent CLP to mimic
sepsis-induced acute kidney injury. Immediately following the sham
surgery or CLP, the mice in three TNG treatment groups received TNG
(National Institute for the Control of Pharmaceutical and
Biological Products, Jilin, China) at a single dose of 50, 20 or 5
mg/kg, respectively, by intraperitoneal injection. At 24 h
following CLP or sham surgery, the body weight of all mice was
determined. The blood was collected in heparinized centrifuge tubes
through the abdominal aorta and following collection of blood
samples, a high dose of anesthetic (110 mg/kg thiopental sodium,
i.p.) was administered and the animals were bled to death. Freshly
isolated serum was used for the assessment of renal function. The
two kidneys were harvested and weighed, and were used for
histological sectioning and biochemical assays.
CLP procedure
CLP was performed according to the procedure used by
Rittirsch et al (16). In
brief, following anesthetizing mice by intraperitoneal injection
300 mg/kg chloral hydrate, a midline incision of 2–3 cm in length
was made to expose the cecum and the adjoining intestine.
Subsequently, 50% of the cecum was ligated and perforated between
the ligation and the tip of the cecum in a mesenteric to
anti-mesenteric direction with an 18-gauge needle. Following
extrusion of a small amount of stool through the puncture site, the
cecum was replaced into the abdomen and the incision was closed
using a sterile 4-0 silk suture. Finally, 30 ml/kg of warm sterile
saline was subcutaneously administered for fluid resuscitation.
Mice in the sham group received the same procedure without CLP.
Assessment of renal function
Blood samples were collected from peritoneal veins
and centrifuged at 2,500 × g at 4°C for 10 min. The levels of blood
urea nitrogen (BUN) and serum creatinine (SCr) were assayed with an
automated biochemical analyzer (TBA-40FR; Toshiba, Tokyo,
Japan).
Biochemical assays
Each kidney tissue sample was weighed using an
analytical balance and 100 mg tissue of each sample was homogenized
in 0.01 M PBS buffer (pH 7.2). Following centrifugation of the
homogenate at 12,000 × g for 30 min at 4°C, the supernatant was
collected and quantitatively assayed for TNF-α and IL-6 using tumor
necrosis factor-α (cat. no. H052) and interleukin-6 (cat. no. H007)
ELISA kits (Nanjing Jiancheng Biological Engineering Institute,
Nanjing, China) according to the manufacturer's protocol.
Determination of nitric oxide (NO) and
prostaglandin E2 (PGE2)
The amount of nitrite (a stable metabolite of NO) in
the kidney tissues was detected by the Griess Reagent System
(Beyotime Institute of Biotechnology, Inc., Haimen, China)
according to the manufacturer's protocol. The level of
PGE2 in the kidney tissues was detected using a PGE2
ELISA kit (cat. no. KB3797; Shanghai Ke Min Biotechnology Co., Ltd,
Shanghai, China,) according to the manufacturer's protocol.
Western blot analysis
The kidney samples were homogenised, lysed in
radioimmunoprecipitation assay buffer [50 mmol/l Tris (pH 7.4), 150
mmol/l NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS,
sodium orthovanadate, sodium fluoride, EDTA and leupeptin] with
protease and phosphatase inhibitors, and centrifuged. The resulting
supernatants containing cytoplasmic proteins were assayed using a
bicinchoninic acid protein assay kit to measure the total protein
content (Beyotime Institute of Biotechnology, Inc.). Nuclear
protein was extracted using a Nuclear Protein Extraction kit (cat.
no. AR0106; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
according to the manufacturer's protocol. Following denaturation,
equal amounts of protein (30 µg/lane) were subjected to 10%
SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes, which were washed in Tris-buffered saline with Tween-20
(TBST, 0.05%) and blocked in 5% skimmed milk (Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany) at 4°C overnight. Next, the membranes
were incubated with rabbit anti-IκBα antibody (cat. no. 9242) and
rabbit anti-NF-κB antibody (cat. no. 8242; both Cell Signaling
Technology, Danvers, MA, USA) diluted at 1:1,000 in 5% skimmed milk
overnight at 4°C and subsequently washed three times for 15 min
prior to being incubated with peroxidase-conjugated anti-mouse
(cat. no. ZB-2305; OriGene Technologies, Inc., Beijing, China) or
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no.
ZB-2301; OriGene Technologies, Inc.) diluted at 1:500 at room
temperature for 1 h. The bound antibodies were visualised with an
enhanced chemiluminescence (ECL) western blot detection system
according to the manufacturer's protocol (GE Healthcare, Chalfont,
UK).
Histopathological evaluation
Kidney specimens were collected and fixed in 10%
formalin for 24 h at room temperature and then embedded in
paraffin. Sections 4 µm thick were cut from formalin-fixed tissues
and stained with hematoxylin and eosin according to the
manufacturer's protocol (cat. no. G1120; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). Specimens were then
examined under a light microscope at a magnification of ×200.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using a one-way ANOVA
with a Dunnett's post hoc test as the multiple comparison method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TNG treatment improves the kidney
coefficient in mice with sepsis-induced AKI
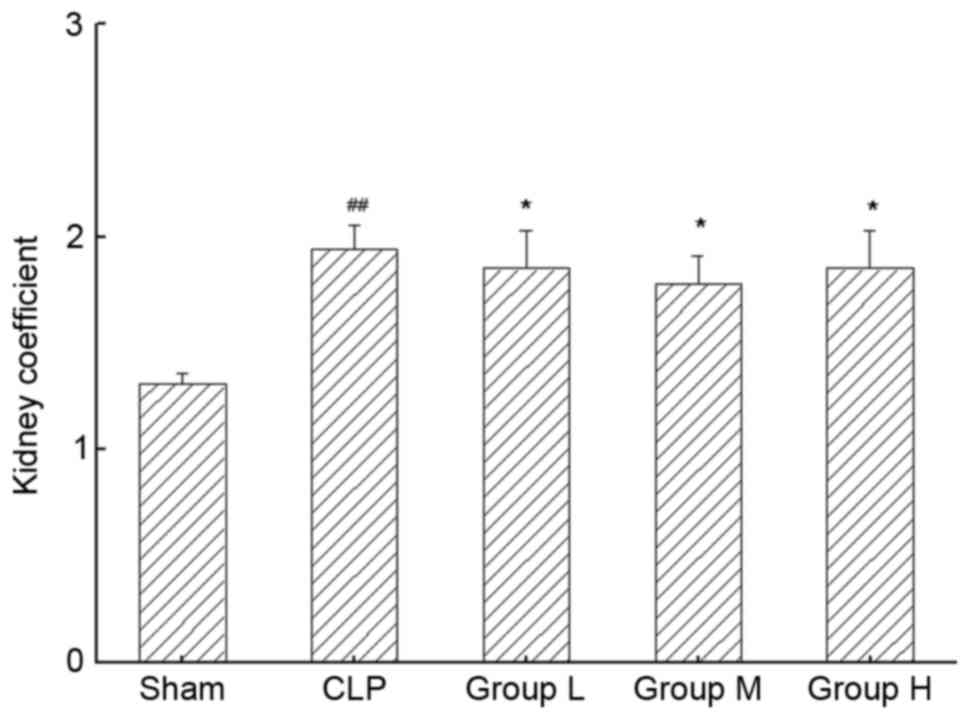
Fig. 1 demonstrated
the effects of TNG treatment on the kidney coefficient in the
different experimental groups. According to the results, the kidney
coefficient in the CLP group was significantly higher than that in
the sham group (P<0.01). TNG treatment caused marked reductions
in the kidney coefficient in the L group compared with that in the
CLP group (P<0.05) and this tendency was also present in the
other two TNG treatment groups (P<0.05).
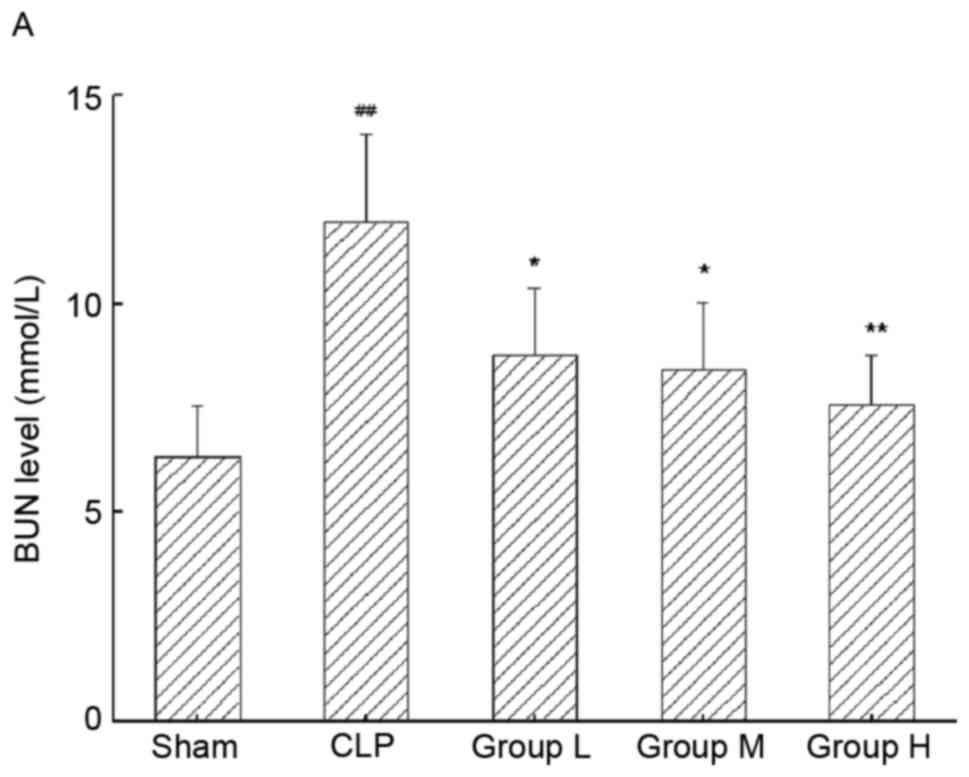
TNG improves kidney function
parameters in mice with sepsis-induced AKI
The BUN and SCr levels in mice with sepsis-induced
AKI were assessed (Fig. 2A and B,
respectively). Following CLP surgery, mice with AKI displayed
significant elevations in the levels of BUN (P<0.01) and SCr
(P<0.01) in renal tissues. Of note, the elevated levels of BUN
and SCr were significantly lowered by the application of TNG
(P<0.05 for each).
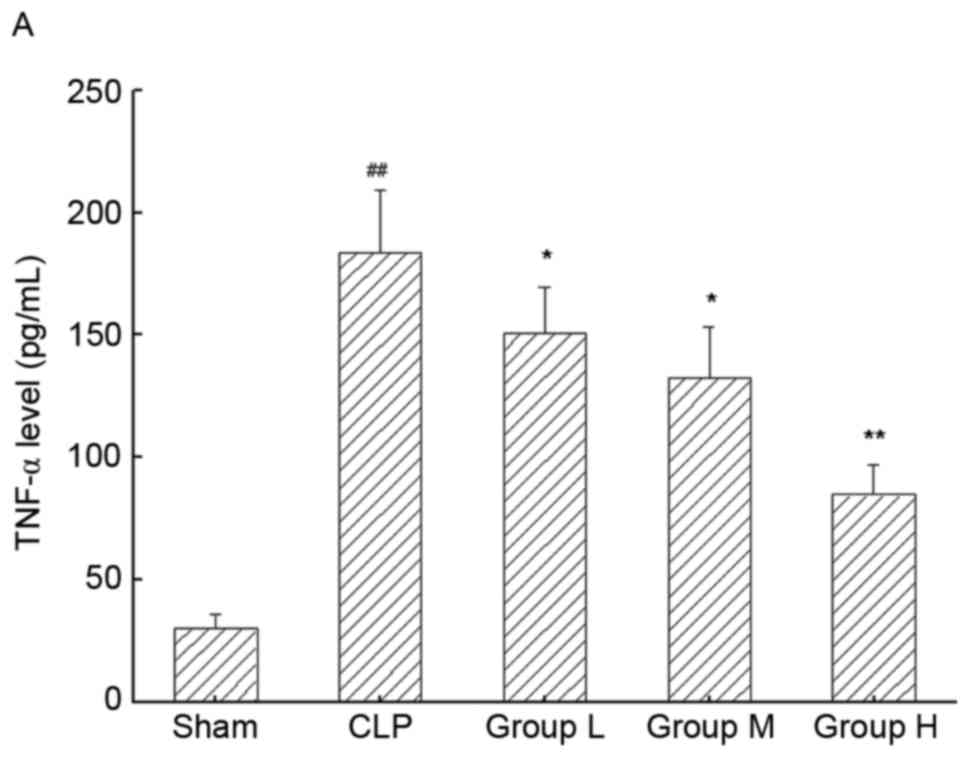
TNG treatment ameliorates inflammatory
response in the kidneys of mice following CLP
To analyze the inflammatory response in the kidneys
of mice, the levels of TNF-α and IL-6 in kidneys were assessed by
ELISA (Fig. 3A and B, respectively).
Compared with the sham group, TNF-α and IL-6 levels were
significantly increased in the CLP group (P<0.01 for each),
which was significantly inhibited by TNG treatment in a
dose-dependent manner (P<0.05 or P<0.01).
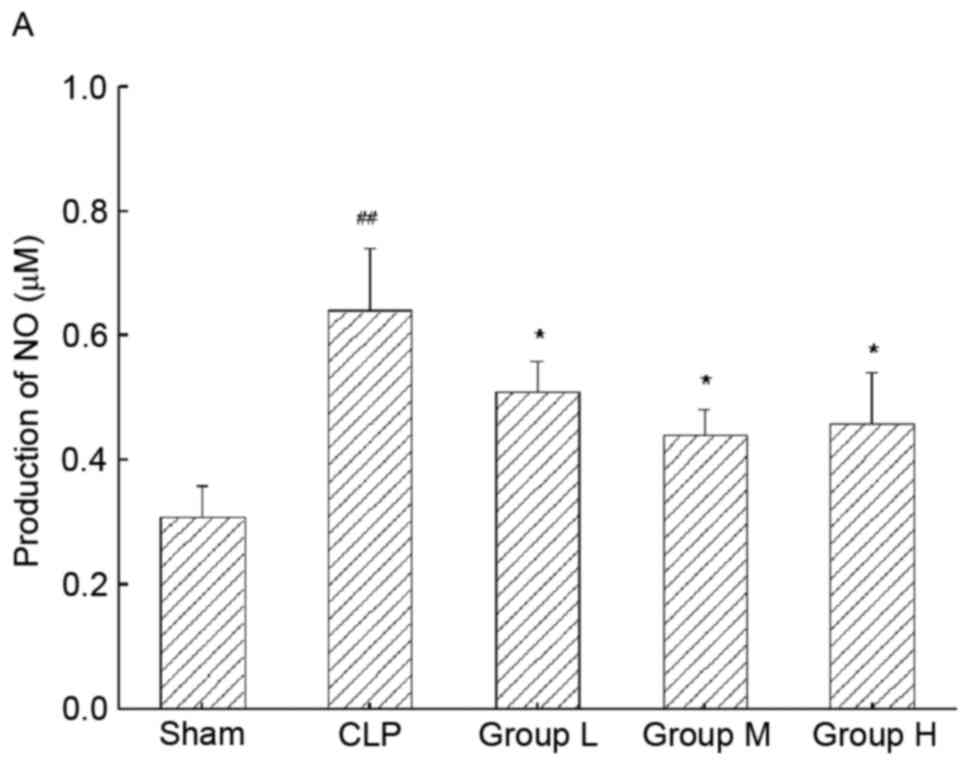
TNG decreases the production of NO and
PGE2 in mice with sepsis-induced AKI
To further demonstrate the anti-inflammatory effect
of TNG, the production of inflammatory mediators and proteins was
detected. As demonstrated in Fig. 4,
sepsis resulted in a significant increase in NO production in
kidney tissue compared with that in the sham group (P<0.01),
whereas TNG significantly inhibited sepsis-induced production of NO
(P<0.05; Fig. 4A). Furthermore,
TNG significantly inhibited sepsis-induced production of
PGE2 (P<0.05; Fig.
4B).
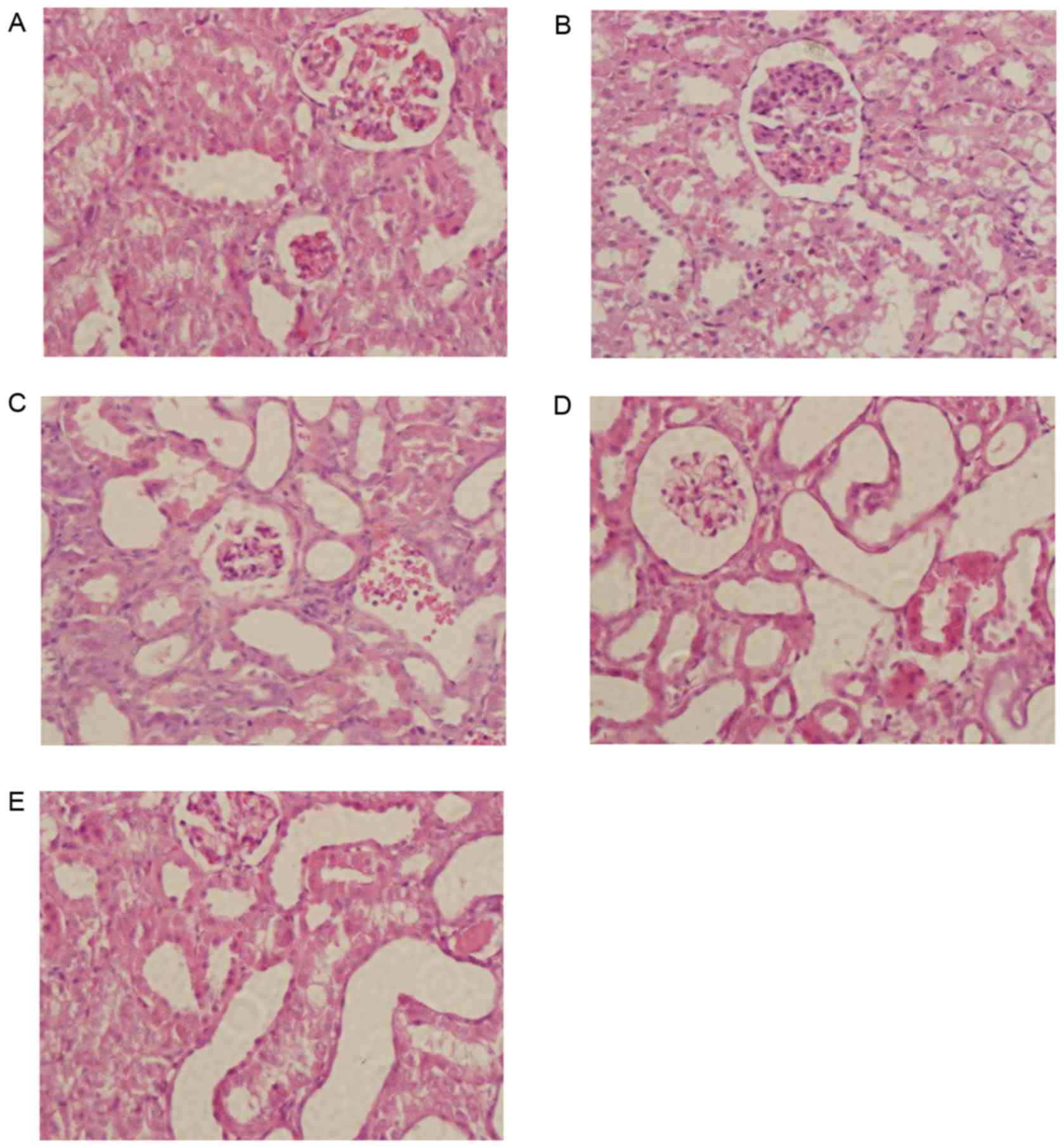
TNG attenuates histological changes in
the kidneys of mice after CLP
Histological analysis of hematoxylin and
eosin-stained kidney samples demonstrated a marked thickening of
the glomerular basement membrane and an obvious expansion of
mesangium in the CLP group compared with the sham group (Fig. 5A and B). Of note, these
histopathological alterations in the kidney were improved in the
TNG-treatment groups (Fig.
5C-E).
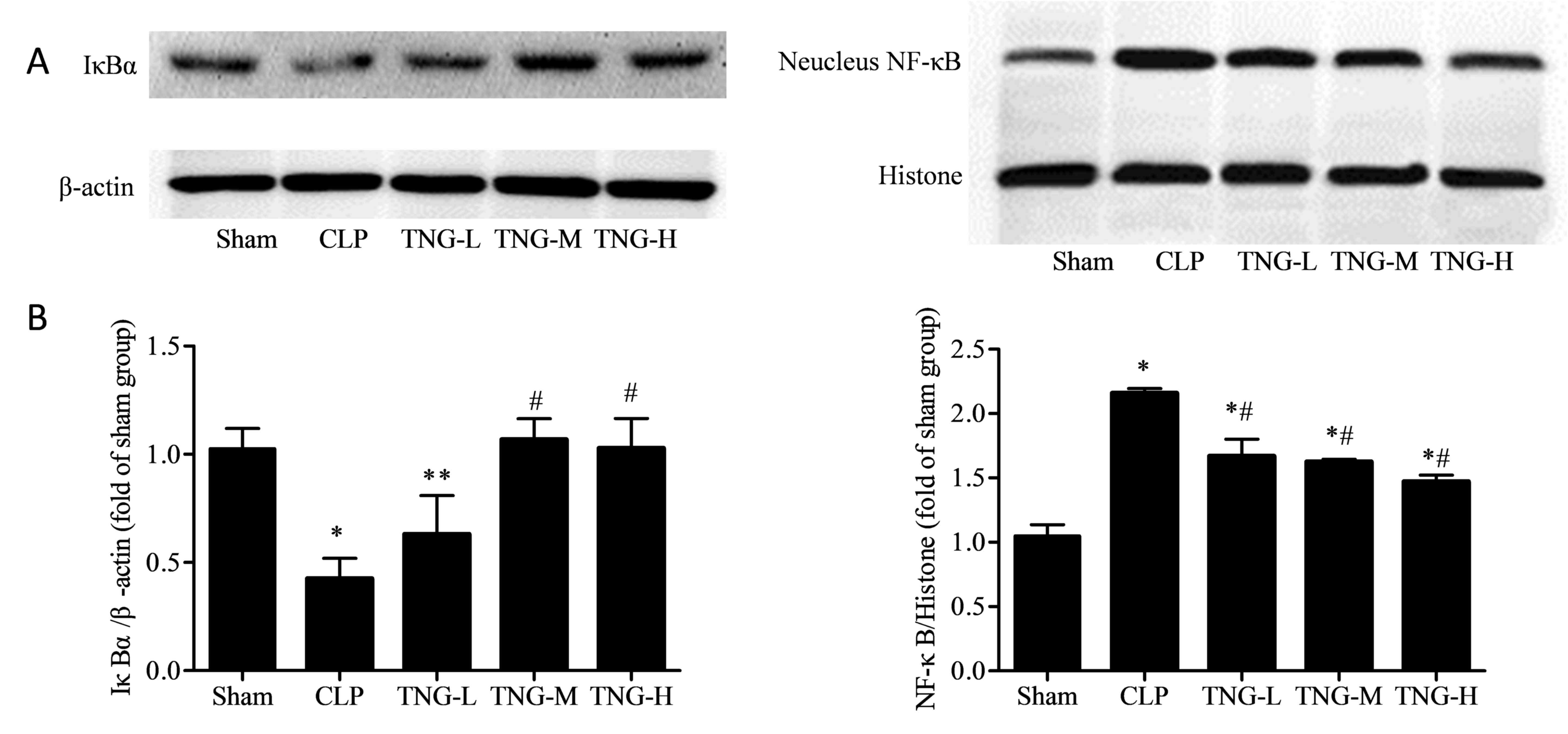
TNG inhibits the NF-κB signaling
pathway in the kidneys of mice induced by AKI
To further evaluate the mechanisms underlying the
anti-inflammatory effect of TNG, the level of nuclear NF-κB and
that of its inhibitor IκBα was assessed (Fig. 6). Western blot analysis demonstrated
that the levels of NF-κB in the nuclei of kidney cells from mice in
the CLP group were significantly increased compared with those in
the sham group (P<0.05), while the expression of IκBα was
decreased (P<0.01). While middle and high dose of TNG treatment
significantly increased IκBα expression compared to that in the CLP
group. IκBα expression in the H group was slightly decreased
compared with that of the M group, however, the difference was not
statistically significant. Following treatment with different doses
of TNG, although the NF-κB levels in the three TNG treatment groups
remained higher than that in the sham group (P<0.01), but were
significantly decreased when compared with the CLP group
(P<0.01).
Discussion
Inflammation is an essential component of the innate
immune response that protects against tissue damage. However, when
unbalanced, inflammation causes tissue damage (17). AKI is thought to be a result of
uncontrolled renal inflammation and has a high mortality rate.
Traditional Chinese medicines have been used for the prevention and
treatment of various diseases in China for several thousands of
years. TNG is an active component of a traditional Chinese
medicinal herb that has been reported to have anti-oxidant and
anti-inflammatory properties (13,14). The
present study examined whether the protective effect of TNG on AKI
induced by CLP is due to its anti-inflammatory properties.
The present study demonstrated that treatment with
TNG in mice with sepsis-induced AKI alleviated the pathological
damage of kidney tissue, attenuated renal dysfunction and inhibited
the release of inflammatory cytokines and mediators, indicating a
profound impact on the severity of AKI. To shed light on the
underlying mechanisms, the present study focused on the NF-κB
pathway. The results demonstrated that TNG significantly inhibited
the activation of NF-κB the in kidneys of mice challenged by CLP.
These results indicated that TNG exerts its anti-inflammatory
effects at least in part through inhibition of the activation of
the NF-κB signaling pathway.
The present study established a mouse model of
sepsis-induced AKI, which is one of the classical methods for
experimental studies on sepsis and also the animal model, which is
the most representative of the pathophysiological processes of
sepsis. The kidney is the most sensitive organ in sepsis. A
progressive decline in the glomerular filtration rate, reflecting
SCr and BUN levels, is the most common characteristic in the
development of AKI, which causes proteinuria, leading to
histological damage in the kidney. Following CLP surgery, mice with
AKI exhibited significant increases in SCr and BUN levels and
pathological abnormalities were observed, representing a decline in
renal function. However, TNG treatment positively affected these
parameters of renal function and effectively restrained the
pathological changes, indicating a renoprotective effect of TNG in
sepsis-induced AKI.
There is a strong association between inflammatory
cytokine levels and the development of sepsis-induced AKI (18,19).
Therefore, the upregulation of inflammatory cytokines, including
TNF-α and IL-6 is considered a reliable marker of sepsis. The
experimental data of the presents study suggested that the
production of TNF-α and IL-6 was significantly induced by AKI, and
was suppressed by treatment with TNG. Based on the above outcome,
it was further demonstrated that TNG had a protective effect on
mice with sepsis-induced AKI.
NO is derived from the oxidation of L-arginine,
which is catalyzed by nitric oxide synthase (NOS). In sepsis, the
expression of inducible (i)NOS induced by inflammatory mediators
and cytokines is significantly increased in immunocytes, such as
neutrophils and macrophages (20).
In the present study, the production of NO was significantly
induced by AKI, which was suppressed by treatment with TNG.
PGE2 is an important inflammatory mediator and has a
significant role in the inflammatory response (21,22). In
the present study, the production of PGE2 was significantly induced
by AKI, which was suppressed by treatment with TNG.
The NF-kB pathway involves an important family of
transcription factors that control the expression of cytokines,
cell adhesion molecules, growth factors and also apoptosis, cell
proliferation, differentiation and survival (23,24).
Studies have demonstrated the activation of NF-κB in the kidneys
during AKI (25–27). The principal pro-inflammatory
mediators in the pathophysiology of sepsis are TNF-α and IL-1β,
which activate NF-κB by triggering a signaling pathway that leads
to the phosphorylation and consequent degradation of the inhibitor
of NF-κB α (IκBα) (28,29). The degradation of IκBα generates a
nuclear localization signal for the NF-κB protein, which then
migrates into the nucleus and stimulates the transcription of
specific genes. The overproduction of pro-inflammatory mediators
enhances adhesion molecules and also leads to deleterious effects
associated with multiple organ failure and shock (30). iNOS and cyclooxygenase (COX)-2 are
two important proteins downstream of the NF-κB signaling pathway
and NF-κB specifically binds to the promoter region of iNOS and
COX-2, which promotes their transcription and ultimately
facilitates the production of NO and PGE2. The present
study demonstrated that following CLP, accumulation of NF-κB was
increased, accompanied with downregulation of the expression of
IκBα. As expected, following treatment with TNG, the NF-κB
signaling pathway was inhibited.
In conclusion, the results of the present study
indicated that TNG significantly ameliorates kidney tissue damage,
improves renal function, and inhibits the release of inflammatory
cytokines and mediators, suggesting that TNG has a protective
effect on AKI caused by sepsis. This renoprotection of TNG may be
due to its anti-inflammatory effects. The present study also
provided evidence that the mechanism of action may involve the
inhibition of the NF-κB signaling pathway. These results indicated
that TNG is a promising candidate for a novel adjuvant therapeutic
strategy for sepsis-induced AKI and that the NF-κB signaling
pathway may be a potential target.
References
|
1
|
Lauer S, Fischer LG, Van Aken HK, Nofer JR
and Freise H: Gadolinium chloride modulates bradykinin-induced
pulmonary vasoconstriction and hypoxic pulmonary vasoconstriction
during polymicrobial abdominal sepsis in rats. Exp Lung Res.
41:270–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bagshaw SM, Uchino S, Bellomo R, Morimatsu
H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, et al:
Septic acute kidney injury in critically ill patients: Clinical
characteristics and outcomes. Clin J Am Soc Nephrol. 2:431–439.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mårtensson J and Bellomo R: Sepsis-induced
acute kidney injury. Crit Care Clin. 31:649–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zarjou A and Agarwal A: Sepsis and acute
kidney injury. J Am Soc Nephrol. 22:999–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singbartl K and Kellum JA: AKI in the ICU:
definition, epidemiology, risk stratification, and outcomes. Kidney
Int. 81:819–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schrier RW and Wang W: Acute renal failure
and sepsis. N Engl J Med. 351:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kellum JA, Kong L, Fink MP, Weissfeld LA,
Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL and Angus DC:
GenIMS Investigators: Understanding the inflammatory cytokine
response in pneumonia and sepsis: Results of the Genetic and
Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med.
167:1655–1663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsujimoto H, Ono S, Efron PA, Scumpia PO,
Moldawer LL and Mochizuki H: Role of Toll-like receptors in the
development of sepsis. Shock. 29:315–321. 2008.PubMed/NCBI
|
|
9
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SF and Malik AB: NF-κB activation as a
pathological mechanism of septic shock and inflammation. Am J
Physiol Lung Cell Mol Physiol. 290:L622–L645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christaki E, Anyfanti P and Opal SM:
Immunomodulatory therapy for sepsis: an update. Expert Rev Anti
Infect Ther. 9:1013–1033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park CH, Choi SH, Koo JW, Seo JH, Kim HS,
Jeong SJ and Suh YH: Novel cognitive improving and neuroprotective
activities of Polygala tenuifolia Willdenow extract, BT-11. J
Neurosci Res. 70:484–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan HL, Li B, Xu J, Wang Y, He Y, Zheng Y
and Wang XM: Tenuigenin protects dopaminergic neurons from
inflammation mediated damage induced by the lipopolysaccharide. CNS
Neurosci Ther. 18:584–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Han T, Zhang L, Yu CH, Wan DG,
Rahman K, Qin LP and Peng C: Effects of tenuifolin extracted from
radix polygalae on learning and memory: A behavioral and
biochemical study on aged and amnesic mice. Phytomedicine.
15:587–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011, PubMed/NCBI
|
|
16
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serhan CN, Chiang N and Van Dyke TE:
Resolving inflammation: Dual anti-inflammatory and pro-resolution
lipid mediators. Nat Rev Immunol. 8:349–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murugan R, Karajala-Subramanyam V, Lee M,
Yende S, Kong L, Carter M, Angus DC and Kellum JA: Acute kidney
injury in non-severe pneumonia is associated with an increased
immune response and lower survival. Kidney Int. 77:527–535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Payen D, Lukaszewicz AC, Legrand M, Gayat
E, Faivre V, Megarbane B, Azoulay E, Fieux F, Charron D, et al: A
multicentre study of acute kidney injury in severe sepsis and
septic shock: Association with inflammatory phenotype and HLA
genotype. PLoS One. 7:e358382012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heemskerk S, Masereeuw R, Russel FG and
Pickkers P: Selective iNOS inhibition for the treatment of
sepsis-induced acute kidney injury. Nat Rev Nephrol. 5:629–640.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ricciotti E and FitzGerald GA:
Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Correa M, Machado J Jr, Carneiro CR,
Pesquero JB, Bader M, Travassos LR, Chammas R and Jasiulionis MG:
Transient inflammatory response induced by apoptotic cells is an
important mediator of melanoma cell engraftment and growth. Int J
Cancer. 114:356–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perkins ND: Integrating cell-signalling
pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden MS and Ghosh S: Shared principles
in NF-κB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basak S and Hoffmann A: Crosstalk via the
NF-kappaB signaling system. Cytokine Growth Factor Rev. 19:187–197.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benedetti G, Fokkelman M, Yan K,
Fredriksson L, Herpers B, Meerman J, van de Water B and de Graauw
M: The Nuclear Factor κB Family Member RelB Facilitates Apoptosis
of Renal Epithelial Cells Caused by Cisplatin/Tumor Necrosis Factor
α Synergy by Suppressing an Epithelial to Mesenchymal
Transition-Like Phenotypic Switch. Mol Pharmacol. 84:128–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Lamki RS, Lu W, Finlay S, Twohig JP,
Wang EC, Tolkovsky AM and Bradley JR: DR3 signaling protects
against cisplatin nephrotoxicity mediated by tumor necrosis factor.
Am J Pathol. 180:1454–1464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nozaki Y, Nikolic-Paterson DJ, Yagita H,
Akiba H, Holdsworth SR and Kitching AR: Tim-1 promotes cisplatin
nephrotoxicity. Am J Physiol Renal Physiol. 301:F1098–F1104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaffey N, Alberts B, Johnson A, Lewis J,
Raff M, Roberts K and Walter P: Molecular biology of the cell. Ann
Bot. 91:4012003. View Article : Google Scholar
|
|
30
|
Thijs A and Thijs LG: Pathogenesis of
renal failure in sepsis. Kidney Int Suppl. 66 Suppl:S34–S37.
1998.PubMed/NCBI
|