Introduction
Tuberculosis (TB) is harmful to human beings. It has
strong infectivity but was almost eradicated 20 years ago due to
the use of anti-TB drugs (1).
However, in the last decade it has prevailed in a recurrent state
due to resistant strain variation. Furthermore, the incidence of TB
has increased worldwide (1) and the
number of patients with laryngeal TB has also increased accordingly
(1). In the past, laryngeal
tuberculosis was almost always associated with advanced pulmonary
infections, but in recent decades, patients who have negative
findings on radiological examinations of the chest, negative sputum
cultures, and negative history have been reported (2). Widespread use of anti-TB agents over a
prolonged period of time has led to a change in the characteristics
of the signs and symptoms of laryngeal TB. For example, severe sore
throat and painful swallowing similar to laryngitis have been
observed, without cough and light fever; therefore, misdiagnosis is
commonly observed (1–3). Furthermore, as the disease has a potent
infectivity, the United States Centers for Disease Control and
Prevention have stressed the importance of acknowledging the
changes in clinical characteristics, diagnostic points, early
diagnosis and timely treatment of the disease (3). For the above reasons, early diagnosis
and treatment are very important. Nowadays, laryngoscopy is a
primary and effective method for detecting laryngeal TB (4). The present retrospective study analyzed
the data of 61 patients at Shanghai General Hospital (Shanghai,
China) who had suffered from laryngeal TB for ~14 years, to provide
a means of early diagnosis to clinical workers.
Patients and methods
Patients
A total of 61 patients who were admitted to Shanghai
General Hospital (male, n=23; female, n=38; age, 22–79 years; mean
age, 47.7 years) and underwent laryngoscopy examination between
January 1998 and December 2012 were included in the present study.
Ethical approval was received from the Ethics Committee of Shanghai
General Hospital (Shanghai, China) and informed written consent was
obtained from all patients in the present study. The time interval
between the onset of symptoms and laryngoscopy examination ranged
between 1 and 17 months (mean, 7.4 months). The 61 cases included
26 cases of acute or chronic laryngitis (first diagnosis), 18 cases
of vocal polyps or throat tumors (first diagnosis), 8 cases of
epiglottitis (first diagnosis), 8 cases of laryngeal lesions and 1
case of laryngeal keratinization disorder. Excluding the 8 cases of
laryngeal lesions, the misdiagnosis rate was 86.8%. The local and
systemic signs and symptoms were recorded for all patients, and the
condition of the throat was observed using a rigid laryngoscope
(8706 CJ; Kal Storz GmbH & Co. KG., Tuttlingen, Germany) or a
fiber laryngoscope (ENF TYPE T3; Olympus Corp., Tokyo, Japan) when
the patient's tongue was too large. All cases under the fiber
laryngoscope, rigid laryngoscope (or support laryngoscope if the
former two methods failed) underwent biopsy, chest X-ray, and
tuberculin purified protein derivative (PPD) skin test. The PPD
test was considered to have a positive result if skin showed red
spots with a diameter >5 mm after 0.0002 PPD was injected into
the skin (2). A total of 36 patients
underwent sputum smear tests for acid-fast bacilli examination; 0.1
ml sputum was smeared to 20×25 mm and stained according to the
Ziehl-Neelsen method (3). A total of
30 patients underwent pathological examination to identify TB
bacterium DNA (via polymerase chain reaction).
Diagnosis criteria
In addition to the laryngoscope results, local
lesions were required to satisfy one of the following criteria: i)
Pathological section was positive for the acid-fast stain; ii)
pathological section exhibited caseous necrosis foci; iii) positive
PPD skin test; iv) presence of Mycobacterium tuberculosis in
the sputum smear and culture; or v) diagnostic treatment was
effective. Diagnostic treatment consisted of short-term trial
anti-TB medications (rifampin, 600 mg daily for 2–3 weeks,
isoniazid, 450 mg daily for 2–3 weeks). Any changes in the
patient's condition, such as less edema or fewer ulcers in the
laryngeal lesions, were considered to potentially indicate
laryngeal TB. Following confirmation, all patients were transferred
to the Shanghai Tuberculosis Hospital (Shanghai, China) for regular
chemotherapy treatment.
Results
Symptoms and history
Patients presented with local symptoms, which
included hoarse voice problems and aphonia in 57/61 cases (93.4%);
sore throat and painful swallowing in 49/61 cases (80.3%), both
hoarse voice problems/aphonia and sore throat/painful swallowing in
42/61 cases (68.9%); cough and sputum production in 20/61 cases
(32.8%); and dysphagia in 9/61 cases (14.8%). Systemic symptoms
included mild and severe fever, weakness, weight loss, a maximum
temperature of 39.5°C in 2/61 cases (mean, 37.9°C) and a minimum of
37.4°C in 16 cases (26.2%). A total of 4 patients had a history of
TB, 2 of whom had been cured 20 years prior, 1 was cured 25 years
prior and 1 case was cured 50 years prior. Of the 61 patients, 41
cases had no history of TB nor any clinical manifestations to
indicate primary laryngeal TB.
Of the 61 cases, 6 had enlarged lymph nodes in the
neck, of which 4 cases were on the left-hand side and 2 cases on
the right-hand side with hard texture and distinct margins.
Furthermore, there were 2 cases of large activity of lymph nodes,
and 1 case of restricted movement of lymph nodes. A maximum
diameter of the lymph nodes was 2.5 cm and a minimum was 0.3 cm,
and tenderness was noted in all 6 cases.
Laryngoscopy examination
A total of 25 cases exhibited 2 or more pathological
changes, including 8 cases involving 3 parts and 2 cases involving
the whole laryngeal cavity. In 36 cases, only one area was
affected: Vestibular folds were associated with 4 cases, 20 cases
were in the vocal cords, 8 cases were in the aryepiglottic folds
and 4 cases were in the epiglottis. The 3 types of lesions were
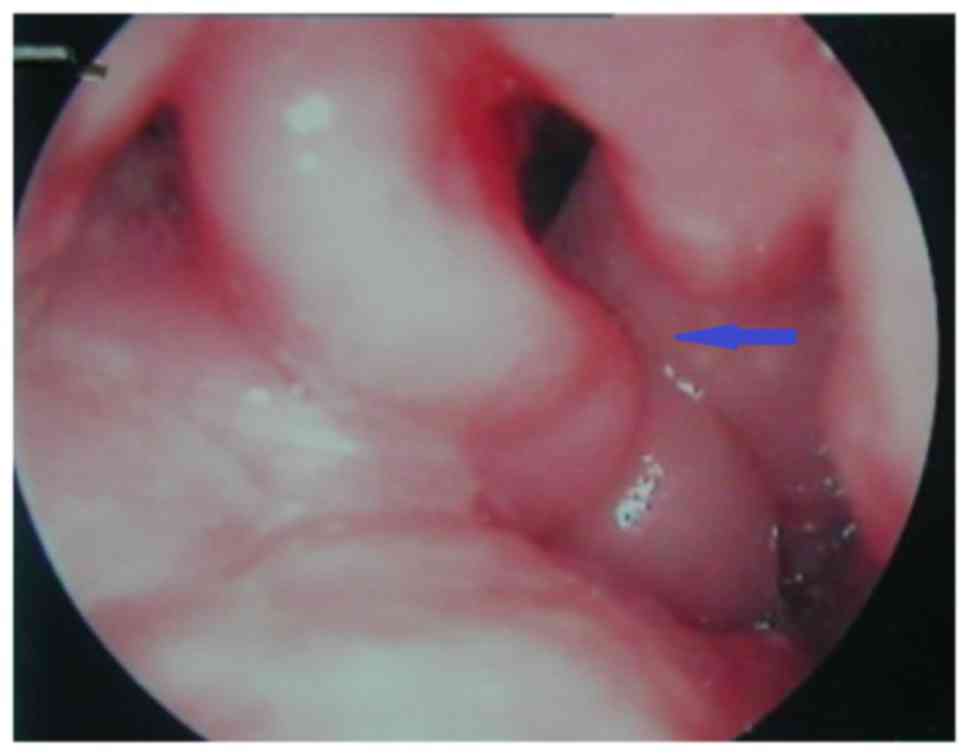
summarized according to their form: Pale edema (24 cases), where
patients exhibited pale edema tissue and spreading of millet white
dots, as shown in Fig. 1;
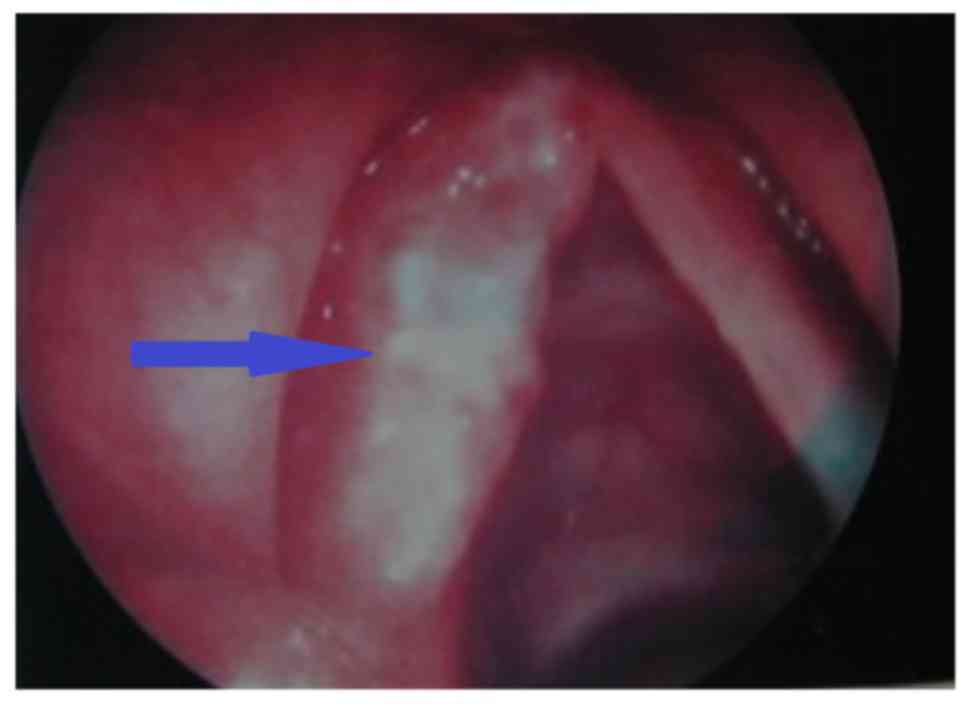
hyperplasia (34 cases), indicated by a white appearance and
polyp-like indications or granuloma vegetation, as shown in
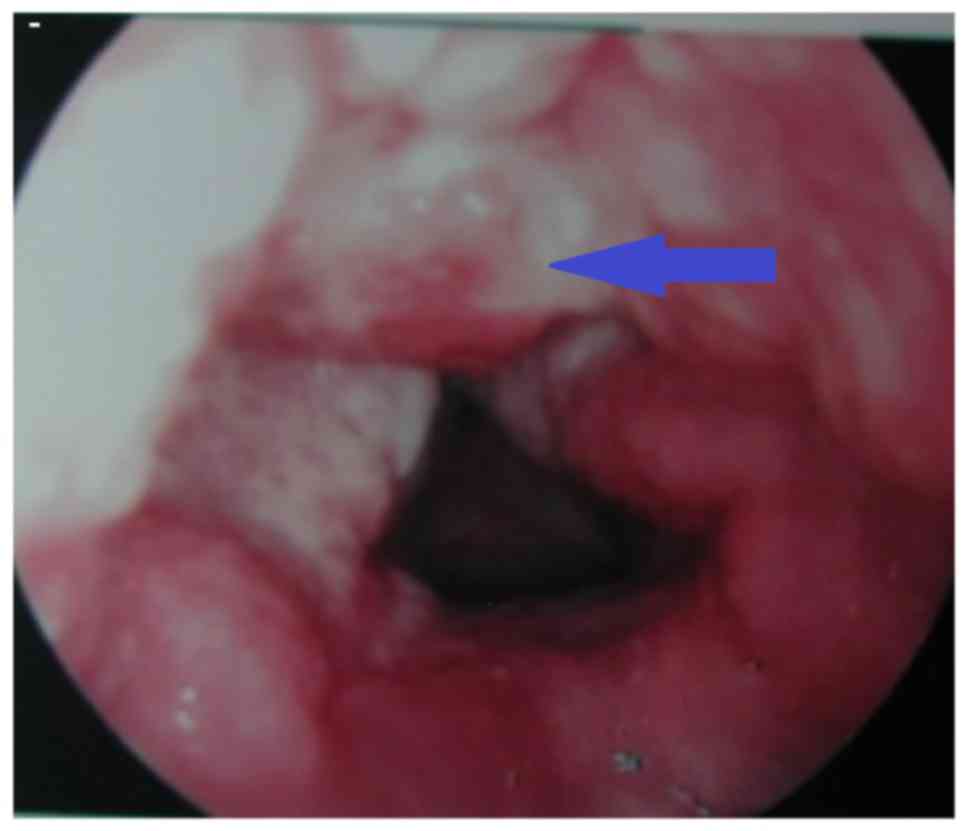
Fig. 2; and ulcer type (3 cases),
defined by the appearance of mucosa ulcer and erosion seepage, as
shown in Fig. 3.
X-ray examination
Of the 61 patients, 40 patients were negative for
laryngeal TB according to X-ray results, 11 cases were diagnosed
with active TB (infiltrating type in 9 cases and blood seeding type
in 2 cases), with a positive rate of 18.0%. A total of 4 cases were
diagnosed with bronchiectasis, 4 cases with pleural effusion and 2
cases with double lung interstitial lesions.
Laboratory and pathology analysis
Of the 61 patients, PPD skin test results were
positive in 53 cases (1:2,000), with a positive rate of 86.9%. A
total of 30 patients were examined for pathological TB bacterium
DNA and 18 cases were positive, with a positive rate of 60.0%. Of
the 36 patients who underwent sputum smear examination for
acid-fast bacilli, 23 cases were positive, with a positive rate of
63.9%. All patients underwent routine biopsy and pathological
examination, and 43 cases were confirmed once, 12 cases were
diagnosed twice and 6 patients were confirmed by biopsy and
pathology >2 times. One patient underwent biopsies 4 times prior
to diagnosis. The majority of pathological changes observed
included squamous cell dysplasia, interstitial phagocytes and giant
cell reaction, the presence of epithelioid cells, Langhans cell
hyperplasia and granuloma, and the presence of necrotic tissue; 26
cases were confirmed by the presence of typical caseous necrosis
foci under the microscope.
Discussion
Laryngeal extrapulmonary TB is the most prevalent
laryngeal granulomatous disease (1).
Widespread use of anti-TB agents over a prolonged time period has
led to a change in the common signs and symptoms of laryngeal TB
(2). Early laryngeal lesions occur
at the posterior larynx, and have been indicated to appear at the
anterior larynx, including the vocal cord, vestibular fold and
epiglottis (4–6). In the present study, 28/36 cases had
single-area involvement at the anterior larynx (77.8%), and only 8
cases involved the posterior larynx, particularly at the
aryepiglottic folds (22.2%). This is similar to previous results
(5,6). Previous findings have indicated that,
laryngeal TB was typically caused by bacterial infection from TB
sputum and the patient being bedridden for a long duration, which
led to the infective sputum bacteria remaining at the posterior
larynx; however, it has more recently been determined that
laryngeal TB spreads predominantly by blood and lymphatic fluid,
indicating a notable change in TB manifestation (5).
Previously, laryngeal TB had typically been
considered secondary to severe TB; therefore, weight loss and mild
laryngeal symptoms occurred (3).
Patients with severe TB typically exhibit severe symptoms,
including low-grade fever, cough and expectoration, weakness,
weight loss, rare hoarseness, and sore throat (5–8).
Currently, hoarseness and sore throat are the most common symptoms
of laryngeal TB, and systemic symptoms and signs are rarely
observed or even absent (3). Of the
61 patients in the present study, 41 cases had no history of TB or
presented TB clinical manifestations, and were considered to have
primary laryngeal TB. Furthermore, 2 cases had a history of TB but
exhibited no signs of recurrence of TB and only 11 laryngeal TB
cases in 61 patients (18%) were considered secondary to active
pulmonary TB according to the X-ray and chest scan. Similarly, this
rate has been observed in various studies (5,6). It is
clear now that laryngeal TB may occur independently of pulmonary TB
and its local symptoms, including hoarseness, sore throat,
dysphagia, serious, and systemic symptoms, which include fever,
night sweats, weakness and weight loss, may be absent. Previous
findings have suggested that the inoculation, widespread use of
long-term anti-TB agents, and the variation of Mycobacterium
tuberculosis L-type bacteria have contributed to the change in
clinical characteristics between laryngeal and pulmonary TB stated
above, which has resulted in the increase in misdiagnosis and
missed diagnosis (6).
Accurate diagnosis of laryngeal TB is dependent on
the patient history, physical examination, chest X-ray, sputum and
PPD examinations, and biopsy results (2,3).
Laryngeal TB is highly contagious and therefore its early diagnosis
is critical (2). Most modern
laryngeal TB cases are not secondary to pulmonary TB. Furthermore,
the clinical characteristics of TB are not obvious, whereas the
local laryngeal symptoms are more noticeable (4). Throat symptoms of laryngeal TB are
easily confused with those of other illnesses, including laryngitis
(4,5). Therefore, missed diagnosis and
misdiagnosis are prone to occur (1,2). In the
present study, 53 of 61 patients were diagnosed with other
laryngeal disorders, such as laryngitis, vocal polyps or throat
tumors, epiglottitis or laryngeal keratinization. In general,
attention should be paid to several diagnostic methods. Initially,
the indication of any history of pulmonary TB (if patients exhibit
laryngeal symptoms with pulmonary TB) should be considered as
potential laryngeal TB cases. In addition, laryngeal lesions should
be carefully examined by all types of laryngoscopes, and if any one
of three types of lesions are observed, a biopsy should be
performed immediately. Furthermore, sputum examinations must be
evaluated, including a direct smear, which is simple and quick,
with a low positive rate (3,5), and sputum culture, which is typically
used and is a longer process but with a high positive rate
(2). Notably, laboratory
examinations should be applied, including the PPD test, where a
positive result strongly suggests TB bacterium infection; however,
this test does not indicate the site of infection (1). Patients who received the PPD test had a
positive rate of 87.9%. In addition, novel technology has been
constructed to detect TB bacterium, including TB bacterium DNA
probe techniques; however, the false-positive and false-negative
rates of these techniques are high and clinical application is
limited to a certain extent (5,6). The
final diagnosis of laryngeal TB is confirmed by histopathological
examination and detection of Mycobacterium tuberculosis.
Slices showing epithelioid cells, macrophages, hyperplasia of
Langhans cells and caseous necrosis are key characteristics
(3,7). If an acid-fast stain is positive,
laryngeal TB may be confirmed; however, the positive rate of
acid-fast stain is considered too low to be reliable alone
(3,8). In some cases, the characteristics of
pathological images are not clear or the acid-fast stain may be
negative, the PPD, PCR tests and fast erythrocyte sedimentation
rate may provide a strong positive result (6). Therefore, the use of these multiple
techniques may provide a clear diagnosis of laryngeal TB.
Diagnostic treatment is also a type of diagnostic method. For some
patients for whom diagnosis cannot be made through repeated
biopsies, short-term trial anti-TB medications (rifampin and
isoniazid) may be used and any changes such as less edema or fewer
ulcers to the laryngeal lesions may indicate laryngeal TB.
During the 9 years between January 1998 and December
2006, the present authors analyzed 33 cases of laryngeal
tuberculosis and the findings were published in the Journal of
Chinese Journal of Clinical Otorhinolaryngology, Head, and Neck
Surgery (4). Subsequently, cases
were collected until December 2012, and a further 28 cases were
detected. Findings in the authors' laryngoscopy room following this
6-year period were similar with what was initially detected in the
first 9 years. In addition, the first 9 years of laryngeal
inspection methods were the same as those used in the following 6
years. Although various methods are available for detecting
laryngeal TB, there are no testing methods that exhibit effective
specificity. Following a total of 15 years of continuous research,
the present findings indicate that detection of laryngeal TB relies
still on rigid laryngoscope and fiber laryngoscopy methods combined
with patient history, PPD, sputum bacteria examinations,
pathological biopsies and acid-fast bacilli examinations for the
final diagnosis. Furthermore, it should be noted that the detection
rate is also associated with the diligent observation and
experience of medical staff.
This study used long-term laryngoscope experience to
observe the modern laryngeal tuberculosis under laryngoscope in
three forms (edema, hyperplasia and ulcer exudation types). It laid
a solid technical foundation for the early diagnosis and treatment
of laryngeal tuberculosis. But due to limitations on the
conditions, only pathological reports without images could be
obtained. This affected the data integrity, and remains to be
improved in future research.
References
|
1
|
Benwill JL and Sarria JC: Laryngeal
tuberculosis in the United States of America: A forgotten disease.
Scand J Infect Dis. 46:241–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizzo PB, Da Mosto MC, Clari M, Scotton
PG, Vaglia A and Marchiori C: Laryngeal tuberculosis: An
often-forgotten diagnosis. Int J Infect. 7:129–131. 2003.
View Article : Google Scholar
|
|
3
|
Rieder HL: The infectiousness of laryngeal
tuberculosis: Appropriate public health action based on false
premises. Int J Tuberc Lung Dis. 13:4–5. 2009.PubMed/NCBI
|
|
4
|
Zhao NJ, Sun YJ and Sun ZF: Clinical
analysis of the diagnosis of laryngeal tuberculosis. Lin Chung Er
Bi Yan Hou Tou Jing Wai Ke Za Zhi. 23:261–263. 2009.(In Chinese).
PubMed/NCBI
|
|
5
|
Shin JE, Nam SY, Yoo SJ and Kim SY:
Changing trends in clinical manifestations of laryngeal
tuberculosis. Laryngoscope. 110:1950–1953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling L, Zhou SH and Wang SQ: Changing
trends in the clinical features of laryngeal tuberculosis: A report
of 19 cases. Int J Infect Dis. 14:e230–e235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yencha MW, Linfesty R and Blackman A:
Laryngeal tuberculosis. Am J Otolaryngol. 21:122–126. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HE: Mycobacterium tuberculosis L
form. Zhonghua Jie He He Hu Xi Za Zhi. 25:579–580. 2002.(In
Chinese).
|