Introduction
Cerebral dural arteriovenous fistulas (DAVFs) are
aberrant vascular communications between dural sinuses and
meningeal arteries and account for 10–15% of all cerebral
arteriovenous diseases (1–3). Although its etiopathogenesis remains
unknown, DAVF is considered to be an acquired disease (4,5) and a
number of etiological factors, including brain trauma (4,6,7) and neurosurgery (8,9) may be
responsible for its development. These lesions are important causes
of hemorrhagic stroke, the degree of which is closely associated
with the development of DAVF and venous drainage. The primary
symptoms and prognosis of DAVF vary (10–12). Due
to the complexity of the vessel architecture the therapies
currently available to treat DAVF, including surgery and
endovascular intervention, are insufficient. Radiotherapy is a
novel treatment that may have a significant effect, however the
prognosis of patients depends on the lesion size and cortical
venous drainage (13). It has been
demonstrated that chronic local poor perfusion may be a key factor
in stimulating angiogenesis of the endocranium, which results in
the formation of DAVFs (14).
Patients with DAVF often exhibit symptoms associated with venous
hypertension (VH), which may be important in the development and
prognosis of DAVFs (15). However,
it remains unclear how important these abnormal changes in
hemodynamics are to the genesis or outcomes of these lesions.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is an important transcriptional factor for cellular responses to a
variety of harmful stresses. It is part of the Kelch-like
ECH-associated protein 1-Nrf2-antioxidant response element
(Keap1-Nrf2-ARE) signaling pathway and regulates numerous genes
associated with ARE, including heme oxygenase-1 (HO-1) and NAD(P)H:
quinine oxidoreductase 1 (NQO1) (16,17).
Nrf2 contributes to the protection of tissues from damage induced
by the outside environment, including damage arising from
inflammation, trauma, ischemia, hemorrhage and cancer (18,19).
Other studies have identified an association between Nrf2 and
angiogenesis (20) and it was
demonstrated that the absence of Nrf2 may suppress cancer cell
angiogenesis and migration in vivo and in vitro
(21–23). However, the association between DAVF
and Nrf2 in the pathogenesis of DAVF remains unclear. The present
study was performed to measure the expression of Nrf2 in a rat DAVF
model and identify the contribution of Nrf2 to the onset of
DAVF.
Materials and methods
Animals
A total of 138 adult male Sprague-Dawley rats (2.5
months old, 350–400 g) were purchased from the Model Animal
Research Center of Nanjing University (Nanjing, China) and this was
approved by the Institutional Experimental Animal Care and Use
Committee of Nanjing Medical University (Nanjing, China). All
animals were kept in a standard and comfortable laboratory
environment at ~25°C and a relative humidity of 70%, with a 12 h
light/dark cycle and free access to food and water for 10 days
prior to the experiments. The 138 rats were randomly assigned to
one of three experimental groups: The control group (n=18), the
sham-surgery group (n=60) and the DAVF group (n=60). Rats in the
DAVF group were subjected to intracranial VH, rats in the
sham-surgery group were subjected to a similar procedure but
without intracranial VH and rats in the control group did not
undergo any surgery. A total of 18 rats from the sham-surgery and
DAVF groups at each time point (1, 4 and 7 days following surgery)
and 18 control rats were used for western blotting, and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
analysis of brain water content. The remaining 6 rats from the
sham-surgery and DAVF groups were sacrificed at day 1 post surgery
and used for immunofluorescence staining for Nrf2. The present
study was approved by the Ethics Committee of Nanjing Medical
University (Nanjing, China).
DAVF animal model
A DAVF rat model was produced according to a method
described previously by Shin et al (24), which was partly modified. Briefly,
rats were anesthetized by intraperitoneal injection of 50 mg/kg
pentobarbital sodium (CAS57-33-0; Haling Biological Technology,
Shanghai, China). An incision was made via the front middle cervix
and then the left common carotid artery (CCA) and the left external
jugular vein (EJV) were separated and exposed. Subsequently, the
CCA and the EJV were crosscut following clamping using temporary
aneurysm clips. An end-to-end anastomosis was performed between the
near-end of CCA and the encephalic end of EJV using 11-0 medical
sutures. The residual end of the CCA and EJV were cauterized by
bipolar coagulation. Subsequently, the draining vein of the
transverse sinus was separated, exposed and cauterized. The skull
was removed over the sagittal sinus and the wall of the sinus was
incised and filled with surgical hemostatic material to form
thrombosis of the sagittal sinus. Surgery incisions were sutured
using 6-0 nylon sutures. The sham group received cervical medial,
post-aurem and frontal incision and suture without the induction of
VH. Rats in the control group did not undergo surgery.
Western blot analysis
The brain tissue at the coronal level 4 mm on either
side to the occluded sinus was collected, homogenized and lysed in
radioimmunoprecipitation assay buffer [1% NP40, 0.1% SDS, 0.5%
sodium deoxycholate, 1 mM EGTA, 1 mM EDTA, 1 mM
Na3VO4, 0.5 mM dithiothreitol, 20 mM NaF, 1
nM phenylmethylsulfonyl fluoride and protease inhibitor cocktail in
PBS (pH 7.4)]. To extract nuclear and cytoplasmic proteins, a
nuclear and cytoplasmic protein extraction kit (Beyotime Institute
of Biotechnology, Nantong, China) was used following the
manufacturer's protocol. Protein concentration was determined using
a BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's protocol. Equal
amounts of total protein (50 µg) were loaded per lane, separated
using 8% SDS-PAGE and transferred to PVDF membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 3% skimmed
milk for 2 h at room temperature. Subsequently, membranes were
incubated with primary antibodies against Nrf2 (cat. no. ab137550;
1:500), NQO-1 (cat. no. ab34173; 1:1,000), HO-1 (cat. no. ab13243;
1:1,000) and histone H3 (cat. no. ab1791; 1:1,000), all purchased
from Abcam (Cambridge, MA, USA), and β-actin (cat. no. sc-130657;
1:2,000) purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA) at 4°C overnight. Membranes were then washed three times with
washing buffer (tris-buffered saline containing 0.05% Tween-20) and
incubated with goat anti-rabbit horseradish peroxidase
(HRP)-conjugated IgG (cat. no. 7074S; 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) at room temperature for 2 h.
Membranes were washed using washing buffer for 15 min, visualized
using immobilon Western chemiluminescent HRP substrate (EMD
Millipore) and exposed to X-ray film (Fuji Hyperfilm, Tokyo,
Japan). Quantification of band density was performed using the
UN-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, USA).
Total RNA extraction and RT-qPCR
To obtain the brain tissues, animals were deeply
anesthetized with pentobarbital sodium and the cerebral cortex was
harvested, immediately frozen in liquid nitrogen and stored at
−80°C until use. Total RNA was isolated from brain tissues at the
coronal level 4 mm either side of the occluded sinus using TRIzol
reagent (Takara Biotechnology Co., Ltd., Dalian, China) following
the manufacturer's protocol. The quantity and purity of the
extracted total RNA were determined at the optical densities of 260
and 280 nm using a NanoDrop™ 2000c (Thermo Fisher
Scientific, Inc.). In order to avoid RNA degradation, part of the
RNA was reverse transcribed to cDNA immediately using a
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.). qPCR
was performed using a previously described method (25). The qPCR reaction mixture was prepared
according to the SYBR® Premix Ex Taq™ kit protocol
(Takara Biotechnology Co., Ltd.), which contained diluted cDNA,
SYBR-Green I Nucleic Acid Stain, 0.2 µM of each gene-specific
primer and nuclease-free water to a final volume of 25 µl. The
primer sequences used in the present study were as follows: Nrf2
forward, 5′-TCAGCGACGGAAAGAGTATGA-3′ and reverse,
5′-CCACTGGTTTCTGACTGGATGT-3′; NQO1 forward, 5′-ATGGTCGGCAGAAGAGC-3′
and reverse, 5′-GGAAATGATGGGATTGAAGT-3′; HO-1 forward,
5′-TCTCCGATGGGTCCTTACACTC-3′ and reverse,
5′-GGCATAAAGCCCTACAGCAACT-3′; and β-actin forward,
5′-CTGAATGGCCCAGGTCTGAG-3′ and reverse, 5′-AAGTCAGTGTACAGGCCAGC-3′.
β-actin was selected as an endogenous reference ‘housekeeping’
gene. Relative changes in target mRNA expression following surgery
was determined using the 2−∆∆Cq method (26).
Immunostaining
For immunofluorescence, consecutive coronal sections
were cut at 4-µm intervals from the hippocampus and the cortex near
the sagittal sinus thrombosis in the DAVF and the sham groups.
Following routine deparaffinization and 2 h blocking in 10% normal
goat serum (Wuhan Boster Biological Technology, Ltd., Wuhan, China)
in PBS at room temperature, sections were incubated overnight with
primary antibodies for Nrf2 (cat. no. ab137550; 1:100, Abcam) at
4°C. On the second day, the slides were washed three times with PBS
and incubated with Alexa Fluor 594®-conjugated Goat
anti-Rabbit IgG secondary antibodies (cat. no. A-11037; 1:200;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Subsequently, slides were washed three times with PBS,
counterstained with DAPI for 2 min at room temperature and covered
with mounting media. Fluorescence was observed using an inverted
microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan) and
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Assessment of brain water content
The change of water content in the brains of rats
subjected to DAVF was detected using a previously reported method
(27). Briefly, the rat brain was
removed and placed a cooling brain matrix for 24 h at 1, 4 and 7
days after surgery. Following separation and removal of the
brainstem and cerebellum, ipsilateral brain hemisphere tissue was
reserved and weighed up as wet weight (ww). Subsequently, the brain
was kept in a drying oven at 80°C for 3 days to dehydrate and
weighed up to obtain the dry weight (dw). At last, the brain water
content was calculated using the following formula: [(ww-dw)/ww]
×100%.
Statistical analysis
At least three separate experiments were performed
for each measurement and the data were expressed as the mean ±
standard deviation. All analyses were performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Data were analyzed by
one-way analysis of variance followed by Tukey's post-hoc test and
P<0.05 was considered to represent a statistically significant
difference.
Results
DAVF significantly aggravates cerebral
edema
All rats in the DAVF group survived following
surgery. To determine the secondary brain edema induced by DAVF,
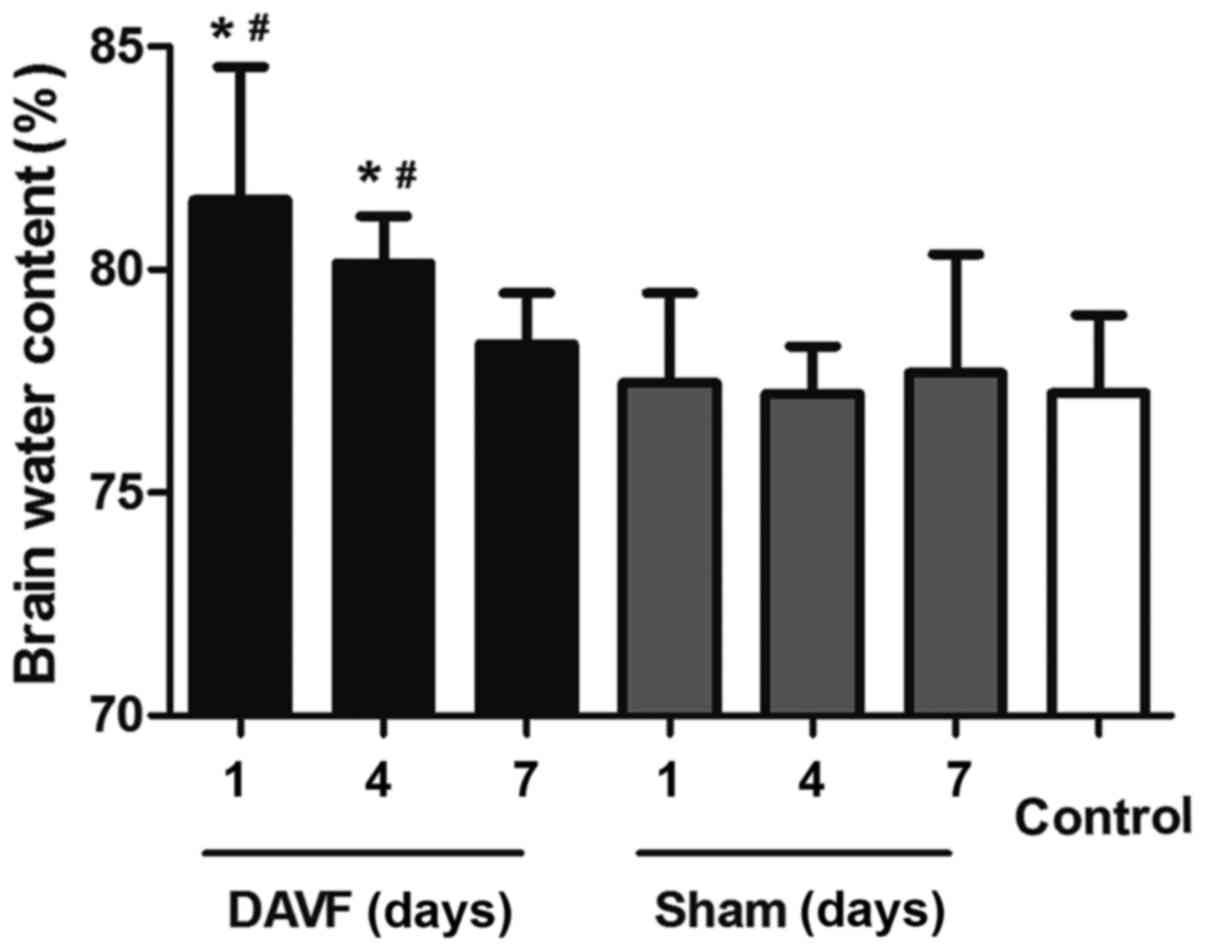
brain water content was measured 1, 4 and 7 days after surgery. As
presented in Fig. 1, no significant
difference was detected in brain water content between the
sham-surgery group and the control group at each time point (1, 4
and 7 days). However, there was a significant aggravation of
cerebral edema 1 day post-surgery in the DAVF group compared with
the sham-surgery group at the same time point and compared with the
control group (P<0.05). Additionally, 4 days following surgery,
the brain water content in the DAVF group decreased but remained
significantly higher than in the sham-surgery and control groups
(P<0.05). Brain water content in the DAVF group returned to
normal levels 7 days post surgery.
DAVF markedly activates Nrf2 in the
rat brain cortex and hippocampus
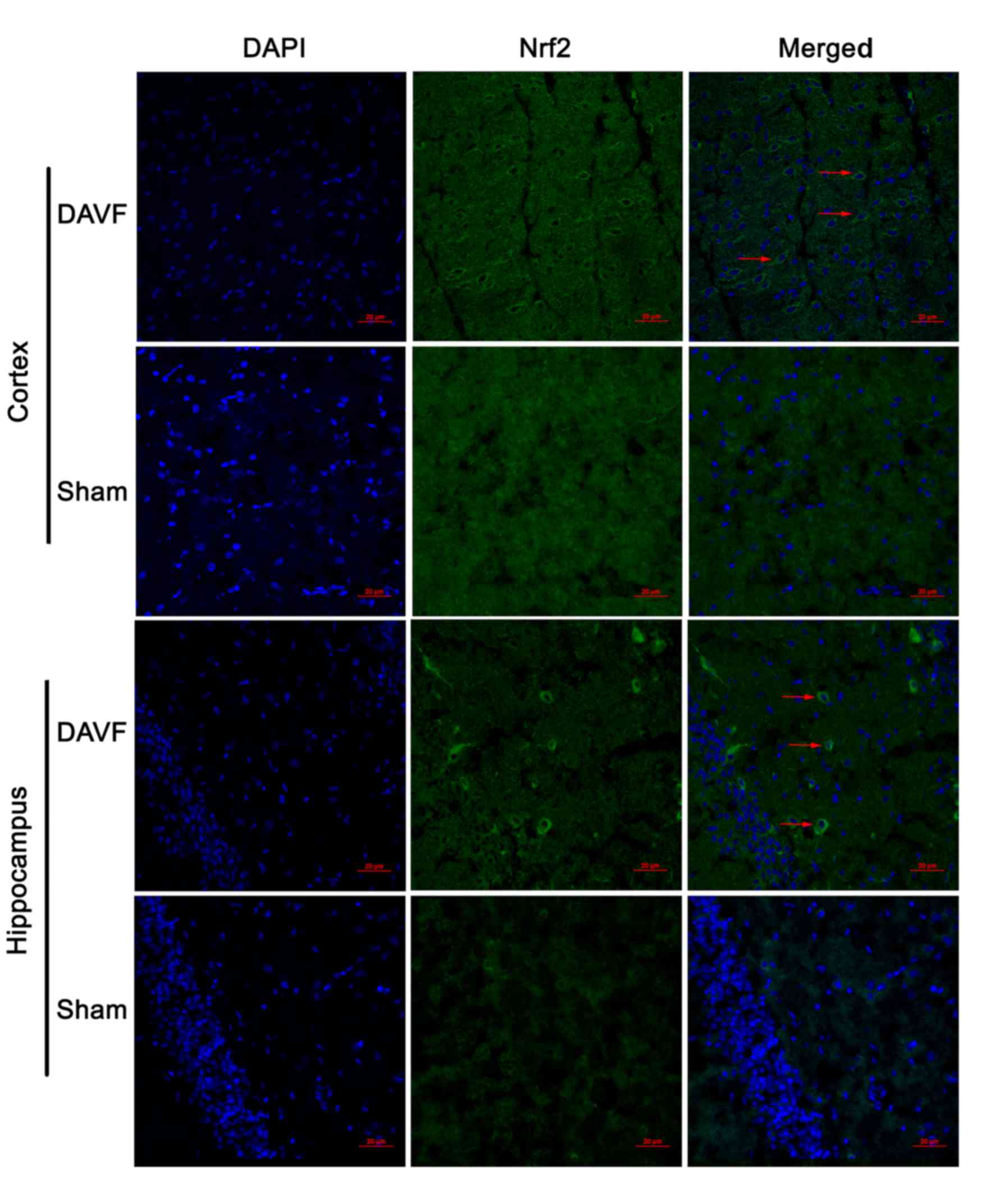
The expression of Nrf2 in the cortex and hippocampus
of rat brain was detected by immunofluorescence 1 day following
surgery. As depicted in Fig. 2, in
the cortex of the rat brain, only a small number of Nrf2-stained
cells were observed in the sham-surgery group, whereas a larger
number of Nrf2-stained cells were observed in the DAVF group.
Similar results were observed in the hippocampus (Fig. 2).
Expression of Nrf2/ARE in DAVF
rats
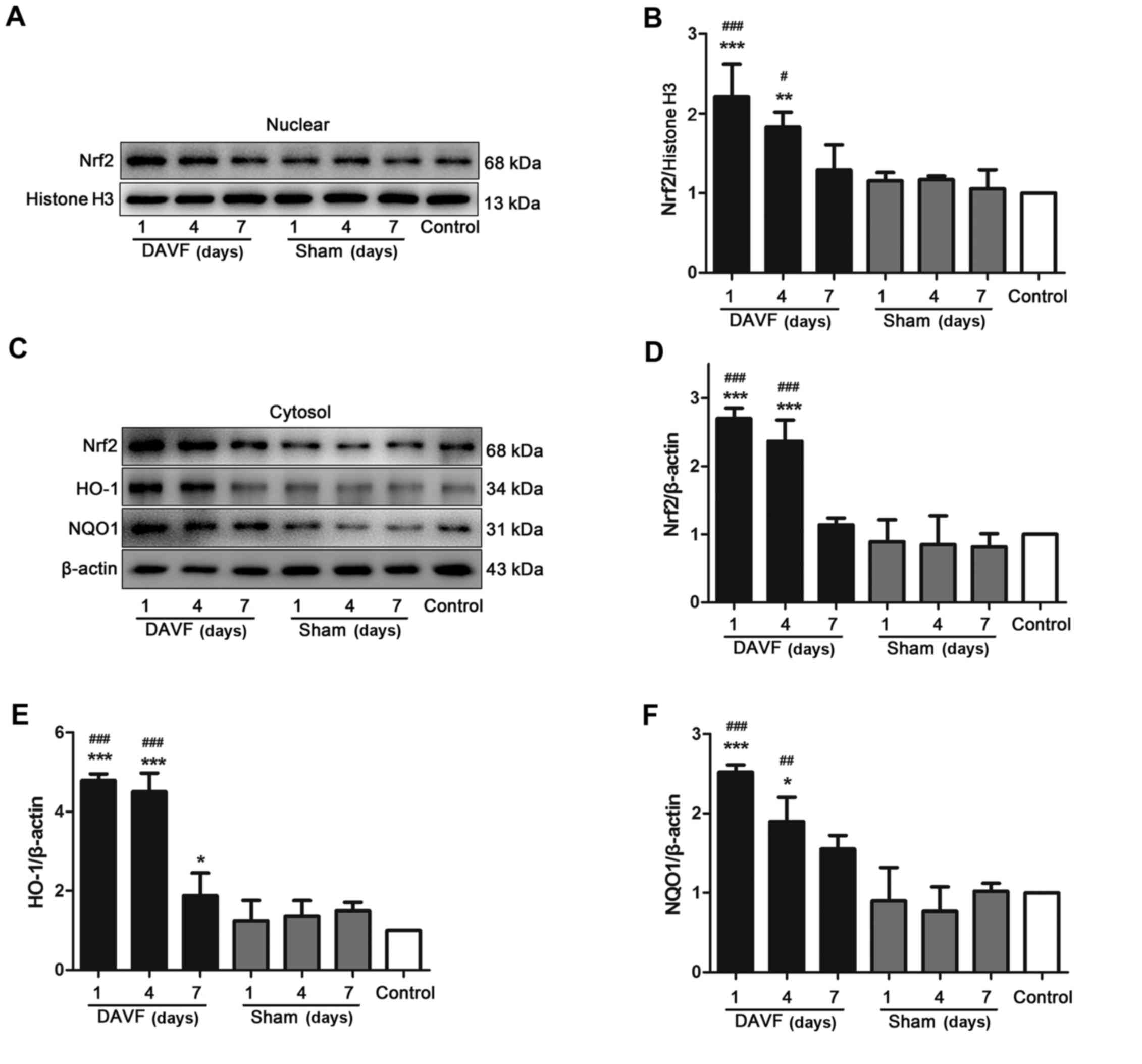
Nrf2 translocation is the primary mechanism by which
the Nrf2-ARE signaling pathway is activated; thus the expression of
Nrf2 in the nucleus was determined using western blot analysis. As
presented in Fig. 3A and B, the
results demonstrated that there was no significant difference in
the expression of nuclear Nrf2 at each time point (1, 4 and 7 days)
between the sham-surgery and the control groups. However, following
DAVF surgery, the expression of nuclear Nrf2 significantly
increased and reached a peak 1 day following surgery (P<0.001).
It began to gradually decrease but remained significantly higher 4
days after surgery compared with the control group (P<0.01).
Nuclear Nrf2 expression returned to normal 7 days post-surgery. The
expression of cytoplasmic Nrf2 was also measured (Fig. 3C and D) and the results were
consistent with those for nuclear Nrf2. Subsequently, the
expression of the Nrf2 downstream target proteins HO-1 and NQO1 was
also measured. It was demonstrated that HO-1 and NQO1 expression
was significantly upregulated 1 and 4 days following DAVF surgery
compared with the sham-surgery and control groups (P<0.05;
Fig. 3E and F).
Expression of Nrf2/ARE mRNA in the
DAVF group
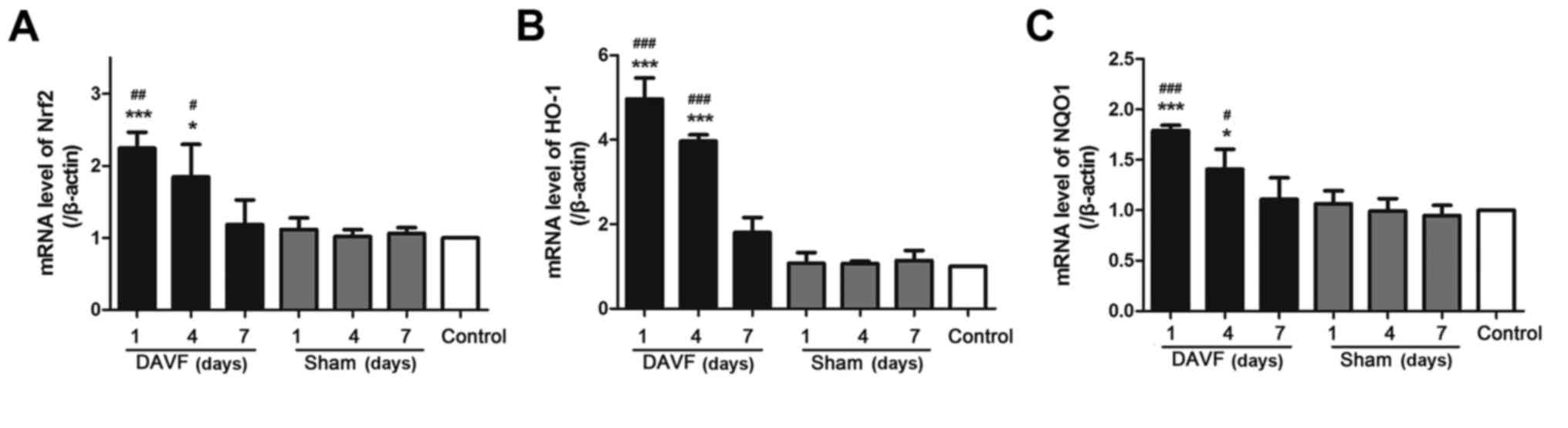
The aforementioned results indicate that DAVF
activates the Nrf2/ARE signaling pathway by increasing the
expression of Nrf2 protein in the nucleus and cytoplasm. To
determine whether DAVF surgery altered the expression of Nrf2/ARE
mRNA, RT-qPCR was performed. As demonstrated in Fig. 4A, no significant differences in Nrf2
mRNA expression were observed between the sham-surgery and control
groups at each time point (1, 4 and 7 days). However, the
expression of Nrf2 mRNA significantly increased 1 day following
DAVF surgery compared with the control and sham-surgery groups
(P<0.01). Nrf2 mRNA expression decreased gradually 4 days
post-surgery but remained significantly higher than in the control
and sham-surgery groups (P<0.05). It then returned to baseline 7
days after surgery. Similar results were obtained regarding the
expression of HO-1 and NQO1 mRNA (Fig.
4B and C).
Discussion
Knowledge of the genesis and progression of brain
arteriovenous malformations and arteriovenous fistulas has improved
due to the development of appropriate experimental animal models.
Numerous studies using an experimental rat model that produced an
arteriovenous fistula between the CCA and the EJV indicated that
intracranial VH may be involved in the development of DAVF
(24,15–31). The
rat DAVF model in the present study was formed by performing
anastomosis between the left CCA and EJV, occluding the
contralateral draining vein and thrombosing the sagittal sinus to
investigate the role of intracranial VH and venous blood flow
obstruction in the development of DAVF (28). The histological changes of the
cerebral venous sinus response to VH were subsequently
investigated.
Although there have been numerous studies
investigating DAVF, the exact mechanisms of its genesis and
development remain unclear. Over the past few decades, knowledge
regarding the pathophysiology of DAVF has improved greatly. Lawton
et al (32) demonstrated that
angiogenesis was important in the genesis and development of DAVF
and identified the major factors influencing angiogenesis,
including hypoxia, ischemia and VH. Zhu et al (33) demonstrated that the hypoxia-inducible
factor 1-α/vascular endothelial growth factor (VEGF) signaling
pathway was activated and contributed to angiogenesis induced by
nonischemic VH in the brain tissues. Additionally, Chen et
al (14) identified that VH may
promote angiogenesis induced by chronic regional poor perfusion and
the upregulation of VEGF matrix metallopeptidase 9 expression.
Regulating the expression of VEGF may be a potential novel
treatment option of controlling angiogenesis in DAVF. VEGF is a
vital pro-angiogenic factor that is upregulated following hypoxia
and ischemia and stimulates the migration and proliferation of
vessel endothelial cells (34).
Immunohistochemistry has been used in DAVF to
demonstrate the histological response to VH (35), and the results indicate that local
tissue hypoxia caused by VH may be the initial step causing
neoangiogenesis in DAVFs. Numerous pro-angiogenic growth factors
have been reported in detail, including platelet-derived growth
factor, fibroblast growth factor and VEGF (36). Previous studies have identified the
close association between DAVF and VEGF (24,29,37).
Kweider et al (36)
demonstrated that VEGF may activate the Nrf2-ARE signaling pathway
in human choriocarcinoma BeWo cells and that this activation could
be completely eliminated using the Nrf2-specific short hairpin RNA.
However, few studies have evaluated the role of Nrf2 in DAVF.
The aim of the present study was to measure the
expression of Nrf2 in mice following surgery to induce DAVF. The
results demonstrated that the expression of Nrf2 protein in the
nucleus and cytoplasm, as well as Nrf2 mRNA, was upregulated and
peaked 1 day following DAVF surgery. Afterwards, expression
gradually decreased but still remained high 4 days post-surgery,
only returning to the baseline 7 days after surgery. It was
observed that the HO-1 and NQO1 were also upregulated. These
results indicate that the expression of Nrf2 increases immediately
following DAVF surgery and that the activation of the Nrf2-ARE
pathway early on may be important in the pathogenesis of DAVF.
Nrf2 is a vital cytoprotective transcription factor
involved in the regulation of detoxifying, anti-inflammatory,
antioxidative and antiapoptotic genes (38), which protect the cells from harmful
damage. Under normal conditions, Nrf2-mediated transcription is
blocked due to the inhibitory effect of cytoplasmic protein Keap1,
which facilitates Nrf2 proteasomal degradation (39). However, following exposure to
oxidative and chemical stress, the Keap1-Nrf2 complex is disrupted
and Nrf2 translocates into the cell nucleus. Nrf2 then activates
its downstream target genes, including HO-1 and NQO1, which are
involved in regulating redox reactions in the cells (40,41).
However, the function of Nrf2 action may be much broader since it
also regulates angiogenesis; for example, it may promote the
formation of blood vessels to protect the retina from oxidative
injury that occurs following hyperoxic stimulation (42). Furthermore, it has been demonstrated
that downregulation of Nrf2 inhibits cancer cell vasculogenesis and
migration in vivo and in vitro (21–23).
These results indicate that the Nrf2/ARE signaling pathway may
potentially contribute to the growth of DAVFs through the
angiogenesis induced by VH.
In conclusion, although there are certain
limitations of the present study, including a lack of long-term
experimental observation and in vitro experiments, taken
together, the results demonstrate that Nrf2 may contribute to the
formation of DAVF via angiogenesis induced by intracranial VH.
However, further studies are essential to determine the precise
mechanisms by which Nrf2 affects the pathophysiology of DAVF.
Acknowledgements
The authors would like to thank Dr. Hao Pan for
technical assistance.
References
|
1
|
Awad IA, Little JR, Akarawi WP and Ahl J:
Intracranial dural arteriovenous malformations: Factors
predisposing to an aggressive neurological course. J Neurosurg.
72:839–850. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaudhary MY, Sachdev VP, Cho SH, Weitzner
I Jr, Puljic S and Huang YP: Dural arteriovenous malformation of
the major venous sinuses: An acquired lesion. AJNR Am J
Neuroradiol. 3:13–19. 1982.PubMed/NCBI
|
|
3
|
Kwon BJ, Han MH, Kang HS and Chang KH: MR
imaging findings of intracranial dural arteriovenous fistulas:
Relations with venous drainage patterns. AJNR Am J Neuroradiol.
26:2500–2507. 2005.PubMed/NCBI
|
|
4
|
Gandhi D, Chen J, Pearl M, Huang J,
Gemmete JJ and Kathuria S: Intracranial dural arteriovenous
fistulas: Classification, imaging findings, and treatment. AJNR Am
J Neuroradiol. 33:1007–1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaudhary MY, Sachdev VP, Cho SH, Weitzner
I Jr, Puljic S and Huang YP: Dural arteriovenous malformation of
the major venous sinuses: An acquired lesion. AJNR Am J
Neuroradiol. 3:13–19. 1982.PubMed/NCBI
|
|
6
|
Cooper CJ, Said S, Nunez A, Quansah R,
Khalillullah S and Hernandez GT: Dural arteriovenous fistula
discovered in patient presenting with recent head trauma. Am J Case
Rep. 14:444–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zaletel M, Surlan-Popovic K, Pretnar-Oblak
J and Zvan B: Moyamoya syndrome with arteriovenous dural fistula
after head trauma. Acta clinica Croatica. 50:115–120.
2011.PubMed/NCBI
|
|
8
|
Nabors MW, Azzam CJ, Albanna FJ, Gulya AJ,
Davis DO and Kobrine AI: Delayed postoperative dural arteriovenous
malformations. Report of two cases. J Neurosurg. 66:768–772. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yassari R, Jahromi B and Macdonald R:
Dural arteriovenous fistula after craniotomy for pilocytic
astrocytoma in a patient with protein S deficiency. Surg Neurol.
58:59–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnwell SL, Halbach VV, Dowd CF,
Higashida RT, Hieshima GB and Wilson CB: A variant of arteriovenous
fistulas within the wall of dural sinuses. Results of combined
surgical and endovascular therapy. J Neurosurg. 74:199–204. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakaki T, Morimoto T, Nakase H, Kakizaki T
and Nagata K: Dural arteriovenous fistula of the posterior fossa
developing after surgical occlusion of the sigmoid sinus. Report of
five cases. J Neurosurg. 84:113–118. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gross BA and Du R: The natural history of
cerebral dural arteriovenous fistulae. Neurosurgery. 71:594–603.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanakita S, Koga T, Shin M, Shojima M,
Igaki H and Saito N: Role of Gamma Knife surgery in the treatment
of intracranial dural arteriovenous fistulas. J Neurosurg. 117
Suppl:S158–S163. 2012.
|
|
14
|
Chen L, Mao Y and Zhou LF: Local chronic
hypoperfusion secondary to sinus high pressure seems to be mainly
responsible for the formation of intracranial dural arteriovenous
fistula. Neurosurgery. 64:973–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terada T, Higashida RT, Halbach VV, Dowd
CF, Tsuura M, Komai N, Wilson CB and Hieshima GB: Development of
acquired arteriovenous fistulas in rats due to venous hypertension.
J Neurosurg. 80:884–889. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villeneuve NF, Lau A and Zhang DD:
Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin
proteasome system: An insight into cullin-ring ubiquitin ligases.
Antioxid Redox Signal. 13:1699–1712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang M, An C, Gao Y, Leak RK, Chen J and
Zhang F: Emerging roles of Nrf2 and phase II antioxidant enzymes in
neuroprotection. Prog Neurobiol. 100:30–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bryan HK, Olayanju A, Goldring CE and Park
BK: The Nrf2 cell defence pathway: Keap1-dependent and -independent
mechanisms of regulation. Biochem Pharmacol. 85:705–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, Fang Q, Zhang J, Zhou D and Wang
Z: Role of the Nrf2-ARE pathway in early brain injury after
experimental subarachnoid hemorrhage. J Neurosci Res. 89:515–523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Florczyk U, Jazwa A, Maleszewska M, Mendel
M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna
S, Bukowska-Strakova K, et al: Nrf2 regulates angiogenesis: Effect
on endothelial cells, bone marrow-derived proangiogenic cells and
hind limb ischemia. Antioxid Redox Signal. 20:1693–1708. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji X, Wang H, Zhu J, Zhu L, Pan H, Li W,
Zhou Y, Cong Z, Yan F and Chen S: Knockdown of Nrf2 suppresses
glioblastoma angiogenesis by inhibiting hypoxia-induced activation
of HIF-1α. Int J Cancer. 135:574–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji XJ, Chen SH, Zhu L, Pan H, Zhou Y, Li
W, You WC, Gao CC, Zhu JH, Jiang K and Wang HD: Knockdown of
NF-E2-related factor 2 inhibits the proliferation and growth of
U251MG human glioma cells in a mouse xenograft model. Oncol Rep.
30:157–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D,
Lee YM, Ku SK, Jung Y and Kwak MK: NRF2 blockade suppresses colon
tumor angiogenesis by inhibiting hypoxia-induced activation of
HIF-1α. Cancer Res. 71:2260–2275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin Y, Nakase H, Nakamura M, Shimada K,
Konishi N and Sakaki T: Expression of angiogenic growth factor in
the rat DAVF model. Neurol Res. 29:727–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JW, Wang HD, Zhong WZ, Li N and Cong
ZX: Expression and cell distribution of metabotropic glutamate
receptor 5 in the rat cortex following traumatic brain injury.
Brain Res. 1464:73–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Wang H, Ding K, Lu X, Li T and Wang
J, Wang C and Wang J: Inhibition of cathepsin S produces
neuroprotective effects after traumatic brain injury in mice.
Mediators Inflamm. 2013:1878732013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bederson JB, Wiestler OD, Brüstle O, Roth
P, Frick R and Yasargil MG: Intracranial venous hypertension and
the effects of venous outflow obstruction in a rat model of
arteriovenous fistula. Neurosurgery. 29:341–350. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kojima T, Miyachi S, Sahara Y, Nakai K,
Okamoto T, Hattori K, Kobayashi N, Hattori K, Negoro M and Yoshida
J: The relationship between venous hypertension and expression of
vascular endothelial growth factor: Hemodynamic and
immunohistochemical examinations in a rat venous hypertension
model. Surg Neurol. 68:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang ST, Rodriguez-Hernandez A, Walker EJ,
Young WL, Su H and Lawton MT: Adult mouse venous hypertension
model: Common carotid artery to external jugular vein anastomosis.
J Vis Exp. 27:504722015.
|
|
31
|
Zou X, Zhou L, Zhu W, Mao Y and Chen L:
Effectiveness of 2-methoxyestradiol in alleviating angiogenesis
induced by intracranial venous hypertension. J Neurosurg.
125:746–753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lawton MT, Jacobowitz R and Spetzler RF:
Redefined role of angiogenesis in the pathogenesis of dural
arteriovenous malformations. J Neurosurg. 87:267–274. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y,
Shen F, Young WL and Yang GY: Expression of hypoxia-inducible
factor-1 and vascular endothelial growth factor in response to
venous hypertension. Neurosurgery. 59:687–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi T, Abe K, Suzuki H and Itoyama Y:
Rapid induction of vascular endothelial growth factor gene
expression after transient middle cerebral artery occlusion in
rats. Stroke. 28:2039–2044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tirakotai W, Bertalanffy H, Liu-Guan B,
Farhoud A and Sure U: Immunohistochemical study in dural
arteriovenous fistulas and possible role of local hypoxia for the
de novo formation of dural arteriovenous fistulas. Clin Neurol
Neurosurg. 107:455–460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kweider N, Fragoulis A, Rosen C, Pecks U,
Rath W, Pufe T and Wruck CJ: Interplay between vascular endothelial
growth factor (VEGF) and nuclear factor erythroid 2-related
factor-2 (Nrf2): Implications for preeclampsia. The Journal of
biological chemistry. 286:42863–42872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Q, Zhang Q, Huang QH, Fang YB, Zhang
ZL, Xu Y and Liu JM: A pivotal role of the vascular endothelial
growth factor signaling pathway in the formation of venous
hypertension-induced dural arteriovenous fistulas. Mol Med Rep.
9:1551–1558. 2014.PubMed/NCBI
|
|
38
|
Lee JM and Johnson JA: An important role
of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem
Mol Biol. 37:139–143. 2004.PubMed/NCBI
|
|
39
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uno K, Prow TW, Bhutto IA, Yerrapureddy A,
McLeod DS, Yamamoto M, Reddy SP and Lutty GA: Role of Nrf2 in
retinal vascular development and the vaso-obliterative phase of
oxygen-induced retinopathy. Exp Res. 90:493–500. 2010.
|