Introduction
Trauma is a common reason for the admission of
patients to an emergency room. Bone fracture combined with soft
tissue contusion is the most common injury seen by an orthopedic
doctor; local tenderness, pain and swelling are the most
troublesome and frequent problems (1). More than 90% of multiply injured
patients with bone fractures are related with the development of
the systemic inflammatory response syndrome and significantly
correlate with multiple organ failure, sepsis and mortality
(1). The inflammatory process
includes release of inflammatory cytokines, activated polymorph
nuclear cells (PMN) and the expression of leukocyte adhesion
molecules (2). Anti-inflammatory
treatment may in certain cases result in delayed infection
(3). Therefore, regulation of
cytokine release following trauma has been considered a potential
strategy (4), and may improve
patients' life quality during acute disease stage. Various
anti-inflammatory medication and analgesics may be selected, but
may cause allergic reaction or more burden to the stomach, liver or
kidney which may become injured during the acute stages of trauma
(5).
Previous results have suggested that bone fracture
elicited a distinct pattern of cytokines associated with organ
injury (6). The data revealed
similar patterns of interleukin-6 (IL-6) and IL-10 after isolated
bilateral femur fracture as compared with other injury ways
(6); however, tumor necrosis
factor-α (TNF-α) and IL-1β exhibit different patterns of a higher
level maintained over a longer time period. A minor unbalance in
serum cytokines or locally produced cytokines may be responsible
for early stage PMN infiltration of the organ (2).
Macrophages and monocytes are the main producers of
TNF-α, that may stimulate the secretion of a variety of pro- and
anti-inflammatory cytokines, for example IL-6, IL-8 and IL-10
(7). Either anti-TNF-α antibodies or
soluble receptors for TNF-α have become a strategy in the treatment
of patients with chronic inflammatory diseases through attenuating
TNF-α (4). Overwhelming production
of pro-inflammatory cytokines such as TNF-α and uncontrolled
production of anti-inflammatory cytokines such as IL-10 can lead to
multiple organ dysfunction and mortality (4). Trauma, taking fracture as an example,
induces inflammation that can cause elevation of proinflammatory
cytokines and induce a series of systemic injury (1).
Freshwater clams (Corbicula fluminea), are a
popular edible shellfish in Asia. Recent experiments noted that
freshwater clam extract (FCE) exerts anti-oxidative and
anti-inflammatory activity (8). FCE
suppresses the release of the pro-inflammatory cytokine TNF-α
production after hemorrhagic shock and decreased the level of liver
injury marker in rats (9). Oral FCE
also significantly decreased chronic hepatic fibrosis triggered by
carbon tetrachloride through attenuating thiobarbituric acid
reactive substances, hydroxyproline and excessive inflammation in
rats (10). To the best of our
knowledge, no prior studies have investigated the effects of FCE
consumption on acute trauma conditions.
The aim of the present study was to determine
whether FCE affected the inflammation induced by unilateral tibial
fracture in a conscious rat model.
Materials and methods
Preparation of FCE and experimental
animals
We prepared FCE and quantitated its
anti-inflammation activity with previous method (11). A total of 32 male Wistar-Kyoto rats
weighing 280–320 g were purchased from the National Animal Center
(Taipei, Taiwan). The rats were housed in our animal center under a
controlled environment at 22±1°C with a 12-h light/dark cycle. Food
and water were provided ad libitum. The experimental
protocol was approved by the Animal Usage Regulation Committee of
Tzu Chi Hospital (Hualien, China).
Conscious rat model of closed tibia
fracture
The animals were anesthetized with ether inhalation
for ~10 min. During the period of anesthesia, polyethylene
catheters (PE-50) were inserted into the femoral artery for
collecting blood samples. The operation was completed within 15
min, and the section wound was as small as possible (<0.5
cm2) (9). Following the
operation, the animal was placed in an individual cage
(Shingshieying Instruments, Hualien, Taiwan). After the rats were
placed properly, left tibial shaft fracture was performed with
three points bending method smoothly (12). The fractured limb was fixed with an
external wooden stick wrapped with elastic bandage, which imitated
the clinical management of the situation.
Experimental design
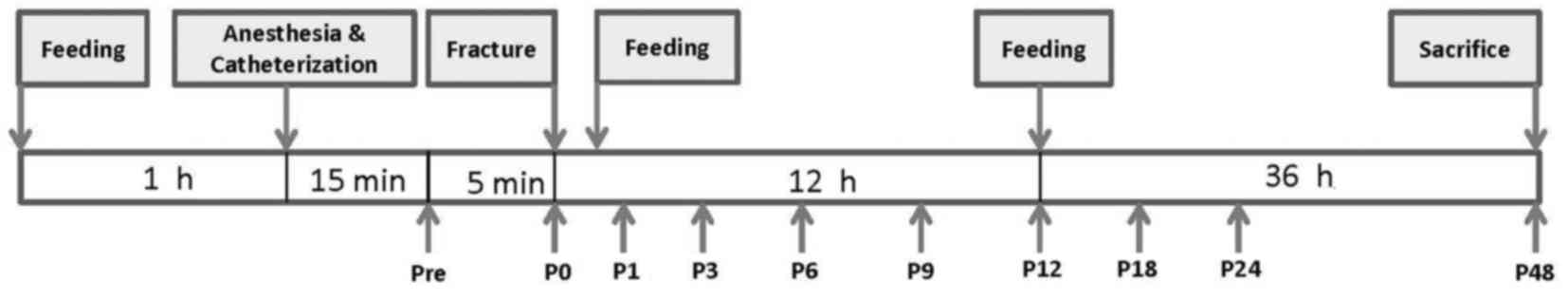
The animals were randomly divided into four groups.
The feeding time-points were prefracture 1 h, and post-fracture 0.5
and 11.5 h. We placed the fed substance in the stomach of the rats
using a dosing syringe. The sham (n=8) and fracture (Fx) groups
(n=8 per group) were fed with 1 ml normal saline (NS) at the three
time-points. The fracture with FCE (Fx+FC) and the non-fracture
with FCE (NFx+FC) groups were fed with FCE (20 mg/kg) at each of
the three time-points. Fig. 1
displays a flow chart detailing the study protocol.
Heart rate (HR) and central
temperature (CT) recordings
The arterial catheter of was connected to a pressure
transducer (Gould Instrument Systems, Cleveland, OH, USA) for
recording mean arterial pressure (MAP) and HR, using a polygraph
recorder (Power Lab; ADInstruments, Mountain View, CA, USA). CT was
estimated and recorded continuously through the anal thermometer
placed in the rectum of the rats.
Blood sample analyses
Arterial blood samples were obtained for baseline
values before fracture. Arterial blood samples (0.5 ml) were
collected before fracture and at 1, 3, 6, 9, 12, 24 and 48 h after
fracture. Half of the serum was stored at −20°C for cytokine
analysis. The concentrations of TNF-α (DY510), IL-6 (DY506) and
IL-10 (DY522) in the samples were measured separately using
enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems,
Minneapolis, MN, USA). All samples were stored at −20°C prior to
testing. The reagents, samples and working standards were brought
to room temperature and prepared according to the manufacturer's
directions. The ELISA was performed following the manufacturer's
instructions. Each sample was run in duplicate and determined using
an automated ELISA reader (Sunrise; Tecan, Grödingen, Austria) at
450/540 nm. The other half of the serum was decanted and stored at
4°C. Biochemical examinations were performed within 1 h after
specimen collection. Serum levels of creatine phosphokinase (CPK)
and lactate dehydrogenase (LDH) were measured using an auto
analyzer (COBAS Integra C111; Roche Diagnostics, Basel,
Switzerland).
Histological examination
Tibial muscles were harvested 48 h after fracture.
These tissue specimens were fixed overnight in 4% buffered
formaldehyde, processed by standard methods, and stained with
hematoxylin and eosin. An observer participated in the pathological
examination in a blind fashion to minimize subjectivity.
Data analysis
Data are expressed as the mean ± standard error of
the mean. The significance of differences in the measured values
between groups was analyzed using one-way analysis of variance. The
significance of the measured values within groups was analyzed with
repeated measurements by t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of FCE on vital signs post
inflammatory response
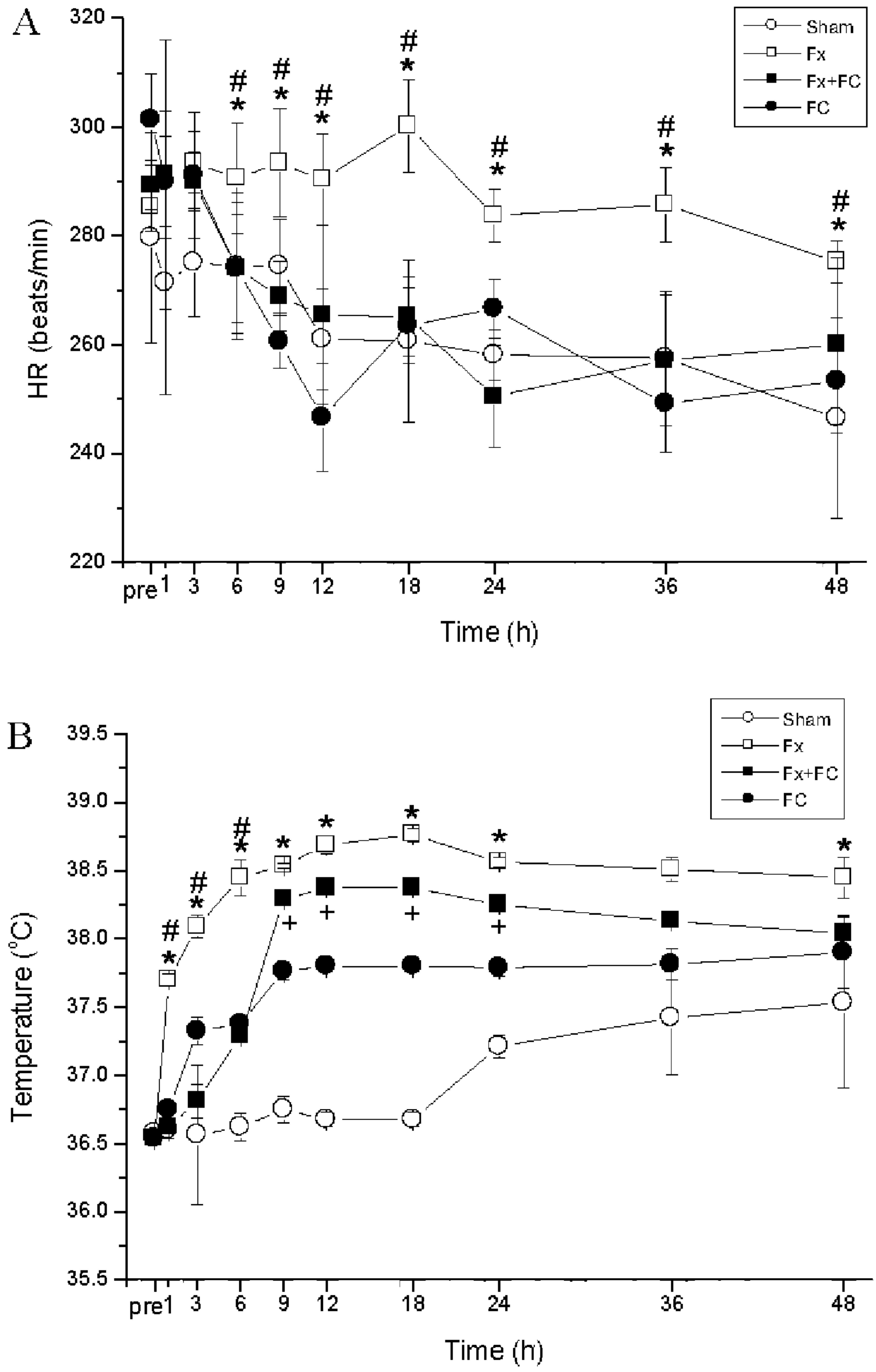
The average HR and CT showed no significant
difference between four groups before blood withdrawal (Fig. 2). The Fx group had significantly
higher HR levels at all the time-points compared to the sham group
(P<0.05) (Fig. 2A). The Fx+FC
group had relatively lower HRs at 3, 6, 9, 12 and 24 h compared to
the Fx group, although HR did not significantly change during the
experimental period. CT of the Fx and sham groups decreased at 6 h
and increased at 9 h. The average CT of the Fx group was
significantly increased at all the time-points compared to the
baseline level (P<0.05) (Fig.
2B). CT of the Fx+FC group decreased at 1 h and increased
steadily between 1 and 9 h, and was unchanged between 9 and 24 h.
The CT of the Fx+FC group was significant lower at 1 and 3 h, and
higher at 6 h, compared to the Fx group.
Effects of FCE on proinflammatory
cytokine serum level
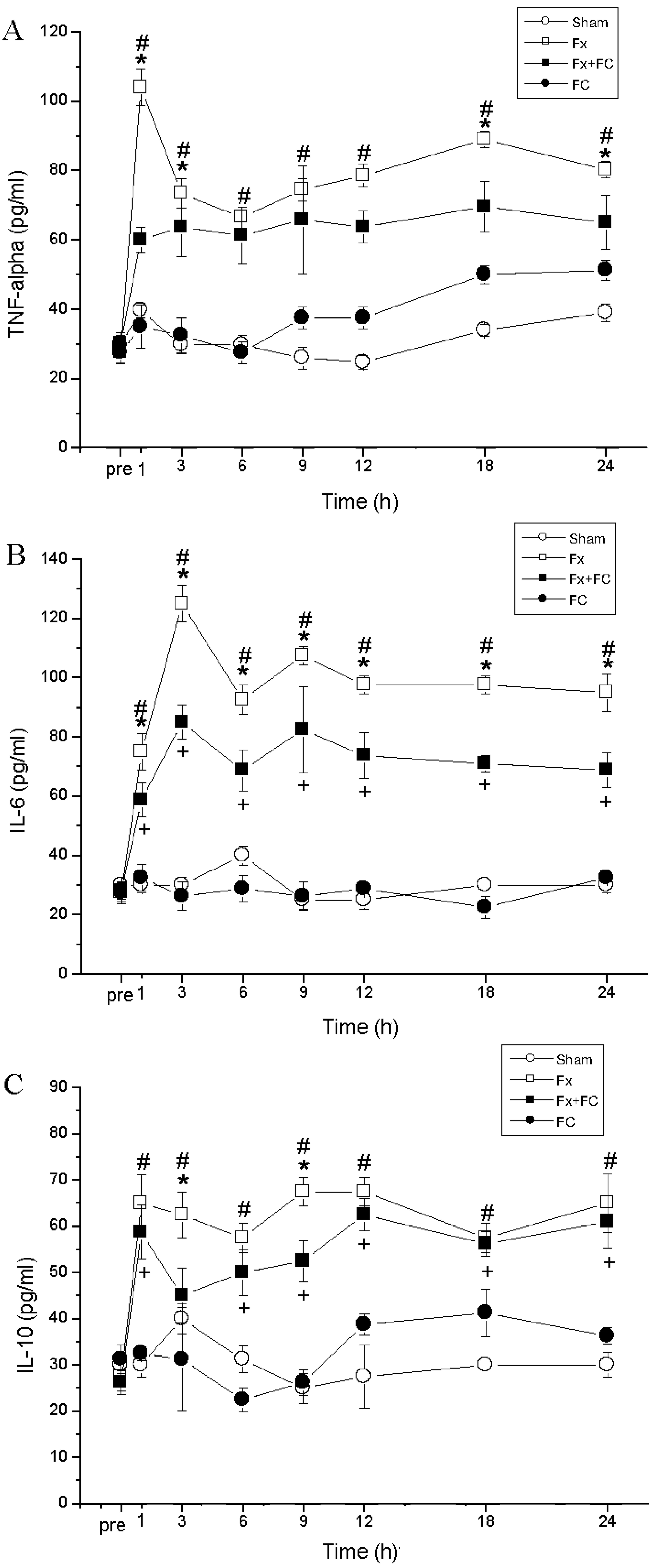
Plasma levels of TNF-α, IL-6 and IL-10 showed no
significant difference between the four groups prior to blood
withdrawal (Fig. 3).
The plasma TNF-α level elevated to the first peak in
the Fx group at 1 h and decreased rapidly at 3 h and mildly at 6 h
(P<0.05) (Fig. 3A). Then it
reached the second peak at 18 h. TNF-α levels in the Fx group were
significantly higher than that of the sham group (P<0.05). TNF-α
levels of the Fx+FC group climbed up to the peak at 1 h and reduced
to a steady level between 3 and 4 h, which was significantly lower
than that of the Fx group at 1, 3, 9, 12, 18 and 24 h.
Serum IL-6 levels of the Fx and Fx+FC groups were
significantly higher compared to the sham group (P<0.05)
(Fig. 3B). Serum IL-6 levels peaked
at 3 and 9 h in the Fx group, and the former one is higher. The
IL-6 level of the Fx+FC group showed a similar pattern to the Fx
group, but was significantly lower at all the time-points
(P<0.05).
Serum levels of IL-10 were significantly higher in
the Fx group compared with the sham group, and it had two reverse
peak at 6 and 18 h after elevation at 1 h (P<0.05) (Fig. 3C). The IL-10 level sof the Fx+FC
group were significantly higher than the sham group at 1, 6, 9, 12,
18 and 24 h, which had two reverse peaks at 3 and 18 h after
elevation at 1 h. IL-10 levels of the Fx+FC group were obviously
reduced compared with the Fx group at 3 and 9 h.
These results suggest that FCE lowered the levels of
the proinflammatory cytokines that had been induced by
fracture.
Effects of FCE on injury related
enzyme post inflammatory response
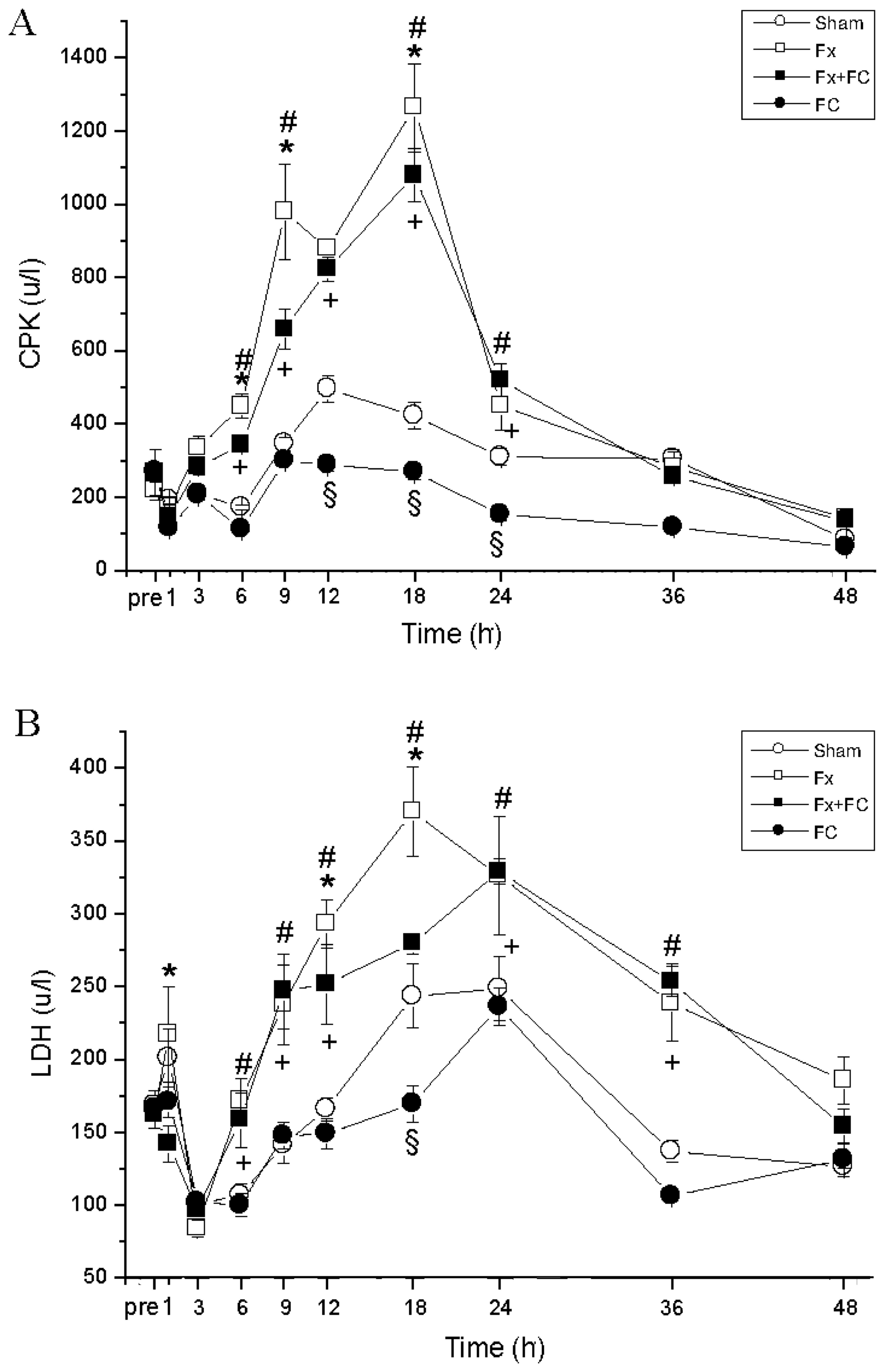
Plasma CPK level reduced mildly at 1 h, then
increased in all four groups (Fig.
4A). The CPK levels of the Fx and Fx+FC groups were
significantly higher than the sham group at 3, 6, 9, 12, 18 and 24
h (P<0.05), and peaked at 18 h. The CPK levels of the Fx+FC
group were lower at 6, 9 and 18 h compared to the Fx group.
Plasma LDH levels of the Fx group peaked at 1 and 18
h (Fig. 4B). The LDH levels of the
Fx and FC groups decreased at 1 and 3 h, and peaked at 24 h. The
LDH levels in the Fx+FC group were significantly lower at 1, 12 and
18 h, compared to the Fx group (P<0.05). LDH levels of the Fx
and FX+FC groups returned to prefracture level at 48 h.
Effects of FCE on the recruitment of
lymphocytes
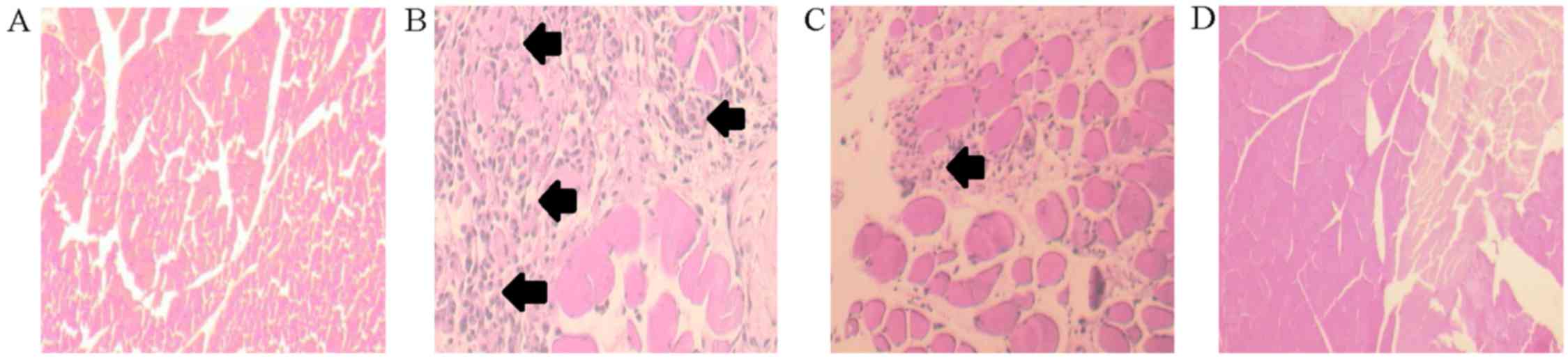
Histopathological analysis of the hematoxylin and
eosin-stained tissue sections was performed. There were no
significant changes over the muscle in the sham group (Fig. 5A); however, this analysis revealed
lymphocyte infiltration and hemorrhage of the muscle in the Fx
group (Fig. 5B). Less grouping of
lymphocytes and hemorrhage was noted in the Fx+FC group (Fig. 5C). There were no significant changes
over the muscle in the FC group (Fig.
5D). These findings suggest that FCE decreased the recruitment
of inflammatory cell induced by fracture.
Discussion
FCE is extracted from the freshwater clam
(Corbicula fluminea) in Taiwan. The nearest compositions of
100 g of powdered FCE include 53.4 g protein, 30.4 g carbohydrate,
1.1 g moisture, 11.0 g crude fat and 4.1 g ash. FCE contains many
bioactivities, including antihypertension, hepatoprotection,
antihypercholesterolemia, and antineoplasm (8). Lin et al (11) found that C. fluminea crude
extracts have the ability to suppress LPS-induced release of
pro-inflammatory cytokines by increasing IκB protein expression. In
the present study it was observed that FCE may effectively reduce
tachycardia that is predominantly caused by pain, fever and the
release of proinflammatory cytokines following fracture in rats.
Instead of analyzing throughout the precise quantities of active
gradients of FCE, we examined its anti-inflammatory effect with
monocyte-like THP-1 cells and LPS (1 µg/ml), as a stimulator of
inflammation. A prior study suggested that clam chloroform water
extract had the reverse effect on LPS-induced inflammation via an
in vitro method (9);
therefore, the present study was designed to elucidate whether it
had a consistent anti-inflammatory effect in an in vivo
study.
Previous studies have investigated the medication
influence on the callus formation and remodeling stages of bone
fracture (13,14), while the influence of FCE on the
acute stage of fracture is seldom studied. People who suffer from
traumatic fracture are often admitted for further surgery, medical
care of soft tissue, and administration of analgesics (1). Asians often choose to receive
traditional medicine therapy over general medical management under
acute trauma condition, such as Chinese herbs or acupuncture
(15,16). As such, FCE is a type of
Asian-accepted Chinese medicine that may be preferred. The goal of
the additional treatment rather than western medicine is to improve
the life quality of the patient at the acute stage, including vital
signs stabilization, soft tissue swelling faster subsidence and
smooth wound healing (16). In
previous studies, FCE appeared to effectively suppress the release
of pro-inflammatory TNF-α after hemorrhagic shock and protect the
liver function of the experimental animals (17,18). The
characteristic of anti-inflammatory and liver protection may
benefit acute stage of traumatic fracture. Establishment of a
conscious rat model (9,17) to observe the effect of FCE on the
acute stage of a fracture can simulate our recording vital signs
two or three times a day for closely observation the influence of
medical therapy on the acute stage. We recorded vital signs of the
rats to monitor their instant conditions during the experimental
period. The change of HR may show pain condition and CT may show
the severity of inflammation. The present results indicate that FCE
effectively stabilized the HR of the rats. The rats using FCE also
revealed more stable and normal CT compared to those treated with
normal saline. The results further suggest that FCE improved the
inflammatory condition, as assessed by pro-inflammatory cytokines,
and relieved pain, as observed via vital signs, caused by
fracturing. This suggests that FCE may be applied as adjuvant
therapy for medical treatment at the acute stage of traumatic
fracture.
In the present study we can see the fractured rats
treated with FCE exhibited lower and more stable levels of CPK,
LDH, TNF-α, IL-6 and IL-10 level during the 24-h period following
fracture. Fracture may be caused by a high energy contusion and
result in injury to the surrounding tissue. Both events may cause
local soft tissue damage and systemic inflammatory response.
Adequate inflammation condition is critical for cascades of bone
healing (19), but hyperinflammatory
status may exacerbate local tissue damage and vital organ injury
(5). The major proinflammatory
cytokines associated with response to trauma are TNF-α and IL-6.
These are predominantly produced by monocytes and macrophages and
cause a variety of frequently additive effects on the inflammatory
process at the acute stage (2).
TNF-α is an early regulator of the immune response and can induce
the release of secondary cytokines, such as IL-6 (2). IL-10 has both proinflammatory and
anti-inflammatory effects. The anti-inflammatory effect of IL-10
can reduce the synthesis of proinflammatory mediators at the later
period of the acute stage of trauma (4). From this result we know that FCE may be
able to relieve the inflammation and soft tissue injury of the
acute stage of fracture.
Decrease lymphocyte infiltration and muscle damage
and hemorrhage were also noted in our Fx+FC group. Adminssistration
of analgesics can have the same effect but may also damage the
liver and the kidney, which have been injured by acute trauma
condition (20,21). This is the first study to our
knowledge that is aimed at intervention at the acute stage of
traumatic fracture and application of FCE in this condition. On the
basis of the results of this study, we hypothesize that FCE can
stabilize the vital signs, improve systemic inflammation and local
soft tissue damage condition. These results suggest that FCE may
decrease fracture-induced inflammatory reactions and have
beneficial regulatory effect on post inflammatory response. Future
clinical trials are considered to be done to investigate the effect
of FCE on chronic phase of fracture healing.
Acknowledgements
This study was supported in part by the Hualien Tzu
Chi Hospital, Buddhist Tzu Chi Medical Foundation (grant no.
TCRD102-57).
References
|
1
|
Giannoudis PV: Current concepts of the
inflammatory response after major trauma: An update. Injury.
34:397–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hildebrand F, Pape HC and Krettek C: The
importance of cytokines in the posttraumatic inflammatory reaction.
Unfallchirurg. 108:793–794. 796–803. 2005.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pape HC, Marcucio R, Humphrey C, Colnot C,
Knobe M and Harvey EJ: Trauma-induced inflammation and fracture
healing. J Orthop Trauma. 24:522–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stensballe J, Christiansen M, Tønnesen E,
Espersen K, Lippert FK and Rasmussen LS: The early IL-6 and IL-10
response in trauma is correlated with injury severity and
mortality. Acta Anaesthesiol Scand. 53:515–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobbe P, Vodovotz Y, Kaczorowski DJ,
Billiar TR and Pape HC: The role of fracture-associated soft tissue
injury in the induction of systemic inflammation and remote organ
dysfunction after bilateral femur fracture. J Orthop Trauma.
22:385–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobbe P, Vodovotz Y, Kaczorowski DJ,
Mollen KP, Billiar TR and Pape HC: Patterns of cytokine release and
evolution of remote organ dysfunction after bilateral femur
fracture. Shock. 30:43–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glass GE, Chan JK, Freidin A, Feldmann M,
Horwood NJ and Nanchahal J: TNF-alpha promotes fracture repair by
augmenting the recruitment and differentiation of muscle-derived
stromal cells. Proc Natl Acad Sci USA. 108:pp. 1585–1590. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang YT, Huang YH, Hour TC, Pan BS, Liu
YC and Pan MH: Apoptosis-inducing active components from Corbicula
fluminea through activation of caspase-2 and production of reactive
oxygen species in human leukemia HL-60 cells. Food Chem Toxico.
44:1261–1272. 2006. View Article : Google Scholar
|
|
9
|
Lee RP, Subeq YM, Lee CJ, Hsu BG and Peng
TC: Freshwater clam extract decreased hemorrhagic shock-induced
liver injury by attenuating TNF-α production. Biol Res Nurs.
14:286–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu CL, Hsu CC and Yen GC:
Hepatoprotection by freshwater clam extract against CCl4-induced
hepatic damage in rats. Am J Chin Med. 38:881–894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CM, Lin YL, Tsai NM, Wu HY, Ho SY,
Chen CH, Liu YK, Chiu YH, Ho LP, Lee RP and Liao KW: Inhibitory
effects of chloroform extracts derived from Corbicula fluminea on
the release of pro-inflammatory cytokines. J Agric Food Chem.
60:4076–4082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
An Y, Friedman RJ, Parent T and Draughn
RA: Production of a standard closed fracture in the rat tibia. J
Orthop Trauma. 8:111–115. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chrastil J, Sampson C, Jones KB and
Higgins TF: Postoperative opioid administration inhibits bone
healing in an animal model. Clin Orthop Relat Res. 471:4076–4081.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savaridas T, Wallace RJ, Muir AY, Salter
DM and Simpson AH: The development of a novel model of direct
fracture healing in the rat. Bone Joint Res. 1:289–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong V, Cheuk DK, Lee S and Chu V:
Acupuncture for acute management and rehabilitation of traumatic
brain injury. Cochrane Database Syst Rev. 3:CD0077002013.
|
|
16
|
Kan'o T, Han JY, Nakahara K, Konno S,
Shibata M, Kitahara T and Soma K: Yokukansan improves distress of
medical staff, and cognitive function and motivation in patients
with destructive and aggressive behaviors after traumatic brain
injury. Acute Med Surg. 1:88–93. 2014. View
Article : Google Scholar
|
|
17
|
Huang KC, Wu WT, Yang FL, Chiu YH, Peng
TC, Hsu BG, Liao KW and Lee RP: Effects of freshwater clam extract
supplementation on time to exhaustion, muscle damage,
pro/anti-inflammatory cytokines, and liver injury in rats after
exhaustive exercise. Molecules. 18:3825–3838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua KF, Chen GM, Ho CL, Chen YL, Chen WJ,
Huang JF, Perng YS and Lin CC: Freshwater clam extract inhibits
inflammatory responses in LPS-activated macrophages by reducing the
activation of mitogen-activated protein kinases and NF-kappaB. Nat
Prod Commun. 7:1435–1440. 2012.PubMed/NCBI
|
|
19
|
Schmidt-Bleek K, Schell H, Schulz N, Hoff
P, Perka C, Buttgereit F, Volk HD, Lienau J and Duda GN:
Inflammatory phase of bone healing initiates the regenerative
healing cascade. Cell Tissue Res. 347:567–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ungprasert P, Cheungpasitporn W, Crowson
CS and Matteson EL: Individual non-steroidal anti-inflammatory
drugs and risk of acute kidney injury: A systematic review and
meta-analysis of observational studies. Eur J Intern Med.
26:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antoine DJ, Dear JW, Lewis PS, Platt V,
Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, et
al: Mechanistic biomarkers provide early and sensitive detection of
acetaminophen-induced acute liver injury at first presentation to
hospital. Hepatology. 58:777–787. 2013. View Article : Google Scholar : PubMed/NCBI
|