Introduction
The degeneration, necrosis and loss of retinal
pigment epithelial (RPE) cells may lead to retina and choroid
damage, which further results in the occurrence of retinal
degeneration diseases and even impaired vision (1,2). RPE has
a key role in maintaining the normal function of the retina,
particularly the photoreceptor in the optic nerve system (3,4). RPE
dysfunction, loss of vision and degradation are associated with
various diseases of the retina, such as retinitis pigment and
age-related macular degeneration (AMD) (5). In recent years, increasing reports have
investigated the role of RPE in cell pyroptosis, death,
inflammasome and lipofuscin phototoxicity (2,6).
LYTAK1 is a novel and specific transforming growth
factor-β (TGF-β)-activated kinase 1 (TAK1) inhibitor, which has
demonstrated anti-ovarian cancer function by suppressing ovarian
cancer growth (7). Research has
explored the capacity of LYTAK1 as an agent for targeting the
pro-oncogenic TAK1 in human cancer (8). LYTAK1 has been demonstrated to have
potential effects against KRAS mutant colorectal cancer (CRC) cells
both in vitro and in vivo, which suggested that
LYTAK1 may be exploited as a therapy method against CRC with KRAS
mutant (9). Recently, research has
indicated that TAK1 is involved in the autophagy process in RPE
cells, suggesting that aberrant activity of this kinase impairs
autophagy and subsequently leads to alterations in the vitality of
RPE cells (10). In addition, a
study by Dvashi et al (11)
demonstrated that inhibition of TAK1 expression accelerated
cellular senescence of RPE cells, which elucidated its role in
mechanisms underlying RPE cellular senescence induction in the
development of dry AMD. Notably, a previous report demonstrated the
suppressive effect of LYTAK1 on the proliferation of RPE cells
(12).
Previous reports have indicated that the
extracellular signal-regulated kinase (ERK)/protein kinase B
(AKT) signaling pathway is involved in ophthalmic diseases
(13,14). Activation of the E-cadherin-mediated
ERK/AKT signaling pathway is associated with cell-cell
interaction, which is important for the proliferation of MSCs
(15). In a previous study,
TGF-β-stimulated aberrant expression of class III β-tubulin via the
ERK signaling pathway was investigated in cultured RPE cells
(16). ERK/AKT
phosphorylation has been demonstrated to be involved in the
regulation of cell proliferation through anoctamin 6 deficiency,
which has a crucial role in C2C12 myoblast proliferation via
regulating the ERK/AKT signaling pathway (17). Furthermore, a study by Chong and
Zheng (18) indicated that
artemisinin could protect human RPE cells from hydrogen
peroxide-induced oxidative damage through activation of the
ERK/cyclic adenosine monophosphate response element-binding
protein signaling pathway. These reports suggest that the
ERK/AKT signaling pathway is involved in the pathological
process of RPE.
The purpose of the present study was to investigate
the role of LYTAK1 in human RPE cells and explore the potential
molecular mechanism of LYTAK1-mediated proliferation of human RPE
cells. In the present study, the results demonstrated that LYTAK1
may suppress the expression of TAK1 and TAK1-binding protein 2 in
TGF-β-induced epithelial-mesenchymal transition (EMT) processes in
RPE cells. In addition to this, LYTAK1 efficiently inhibited
proliferation of RPE cells through regulation of TGF-β-mediated EMT
via the ERK/AKT signal pathway.
Materials and methods
Cell culture and experimental
groups
The ARPE-19 human RPE cell line was obtained from
PromoCell GmbH (Heidelberg, Germany) and cultured in Dulbecco's
modified Eagle's medium supplemented with 1%
penicillin/streptomycin sulfate, 1% L-glutamine and 10%
fetal bovine serum (all from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a humidified atmosphere containing 5%
CO2 at 37°C. TGF-β1 and LYTAK1 were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Cells were treated
with TGF-β1 + LYTAK1 (25 µM) for 48 h to analyze the effects of
TGF-β1 and/or LYTAK1 on RPE cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The ARPE-19 human RPE cell line was cultured as
above. When the cells reached 85% confluence, the cells were
randomly divided into three groups: Control group, TGF-β1 treatment
group (10 µM, 48 h treatment) and TGF-β1 (10 µM) + LYTAK1 (25 µM)
treatment group (48 h treatment) at 37°C. Total RNA was isolated
from RPE cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and transcribed into cDNA using a Super Script
VILO cDNA Synthesis kit (Life Technologies) according to the
protocol provided by the manufacturer. All forward and reverse
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.) and are indicated in Table I.
Thermocycling conditions included 45 amplification cycles,
denaturation at 96°C for 120 sec, primer annealing at 62°C for 30
sec with touchdown to 58°C for 45 sec, and applicant extension at
72°C for 60 sec). Relative mRNA expression changes were calculated
by the 2−ΔΔCq method (19). The results were expressed as the
n-fold compared to the control.
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
|
| Sequence
direction |
|---|
|
|
|
|---|
| Gene name | Reverse | Forward |
|---|
| TAK1 |
5′-TCTATTTCACTCACACCAGCCCG-3′ |
5′-ATCCAAAGTACCGTTGAGGCTCC-3′ |
| Cadherin |
5′-CTCTTGTTGCCGTGATGAGA-3′ |
5′-GTTTATAGCCTGGGCACGAA-3′ |
| Fibronectin |
5′-AACATTTCTCAGCTATTGGCTT-3′ |
5′-CCATTGCAAATCGCTGCCAT-3′ |
| α-SMA |
5′-CACCATTCTGCCCAGGAGCA-3′ |
5′-TCCTGCTGGTCCTATTGGT-3′ |
| ERK |
5′-GCATCACTACACCCGAACAGA'-3′ |
5′-CAAGAACGGTCAGCAGGAAT-3′ |
| AKT |
5′-TGAGAGAAGCCACGCTGTC-3′ |
5′-CGGAGAACAAACTGGATGAA-3′ |
| TGF-β |
5′-ATCCATGTGTGACCATGAGGAAATG-3′ |
5′-TCGGCTAGTTAGGGTACACTTC-3′ |
| β-actin |
5′-CGGAGTCAACGGATTTGGTC-3′ |
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Western blot analysis
Cells were homogenized in 1X
radioimmunoprecipitation assay buffer and western blotting was
performed to analyze the protein expression. Briefly, protein
concentrations were examined using a BCA protein assay (Invitrogen;
Thermo Fisher Scientific, Inc.) and protein samples (40 µg) was
separated by 15% SDS-PAGE. Proteins were transferred into
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and the primary antibodies used in the immunoblotting assays
were TGF-β1 (1:1,000, ab66043), ERK (1:2,000, ab54230), AKT
(1:2,000, ab8805), cadherin (1:1,000, ab6528), fibronectin
(1:1,000, ab2413), α-smooth muscle actin (α-SMA; 1:1,000, ab5831;
all from Abcam, Shanghai, China) and β-actin (1:2,000, G8140;
United States Biological, Salem, MA, USA) for 12 h at 4°C following
blocking with 5% skimmed milk for 1 h at 37°C. Horseradish
peroxidase-conjugated IgG (Bio-Rad Laboratories, Inc.) was used at
a 1:5,000 dilution for 2 h at 37°C and detected using a Western
Blotting Luminol reagent (sc-2048; Santa Cruz Biotechnology Inc.,
Dallas, TX, USA).
Cell proliferation assay
RPE cell proliferation was detected using a Cell
Counting kit-8 (CCK-8; 96992MSDS; Merck KGaA; Darmstadt, Germany),
according to the manufacturer's instructions. Briefly, RPE cells
were cultured in 48-well plates at a density of 1×104
cells/well in Dulbecco's modified Eagle's medium (DMEM,
Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 5%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) for
24 h at 37 °C. RPE cells were treated with LYTAK1 (20 µM) for 48 h
at 37°C. Finally, 10 µl CCK-8 solution was added to each well and
incubated for 2 h at 37°C. The results were measured using a
microplate reader at 450 nm.
Transfection with small interfering
(si)RNA
All siRNA were synthesized by Invitrogen (Thermo
Fisher Scientific, Inc.) including siRNA-TGF-β1 (Si-TGF-β1 sense,
5′-CCAGAAAUACAGCAACAAUUU-3′ anti-sense,
5′-AUUGUUGCUGUAUUUCUGGUU-3′) or siRNA-vector (Si-vector sense,
5′-AAGTCGAGTCGCGTATGCAGGGCCTG-3′ anti-sense,
5′-AACCTGCATACGCGACTCGACC-3′). RPE cells (1×106 cells)
were transfected with 100 pmol Si-TGF-β1 targeting TGF-β1 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) or Si-vector as a
control (Applied Biosystems; Thermo Fisher Scientific, Inc.) using
a Cell Line Nucleofector kit L (Lonza Group, Ltd., Basel,
Switzerland), according to the manufacturer's instructions.
Wound healing assay
The wound healing assay was performed by following
the protocol provided in previous literature (20). RPE cells were cultured in DMEM in a
12-well plate and treated with Si-TGF-β1 or Si-vector for 48 h at
37°C. A wound was created in the cells. After washing with DMEM
medium to remove cell debris, the cells were allowed to migrate for
48 h at 37°C, followed by observation under a light microscope
(BZ-9000; magnification, ×40; Keyence Corporation, Osaka,
Japan).
Statistical analysis
Data were presented as the mean ± standard
deviation. Statistical differences were analyzed by one-way
analysis of variance followed by multiple comparisons performed
with post hoc Bonferroni tests. SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of LYTAK1 on TAK1 expression
in TGF-β1-induced RPE cells
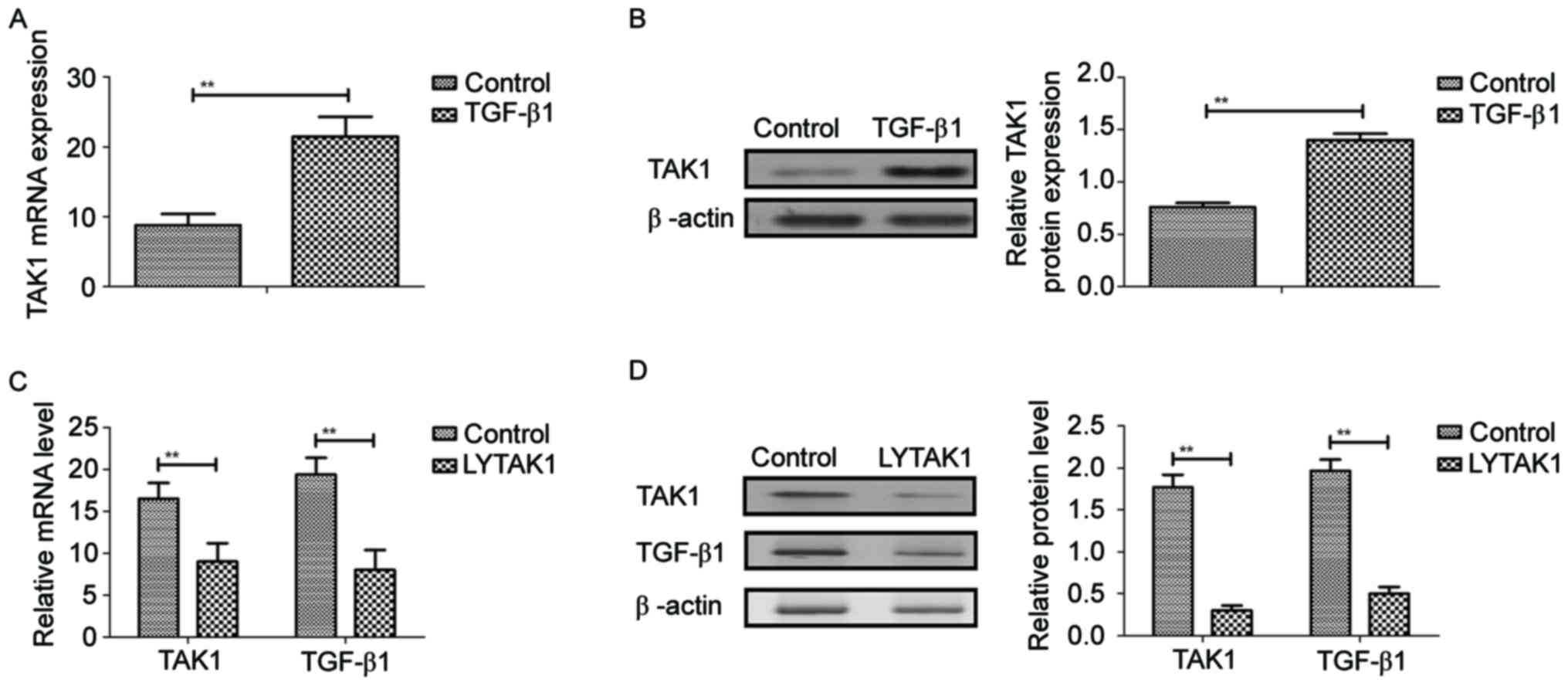
TAK1 expression was analyzed in TGF-β1-induced RPE
cells. Results demonstrated that TGF-β1 significantly induced TAK1
gene and protein expression in RPE cells compared with the control
(P<0.01; Fig. 1A and B). As
indicated in Fig. 1C and D, LYTAK1
significantly inhibited gene and protein expression levels of TAK1
and TGF-β1 in RPE cells compared with the controls. These results
suggested that LYTAK1 inhibited TAK1 expression induced by TGF-β1
in RPE cells.
Effects of LYTAK1 on TAK1-binding
protein expression in TGF-β-induced EMT of RPE cells
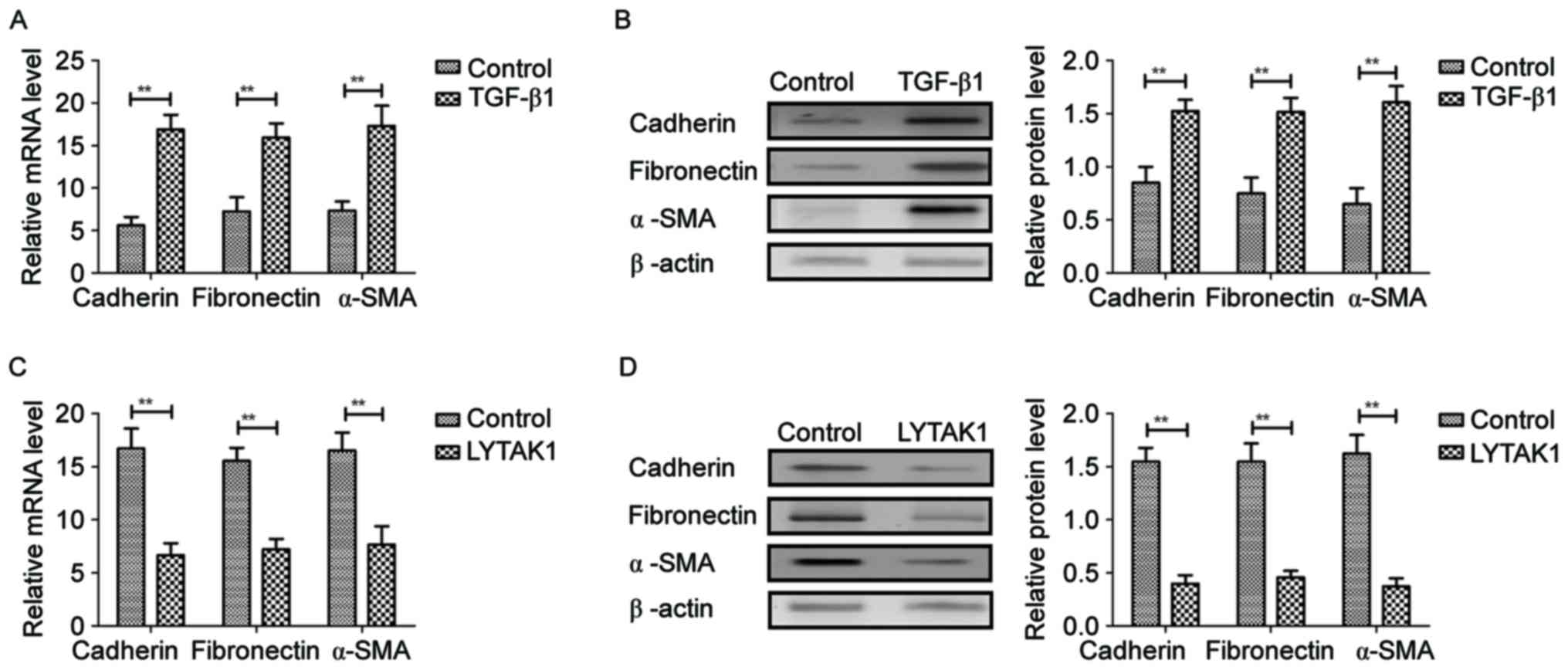
In order to analyze the effects of LYTAK1 on
TGF-β-induced EMT of RPE cells, expression levels of mesenchymal
markers were investigated in the present analysis. It was observed
that TGF-β1 significantly upregulated mRNA and protein expression
levels of cadherin, fibronectin and α-SMA in human RPE cells
compared with the controls (P<0.01; Fig. 2A and B). However, results
demonstrated that LYTAK1 administration significantly inhibited
mRNA and protein expression levels of cadherin, fibronectin and
α-SMA in human RPE cells compared with the controls (P<0.01;
Fig. 2C and D). These data suggested
that LYTAK1 targeting of TAK1 inhibits TAK1-binding protein
expression in TGF-β-induced EMT of RPE cells.
LYTAK1 ameliorates proliferation of
RPE cells and decreases ERK and AKT expression and
phosphorylation
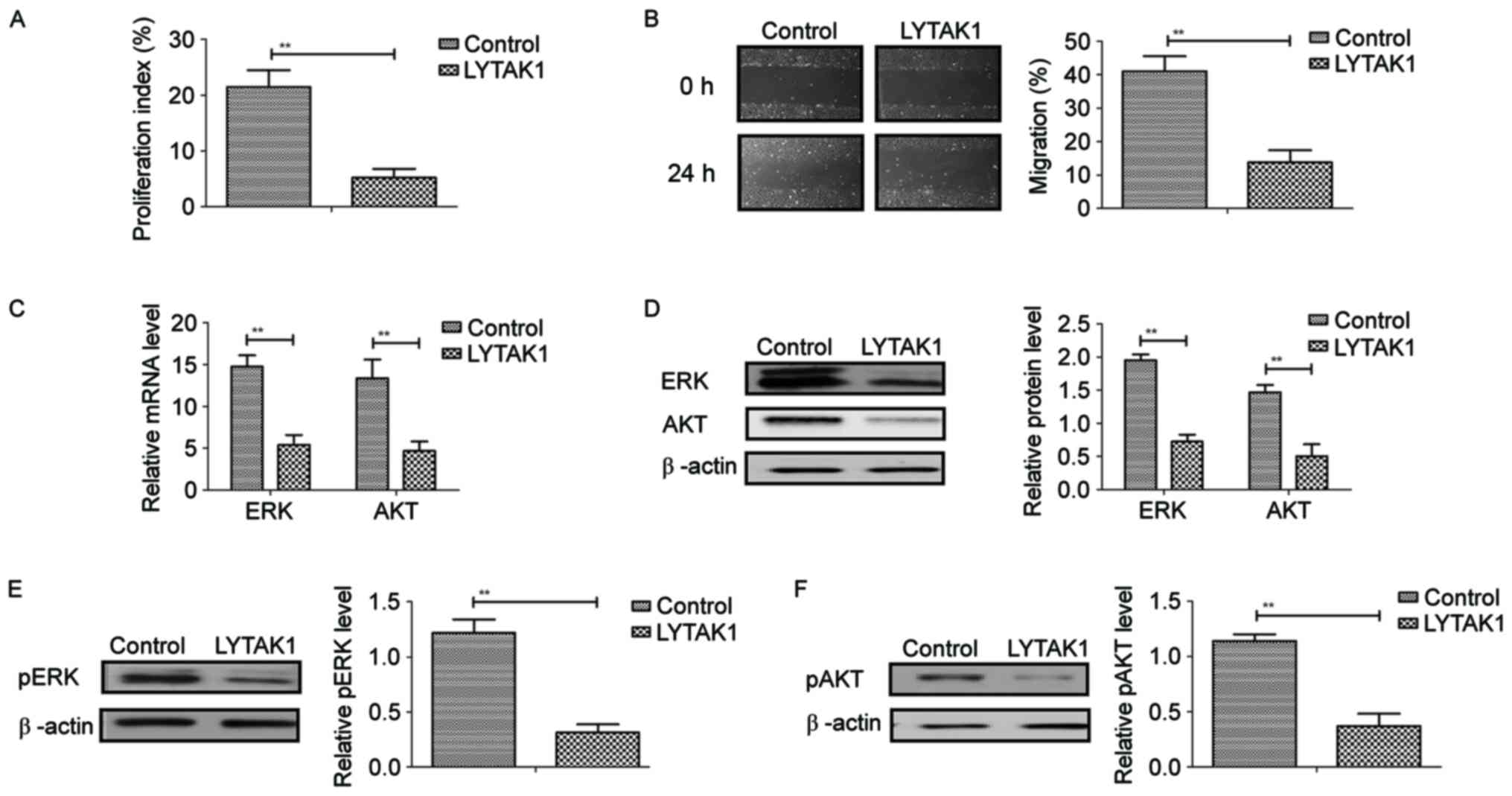
As demonstrated in Fig.
3A and B, LYTAK1 had significant inhibitory effects on RPE cell
proliferation and migration following treatment for 24 h compared
with the controls (P<0.01). Results indicated that LYTAK1
administration significantly decreased mRNA and protein expression
levels of ERK and AKT in RPE cells compared with the controls
(P<0.01; Fig. 3C and D).
Phosphorylation levels of ERK and AKT were significantly
downregulated by LYTAK1 in TGF-β-induced RPE cells compared with
controls (P<0.01; Fig. 3E and F).
These results suggested that LYTAK1 inhibited the proliferation of
RPE cells and decreased ERK and AKT expression and phosphorylation
in TGF-β-induced EMT of RPE cells.
LYTAK1 regulates proliferation of RPE
cells through inhibition of TGF-β-mediated EMT via the ERK/AKT
signaling pathway
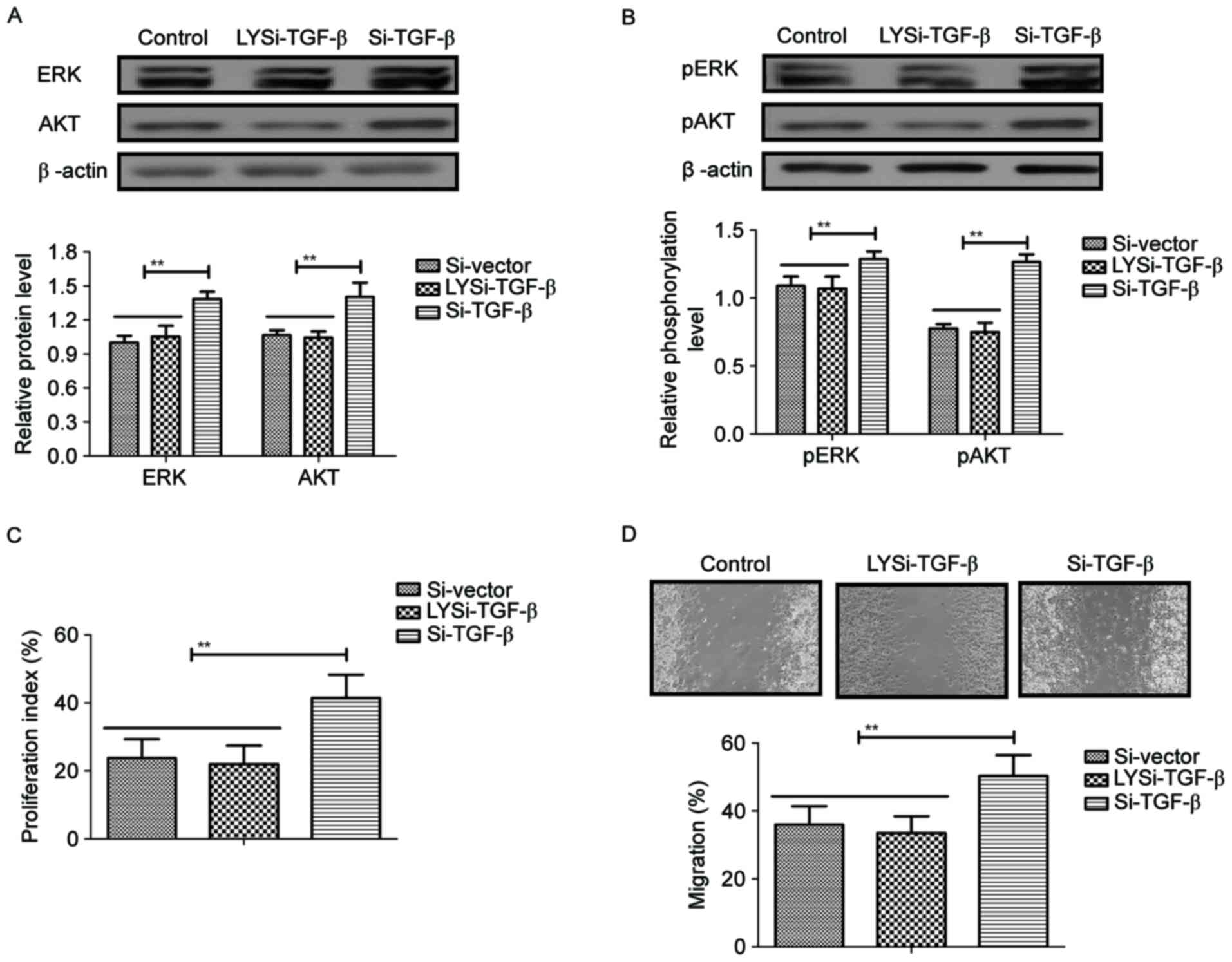
In order to analyze the potential mechanism of
LYTAK1-inhibited proliferation of RPE cells, the ERK/AKT
signaling pathway was investigated in RPE cells. It was
demonstrated that knockdown of TGF-β by Si-TGF-β blocked
LYTAK1-inhibited expression (LYSi-TGF-β) and phosphorylation levels
of ERK and AKT in RPE cells compared with cells transfected with
si-TGF-β (P<0.01; Fig. 4A and B).
LYTAK1-inhibited proliferation of RPE cells was also canceled by
knockdown of TGF-β (P<0.01; Fig.
4C). Results also demonstrated that knockdown of TGF-β
abolished the migration inhibition induced by LYTAK1 (P<0.01;
Fig. 4D). These results indicated
that LYTAK1 regulated proliferation of RPE cells through inhibition
of TGF-β-mediated EMT via the ERK/AKT signaling pathway.
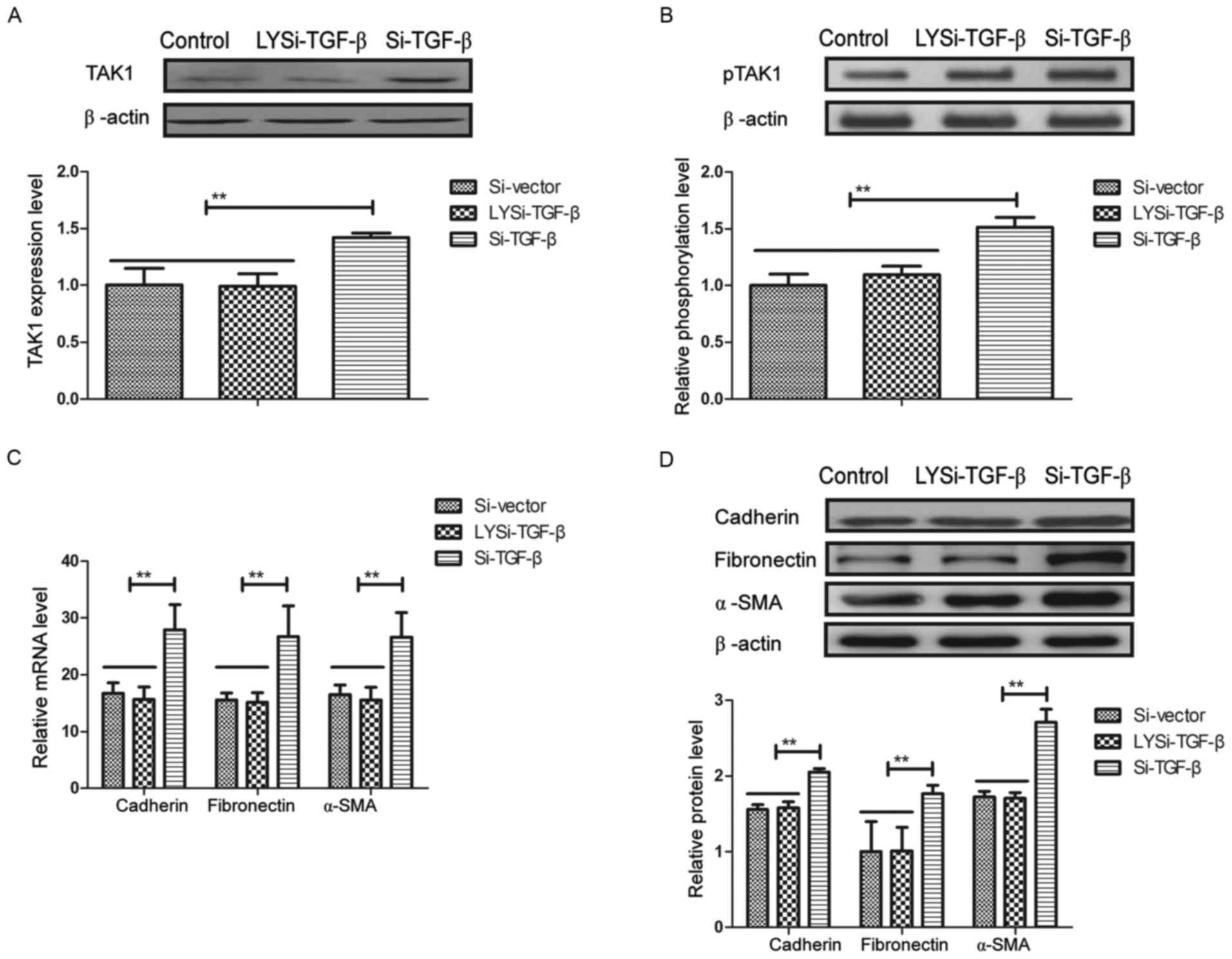
Knockdown of TGF-β inhibits
phosphorylation and activity of TAK1 in RPE cells
Effects of TGF-β knockdown on phosphorylation and
activity of TAK1 in RPE cells were further analyzed in RPE cells.
As demonstrated in Fig. 5A,
expression and phosphorylation levels of TAK1 were significantly
decreased by Si-TGF-β and Si-TGF-β abolished the inhibition induced
by LYTAK1 in RPE cells (P<0.01). Activity of TAK1 in RPE cells
mediated by LYTAK1 was also significantly abolished by Si-TGF-β in
RPE cells (P<0.01; Fig. 5B).
LYTAK1 downregulation of mRNA and protein expression levels of
cadherin, fibronectin and α-SMA were significantly inhibited by
TGF-β knockdown in human RPE cells (P<0.01; Fig. 5C and D). These results suggested that
knockdown of TGF-β inhibited phosphorylation and activity of TAK1
in RPE cells.
Discussion
Currently, proliferation and migration of RPE cells
has an essential role in the progression of proliferative
vitreoretinopathy (PVR) and other fibro-proliferative eye diseases
(21,22). Evidence has indicated that viability
of RPE cells is associated with various ophthalmic diseases through
regulation of EMT molecular markers (22). Research has also demonstrated that
RPE cell apoptosis is influenced by a combination of macrophages
and soluble mediators in the progression of AMD (11). Additionally, our previous study
suggested that TAK1 inhibitor could suppress proliferation and EMT
in RPE cells (12). In the present
study, the molecular mechanism of TAK1 inhibitor (LYTAK1) was
further explored in EMT of RPE cells. The present results indicated
that LYTAK1 administration suppressed TAK1 gene and protein
expression in human RPE cells, which further inhibited
proliferation and migration of human RPE cells in vitro. The
dose of LYTAK1 used in the present study has no toxicity to RPE
cultured cells as the total ERK and AKT proteins were downregulated
in cultured RPE cells. Notably, the findings indicated that LYTAK1
inhibited RPE cell proliferation mediated by the TGF-β-mediated
EMT/ERK/AKT signaling pathway, suggesting that TAK1
is a potential target for the treatment of RPE diseases.
Activity of RPE cells is involved in the
pathogenesis of PVR, which derives from EMT and further leads to
proliferation and migration of RPE cells (23,24). The
pathological significance of the EMT process in the progression of
PVR is becoming increasingly recognized. A study by Sheridan et
al (25) indicated that
migration of RPE cells is related to the pathology of PVR by
regulation of matricellular proteins, thrombospondin-1 and
osteonectin. In addition, research has suggested that proliferation
and migration of RPE-derived cells contributes to in taut
subretinal strands from patients with PVR (26). Furthermore, inhibition of DNA
methylation and methyl-CGP-binding protein 2 suppresses RPE
transdifferentiation, which further leads to PVR (27). The results of the present study
demonstrated that LYTAK1 downregulated TGF-β1 expression levels and
cadherin, fibronectin and α-SMA in EMT of human RPE cells, which
further resulted in inhibition of RPE cell proliferation.
Previous research has indicated that TGF-β
overexpression aggravates the proliferation and is associated with
apoptosis of RPE cells in vitro (28). A study by Alge-Priglinger et
al (29) suggested that negative
regulation of RPE cell attachment by carbohydrate-dependent cell
surface galectin-3 protein by inhibition of the
ERK-mitogen-activated protein kinase pathway disclosed a promising
nouveau perspective for treatment and prophylaxis of early PVR.
Research has also suggested that inhibition of the phosphoinositide
3-kinase/AKT and MEK/ERK signaling pathways
suppresses proliferation, migration and collagen I mRNA expression
in human RPE cells (30).
Additionally, regulation of AKT pathways inhibits the migration of
RPE cells, which further contributes to the improvement of PVR
(31–33). In the present study, results
demonstrated that the ERK/AKT signaling pathway was blocked
by LYTAK1 in human RPE cells. Furthermore, results indicated that
LYTAK1 regulated proliferation of RPE cells through inhibition of
TGF-β-mediated EMT via the ERK/AKT signaling pathway, while
knockdown of TGF-β inhibited phosphorylation and activity of TAK1
and decreased migration of RPE cells inhibited by LYTAK1.
In conclusion, the present study identified the role
of TAK1 inhibitor in TGF-β1-induced EMT in human RPE cells.
Findings suggested that LYTAK1 not only inhibited TAK1 expression
and TAK1-binding protein expression induced by TGF-β1, but also
decreased ERK and AKT expression and phosphorylation in RPE cells.
Notably, the underlying mechanism of LYTAK1 affects TGF-β-mediated
EMT via the ERK/AKT signaling pathway in RPE cells.
Therefore, the present results suggest that inhibition of TAK1
activity may be promising for the treatment of PVR.
Acknowledgements
This study was supported by the Health Science and
Technology Projects of Yunnan Province (grant no. 2016NS239) and
the Yunnan Applied Basic Research Projects-Union Foundation [grant
no. 2017FE468(−119)].
References
|
1
|
Devarajan G, Niven J, Forrester JV and
Crane IJ: Retinal pigment epithelial cell apoptosis is influenced
by a combination of macrophages and soluble mediators present in
age-related macular degeneration. Curr Eye Res. 41:1235–1244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen M, Rajapakse D, Fraczek M, Luo C,
Forrester JV and Xu H: Retinal pigment epithelial cell
multinucleation in the aging eye-a mechanism to repair damage and
maintain homoeostasis. Aging Cell. 15:436–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joshi R, Mankowski W, Winter M, Saini JS,
Blenkinsop TA, Stern JH, Temple S and Cohen AR: Automated
measurement of cobblestone morphology for characterizing stem cell
derived retinal pigment epithelial cell cultures. J Ocul Pharmacol
Ther. 32:331–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanus J, Anderson C, Sarraf D, Ma J and
Wang S: Retinal pigment epithelial cell necroptosis in response to
sodium iodate. Cell Death Discov. 2:160542016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee GY, Kang SJ, Lee SJ, Song JE, Joo CK,
Lee D and Khang G: Effects of small intestinal submucosa content on
the adhesion and proliferation of retinal pigment epithelial cells
on SIS-PLGA films. J Tissue Eng Regen Med. 11:99–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brandstetter C, Patt J, Holz FG and Krohne
TU: Inflammasome priming increases retinal pigment epithelial cell
susceptibility to lipofuscin phototoxicity by changing the cell
death mechanism from apoptosis to pyroptosis. J Photochem Photobiol
B. 161:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ying L, Chunxia Y and Wei L: Inhibition of
ovarian cancer cell growth by a novel TAK1 inhibitor LYTAK1. Cancer
Chemother Pharmacol. 76:641–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Zheng B, Ji J, Shen F, Min H, Liu
B, Wu J and Zhang S: LYTAK1, a novel TAK1 inhibitor, suppresses
KRAS mutant colorectal cancer cell growth in vitro and in vivo.
Tumour Biol. 36:3301–3308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green YA, Ben-Yaakov K, Adir O, Pollack A
and Dvashi Z: TAK1 is involved in the autophagy process in retinal
pigment epithelial cells. Biochem Cell Biol. 94:188–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dvashi Z, Green Y and Pollack A: TAK1
inhibition accelerates cellular senescence of retinal pigment
epithelial cells. Invest Ophthalmol Vis Sci. 55:5679–5686. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Mei Y, Lei H, Tian R, Ni N, Han F,
Gan S and Sun S: LYTAK1, a TAK1 inhibitor, suppresses proliferation
and epithelialmesenchymal transition in retinal pigment epithelium
cells. Mol Med Rep. 14:145–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou WW, Chen KC, Wang YS, Wang JY, Liang
CL and Juo SH: The role of SIRT1/AKT/ERK pathway in ultraviolet B
induced damage on human retinal pigment epithelial cells. Toxicol
In Vitro. 27:1728–1736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Q, Cao C, Lu S, Kivlin R, Wallin B,
Chu W, Bi Z, Wang X and Wan Y: MEK/ERK pathway mediates UVB-induced
AQP1 downregulation and water permeability impairment in human
retinal pigment epithelial cells. Int J Mol Med. 23:771–777. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ,
Lee SW, Jeon HB and Kim HS: Spherical bullet formation via
E-cadherin promotes therapeutic potency of mesenchymal stem cells
derived from human umbilical cord blood for myocardial infarction.
Mol Ther. 20:1424–1433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung EJ, Chun JN, Jung SA, Cho JW and Lee
JH: TGF-β-stimulated aberrant expression of class III β-tubulin via
the ERK signaling pathway in cultured retinal pigment epithelial
cells. Biochem Biophys Res Commun. 415:367–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao P, Torcaso A, Mariano A, Xu L, Mohsin
S, Zhao L and Han R: Anoctamin 6 regulates C2C12 myoblast
proliferation. PLoS One. 9:e927492014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chong CM and Zheng W: Artemisinin protects
human retinal pigment epithelial cells from hydrogen
peroxide-induced oxidative damage through activation of ERK/CREB
signaling. Redox Biol. 9:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stamp ME, Brugger MS, Wixforth A and
Westerhausen C: Acoustotaxis -in vitro stimulation in a wound
healing assay employing surface acoustic waves. Biomater Sci.
4:1092–1099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon OW, Song JH and Roh MI: Retinal
Detachment and Proliferative Vitreoretinopathy. Dev Ophthalmol.
55:154–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai FH, Lo EC, Chan VC, Brelen M, Lo WL
and Young AL: Combined pars plana vitrectomy-scleral buckle versus
pars plana vitrectomy for proliferative vitreoretinopathy. Int
Ophthalmol. 36:217–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García S, López E and López-Colomé AM:
Glutamate accelerates RPE cell proliferation through ERK1/2
activation via distinct receptor-specific mechanisms. J Cell
Biochem. 104:377–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palma-Nicolás JP, López E and López-Colomé
AM: Thrombin stimulates RPE cell motility by PKC-zeta- and
NF-kappaB-dependent gene expression of MCP-1 and CINC-1/GRO
chemokines. J Cell Biochem. 110:948–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheridan CM, Magee RM, Hiscott PS, Hagan
S, Wong DH, McGalliard JN and Grierson I: The role of matricellular
proteins thrombospondin-1 and osteonectin during RPE cell migration
in proliferative vitreoretinopathy. Curr Eye Res. 25:279–285. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winkler J and Hoerauf H: TGF-ß and
RPE-derived cells in taut subretinal strands from patients with
proliferative vitreoretinopathy. Eur J Ophthalmol. 21:422–426.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He S, Barron E, Ishikawa K, Khanamiri H
Nazari, Spee C, Zhou P, Kase S, Wang Z, Dustin LD and Hinton DR:
Inhibition of DNA methylation and methyl-CpG-binding protein 2
suppresses RPE transdifferentiation: Relevance to proliferative
vitreoretinopathy. Invest Ophthalmol Vis Sci. 56:5579–5589. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Enzmann V, Hollborn M, Wiedemann P and
Kohen L: Minor influence of the immunosuppressive cytokines IL-10
and TGF-beta on the proliferation and apoptosis of human retinal
pigment epithelial (RPE) cells in vitro. Ocular Immunol Inflamm.
9:259–266. 2001. View Article : Google Scholar
|
|
29
|
Alge-Priglinger CS, André S, Schoeffl H,
Kampik A, Strauss RW, Kernt M, Gabius HJ and Priglinger SG:
Negative regulation of RPE cell attachment by
carbohydrate-dependent cell surface binding of galectin-3 and
inhibition of the ERK-MAPK pathway. Biochimie. 93:477–488. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin D, Zheng XX and Jiang YR: Apelin-13
induces proliferation, migration, and collagen I mRNA expression in
human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol
Vis. 19:2227–2236. 2013.PubMed/NCBI
|
|
31
|
Tang B, Cai J, Sun L, Li Y, Qu J, Snider
BJ and Wu S: Proteasome inhibitors activate autophagy involving
inhibition of PI3K-Akt-mTOR pathway as an anti-oxidation defense in
human RPE cells. PLoS One. 9:e1033642014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bulloj A, Duan W and Finnemann SC: PI
3-kinase independent role for AKT in F-actin regulation during
outer segment phagocytosis by RPE cells. Exp Eye Res. 113:9–18.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheung YH, Sheridan CM, Lo AC and Lai WW:
Lectin from Agaricus bisporus inhibited S phase cell population and
Akt phosphorylation in human RPE cells. Invest Ophthalmol Vis Sci.
53:7469–7475. 2012. View Article : Google Scholar : PubMed/NCBI
|