Introduction
Nasal polyps, a common and frequently-occurring
disease in the upper respiratory tract, have a negative effect on
patient quality of life and are considered a heavy economic burden
for patients, their families and society in general (1,2). Nasal
polyps are divided into two types, according to the inflammatory
cells affected. One type consists primarily of eosinophils while
the other consists primarily of neutrophils (1,2). Each
type is associated with different immune histopathologic features.
Patients with the former type are predominantly encountered in
Europe and the United States and other Western countries, while
patients with the latter type are typically encountered in Asia
(1,3). The cause of nasal polyps is not
entirely clear; however, multiple factors, such as viruses,
bacteria, fungi and allergens have been implicated (1–3).
Although the use of intranasal corticosteroids and functional
endoscopic sinus surgery may significantly improve the cure rate of
nasal polyps, ~10% of patients will continue to experience
recurrent nasal polyps post-surgery (4). Therefore, the internal mechanisms of
nasal polyp formation require further elucidation.
In vivo studies of human diseases are often
limited in clinical settings due to ethical considerations and
other issues pertaining to patient safety; therefore,
disease-related animal models have been developed as a more
effective means of conducting such in vivo studies. Mice are
the typical laboratory animals used in disease models due to their
extensive availability, affordability and the closely related
genetic complements of mice and humans (5). Although several studies have been
conducted using mouse models of chronic rhinosinusitis (6–8), few
studies have used mouse models of nasal polyps. This paucity of
research has not been conducive to the study of the pathophysiology
of nasal polyps and has stunted the formulation of strategies for
the prevention of the disease. Kim et al (5,9)
successfully established the first mouse model of eosinophilic
nasal polyps using ovalbumin combined with staphylococcal
enterotoxin. However, to date, mouse models of neutrophilic nasal
polyps have not been reported domestically or internationally.
The bacterial endotoxin is a unique cell wall
structure of Gram-negative bacilli and is an exogenous pyrogen.
Thus, bacterial endotoxins are able to activate neutrophils to
release various types of endogenous pyrogen, which affect the
thermoregulatory center to cause fever (10). Lipopolysaccharides (LPS), one of the
principal chemical components of the bacterial endotoxin, are
located in the outer layer of the Gram-negative bacterial cell wall
and consist of three parts: Lipid A, core polysaccharide and
O-specific polysaccharide. In addition to its toxic effects, LPS is
able to induce an immune response (6,10).
Although neutrophilic nasal polyps have been associated with a
variety of bacterial infections, refractory nasal polyps have been
revealed to be closely associated with Gram-negative bacteria
infection and the endotoxins and LPS released by Gram-negative
bacteria are considered to be important pathogenic mechanisms
(11,12). While endotoxin may be a noninfectious
inflammatory factor, it is able to regulate the release of
inflammatory mediators, resulting in chronic rhinosinusitis
(10). Kim et al (6) dropped LPS into the nasal cavities of
rats in order to establish animal models of rhinosinusitis and 4
days following administration, the sinus mucosa of rats were
observed to be significantly thicker. This finding further
confirmed that noninfectious inflammatory factors were involved in
the occurrence and development of inflammatory diseases (10). Based on the known effects of LPS and
the results of the aforementioned studies, the present study was
conducted to explore the feasibility of establishing a mouse model
of nasal polyps with sufficient quantities of LPS administered over
a longer time frame. Furthermore, the present study aimed to
analyze the histopathological features of immunologic tissues in
the mouse model in order to further elucidate the roles and
mechanisms of LPS in the formation of nasal polyps.
Materials and methods
Animals
A total of 30 female C57BL/6J mice (6–8 weeks old;
weighing 1,823 g) raised and maintained under specific pathogen
free conditions were purchased from the Experimental Animal Center
of Wuhan University (Wuhan, China). The mice were maintained under
standard conventional conditions, consisting of a 12-h light/dark
cycle, temperature of 18–22°C and humidity of 50–60%. Food and
water were available ad libitum. Mice were kept in the
Renmin Hospital of Wuhan University. The present study protocol was
approved by the Institutional Animal Care and Use Committee of
Renmin Hospital of Wuhan University.
Experimental protocol
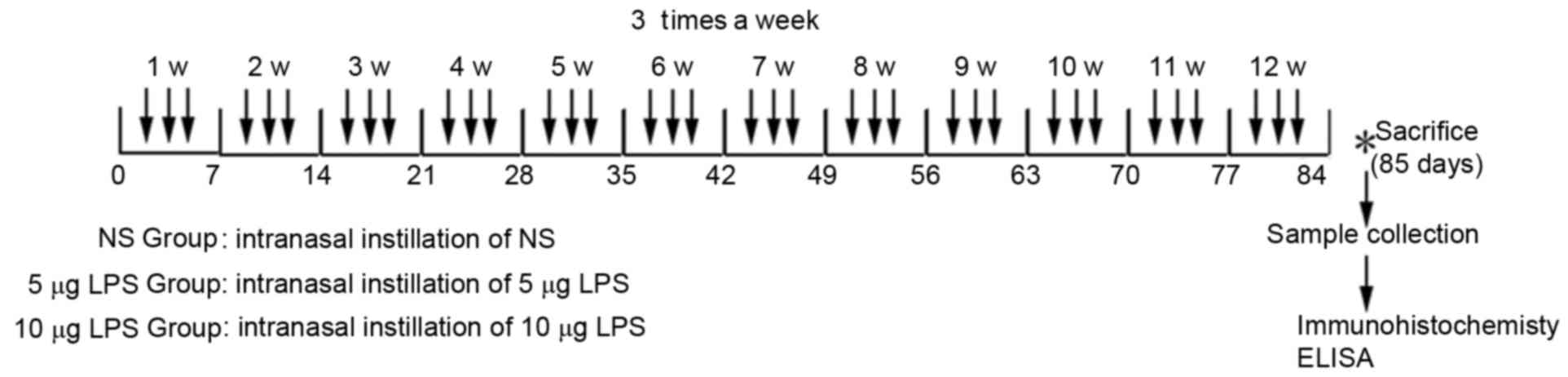
Mice were randomly divided into three groups of 10.
For the normal control group (NS group), 20 µl of sterile normal
saline solution was dropped into the nasal cavities three times a
week for 3 consecutive months. Mice in the low-(5 µg LPS group) and
high-dose (10 µg LPS group) model groups received 5 or 10 µg of LPS
(from Escherichia coli; Sigma-Aldrich, Merck Millipore,
Darmstadt, Germany), respectively, in 20 µl of sterile normal
saline solutions with a 10-µl transferpettor three times a week for
3 consecutive months (Fig. 1).
Histopathological analysis
Nasal tissue samples were handled in accordance with
previously described methods (7).
However, the lung, liver and kidney were not decalcified. In order
to facilitate direct observation of the distribution status of
inflammatory cells of the sinuses, 5 mice in each group were
randomly selected. Nasal lavage was not performed and the mice were
anesthetized by intraperitoneal injection of 1% sodium
pentobarbital (50 mg/kg), sacrificed with no pain and decapitated
24 h after the last nasal dropping. Tissue samples were fixed,
decalcified and embedded in paraffin. Coronal sections with a
thickness of 4 µm were subsequently obtained. The nasal tissues
were stained with hematoxylin-eosin to preliminarily assess the
degree of inflammation and to observe if polyp formation was
detected. The standards to determine the formation of nasal polyps
in mice were in accordance with previously published standards,
such as distinct mucosal bulges with neutrophilic infiltration
and/or microcavity formation (5,9).
Periodic acid-Schiff staining was used to reveal hypertrophy and
hyperplasia of goblet cells. Four continuous sections with similar
sinus anatomy in each mouse were selected for observation at a
magnification of ×400 (highpower fields), using the BX51 upright
microscope (Olympus Corporation, Tokyo, Japan). Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
was used to judge the area and density of the dyed region and the
integrated optical density (IOD) value of the immunohistochemistry
section. Goblet cells were indicated as the number of cells per mm
length of the epithelial basement membrane (N/mm). Nasal polyps in
a mouse were indicated as the total number of polyps in the nasal
cavities of each mouse.
Toll-like receptor 4 (TLR4), cluster
of differentiation (CD) 68 and myeloperoxidase (MPO)
immunohistochemistry
Immunohistochemistry was performed in accordance
with previously described methods (13). Paraffin sections were routinely
dewaxed, rehydrated and incubated with 3%
H2O2 at room temperature for 10 min to
inactivate endogenous peroxidase. The sections were placed into
0.01 M citrate antigen retrieval solution, repaired by microwaving
at 100°C for 10 min and cooled to room temperature prior to washing
three times with phosphate-buffered saline (PBS) for 5 min each.
Sections were subsequently blocked with 5% normal goat serum at
room temperature for 30 min and incubated overnight at 4°C with
anti-mouse TLR4 (cat. no. ab13556), CD68 (cat. no. ab125212) or MPO
(cat. no. ab9535) polyclonal antibodies (Abcam, Cambridge, UK) at a
dilution of 1:100. Following washing, the sections were incubated
with horseradish peroxidase-labeled Streptavidin-Biotin Complex
(1:200, cat. no. SA1029, Wuhan Boster Biotech Co., Ltd., Wuhan
China) at 37°C in a humidified chamber for 30 min and subsequently
washed three times with PBS for 5 min each. The sections were
developed with diaminobenzidine and washed immediately with
running water when brown particles appeared in the cytoplasm to
terminate the reaction. The sections were secondarily stained with
hematoxylin for 2 min. The Image-Pro Plus version 6.0 software
(Media Cybernetics, Inc.) was used to judge the area and density of
the dyed region and the IOD value of the immunohistochemistry
section, and immunohistochemical results were expressed as mean
optical density.
Nasal lavage and ELISA
Nasal lavage was performed according to a previously
described method with slight modification (13). Mice underwent partial tracheotomy
under deep anesthesia by intraperitoneal injection of 1% sodium
pentobarbital (50 mg/kg). A 22-gauge catheter was inserted into the
posterior naris from the opening of the trachea and along the
direction of the nostrils. Sterile saline solution (1 ml) was
perfused gently into the nasal cavities, lavage fluid was collected
from the anterior naris, centrifuged at 220 × g and 4°C for 10 min,
and the supernatant was stored at −20°C. Mice were sacrificed by
dislocation with no pain under anesthesia following the nasal
lavage. Cytokine expression levels in the supernatant were detected
by ELISA. Interleukin (IL)-4 ELISA kits (cat. no. DY404) were
purchased from R&D Systems Inc., Minneapolis, MN, USA.
Interferon (IFN)-γ (cat. no. EK0375), tumor necrosis factor (TNF)-α
(cat. no. EK0527) and IL-17 (cat. no. EK0431) ELISA kits were
purchased from Wuhan Boster Biotech Co., Ltd. The detection
sensitivities of all ELISA detection kits were <2 pg/ml. All
operation methods were performed in strict accordance with the
instructions provided in the kits.
Statistical analysis
All data are expressed as mean ± standard error of
the mean. The non-parametric Kruskal-Wallis test was used for
comparing among the different groups. The Mann-Whitney U Test was
used for pair-wise comparisons of any detected statistically
significant differences. IBM SPSS Statistics 19.0 (IBM SPSS,
Armonk, NY, USA) software was used for statistics analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
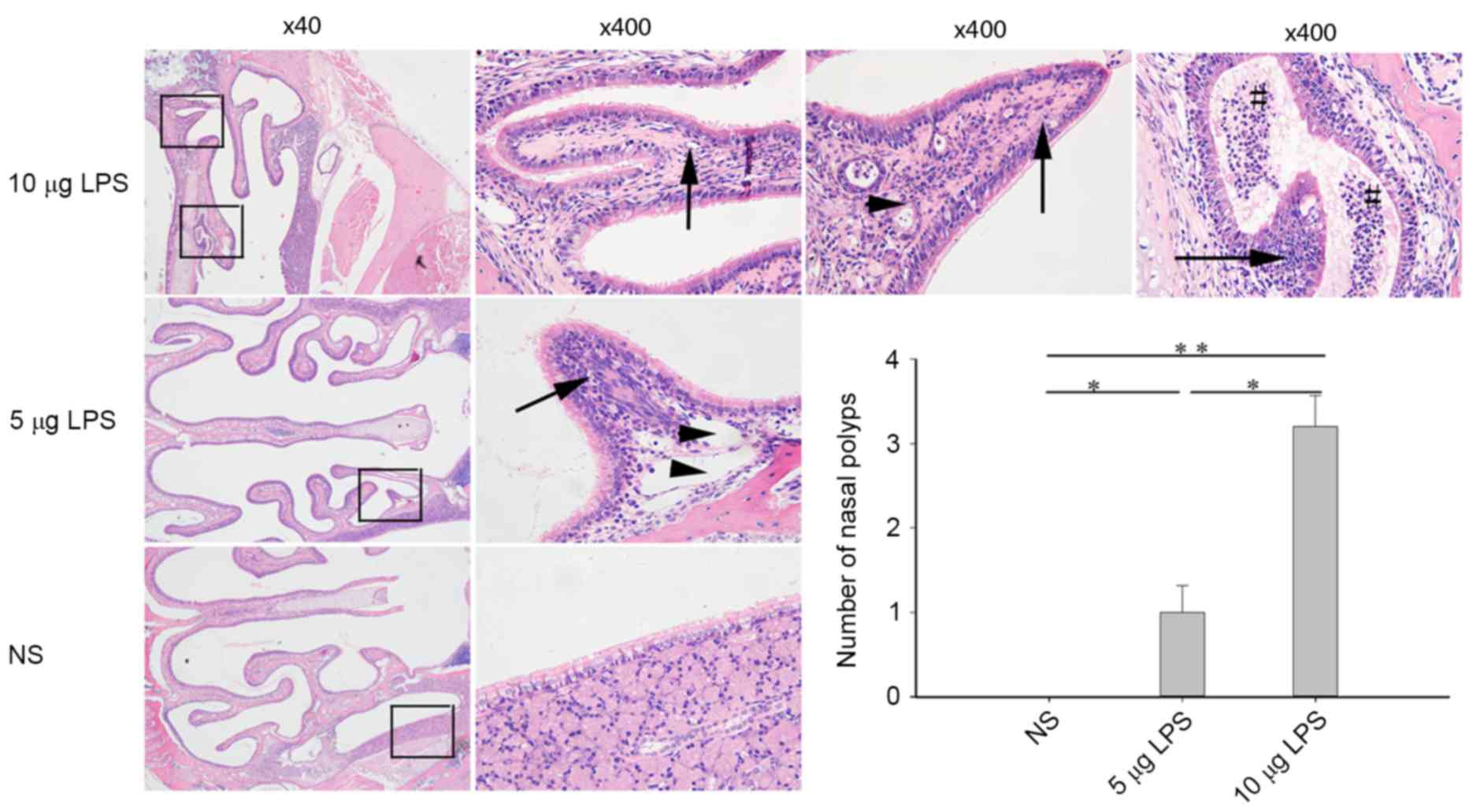
LPS induces nasal polyp formation in
model mice
The sinus mucosa in the control mice was intact and
evenly distributed. A small number of deciduous epithelial cells
and infiltrative inflammatory cells were observed within the
sinuses and no nasal polyp formation was indicated. Compared with
the control group, model mice exhibited sinus mucosa that were not
intact, which were accompanied by a large number of deciduous
epithelial cells and infiltrative inflammatory cells within the
sinuses. Furthermore, part of the mucosa proliferated to form
obvious single or multiple polyps that protruded into the nasal
cavities. These between-group differences were statistically
significantly different and the number of polyps observed were
significantly increased in the 5 µg LPS group (P<0.05) and the
10 µg LPS group (P<0.01) compared with the NS group. The nasal
polyps were most obvious in the high-dose group and were located
predominantly at the top of the nasal cavities and the lateral wall
of the nasal cavities (Fig. 2).
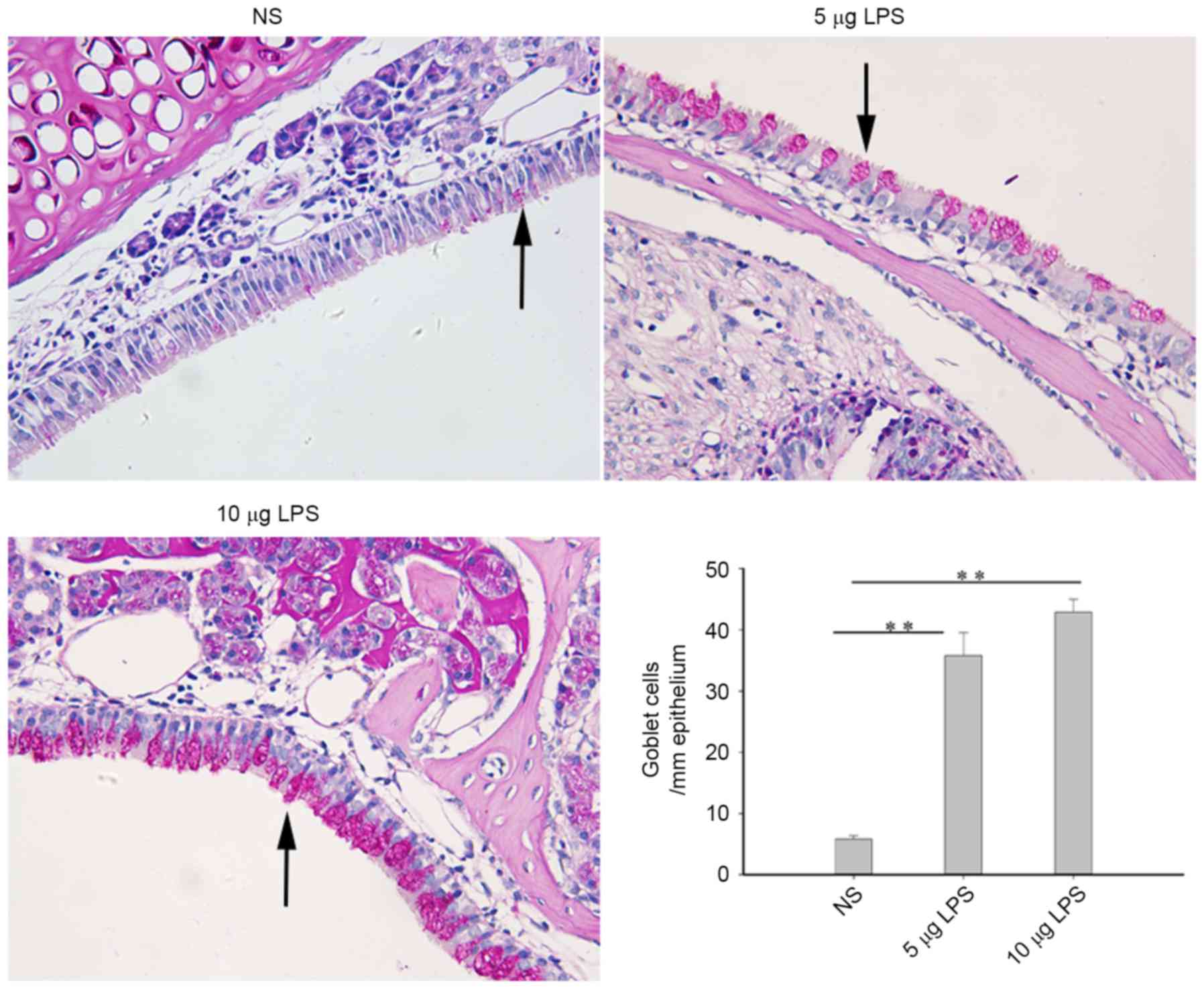
LPS increases nasal mucus gland
metaplasia in model mice
Mucus gland metaplasia is an important feature of
nasal polyps. Goblet cells were rarely observed in the sinus mucosa
of the control mice, such that only a small quantity of mucus
glands were observed under the sinus mucosa. Compared with the
control group, nasal goblet cells and mucus glands were
significantly increased in the mouse model groups (P<0.01);
however, no statistically significant differences were detected
between the 5 µg LPS group and 10 µg LPS group (Fig. 3).
LPS increases expression of TLR4, CD68
and MPO in the nasal tissues of model mice
The principal detected inflammatory cells present in
the nasal cavities and sinuses of mice were verified by
immunohistochemistry. Few CD68+ and MPO+
cells were indicated in the nasal cavities and sinuses of the
control mice. Compared with the NS group, CD68+ and
MPO+ cells were significantly increased in the 5 µg LPS
group (P<0.01) and 10 µg LPS group (P<0.01; Fig. 4). Furthermore, the present findings
revealed a significantly higher number of TLR4+ cells
were present in the nasal tissues of the 5 µg LPS group (P<0.01)
and 10 µg LPS group (P<0.01) when compared with the NS group
(Fig. 4). While the number of
MPO+ and TLR4+ cells present in the nasal
tissue of mice in the 10 µg LPS group were significantly increased
when compared with the 5 µg LPS group (P<0.05), no significant
difference was observed in the number of CD68+ cells
between these groups (Fig. 4).
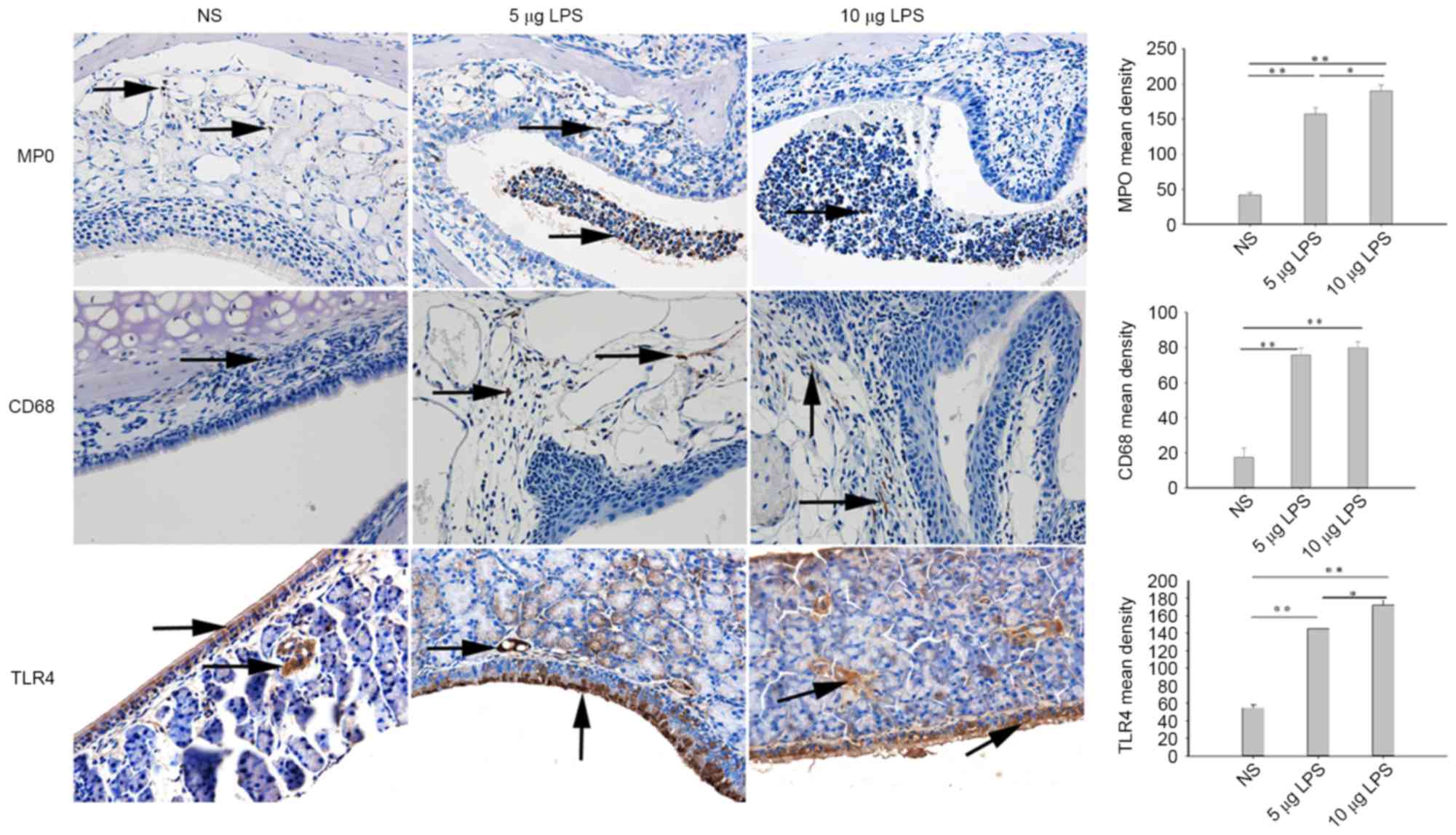 | Figure 4.LPS increased MPO, CD68, and TLR4
expression levels in the nasal mucosa, as detected by
immunohistochemistry using a Streptavidin-Biotin Complex (SABC) in
the nasal mucosa of mice (magnification, ×400). Yellow indicates
MPO, CD68, and TLR4-positive cells. *P<0.05; **P<0.01. Data
are presented as the mean ± standard error of the mean. LPS,
lipopolysaccharides; MPO, myeloperoxidase; TLR4, Toll-like receptor
NS, normal saline; NS group, control mice received normal saline
solution; 5 µg LPS group, mice received 5 µg of LPS; 10 µg LPS
group, mice received 10 µg of LPS. |
Facilitating effects of LPS on the
secretions of Th1 and Th17-related cytokines in the nasal lavage
fluids of model mice
Inflammatory cytokines have an important role in the
process of nasal polyp formation (1). The expression levels of inflammatory
cytokines IFN-γ, TNF-α, IL-17 and IL-4 in the nasal cavities of
mice in the control group were low. Compared with the NS group, the
IFN-γ, TNF-α and IL-17 protein levels exhibited in the nasal lavage
fluids were significantly upregulated in the 5 µg LPS group
(P<0.01) and 10 µg LPS group (P<0.05; Fig. 5). IL-4 levels were significantly
increased in the 5 µg LPS group (P<0.05) when compared with the
NS group; however, no significant difference in IL-4 levels was
observed between the 10 µg LPS group and the NS group (Fig. 5).
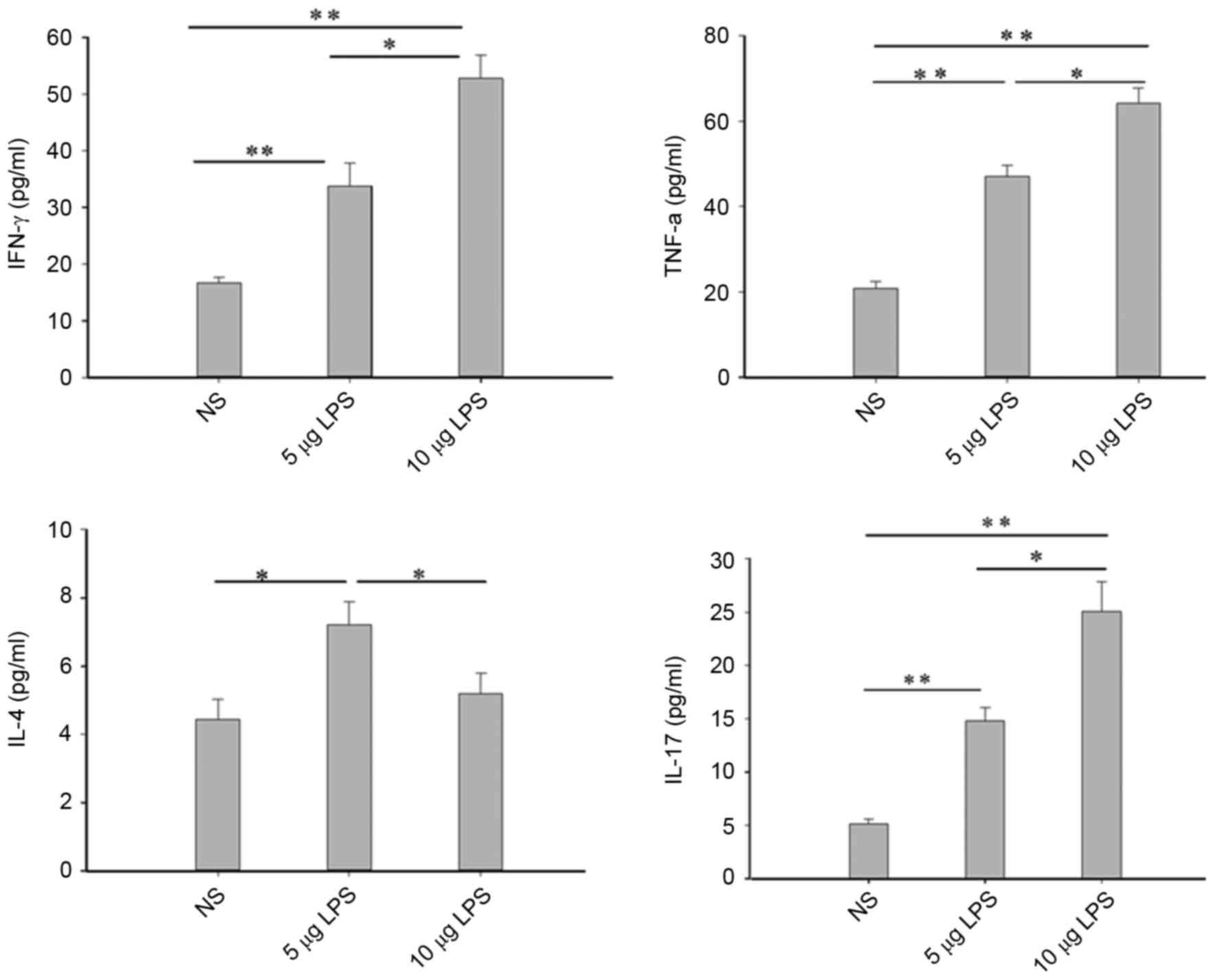 | Figure 5.LPS increased IFN-γ, TNF-α, IL-17 and
IL-4 (only in the 5 µg LPS group) levels in NLF. Cytokine levels in
NLF were assessed by ELISA. *P<0.05; **P<0.01. Data are
presented as the mean ± standard error of the mean. LPS,
lipopolysaccharides; IFN, interferon; IFN-γ, gamma interferon;
TNF-α, tumor necrosis factor-α; IL, interleukin; NLF, nasal lavage
fluid; NS, normal saline; NS group, control mice received normal
saline solution; 5 µg LPS group, mice received 5 µg of LPS; 10 µg
LPS group, mice received 10 µg of LPS. |
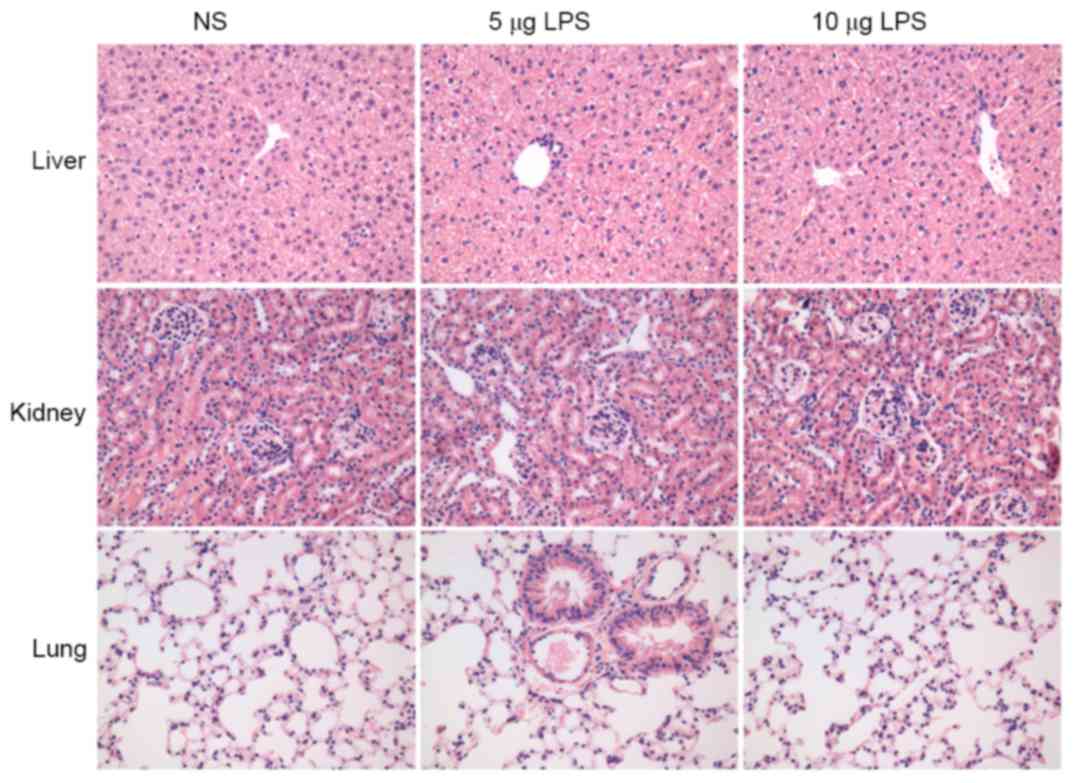
Safety of LPS used in nasal cavities
of mice
To evaluate if LPS causes pathological damage to the
vital organs of experimental animals, routine hematoxylin and eosin
staining of the sections of the murine lung, liver and kidney of
the mice in different groups was performed. No inflammation,
necrosis or structural disorders in the lung, liver and kidney were
observed in all groups, suggesting that LPS had no systematic toxic
or side effects on the vital organs of the experimental animals
(Fig. 6).
Discussion
Nasal polyps may be divided into two types,
eosinophilic and neutrophilic. The eosinophilic nasal polyps are
accompanied by significantly increased Th2 cells and M2 macrophages
that may be established in animal models (5,14).
However, neutrophilic nasal polyps with more Th1 and Th17 cells are
typically observed in those from Asian countries (1,3). To the
best of our knowledge, this is the first time a successfully
established mouse model of nasal polyps using LPS has been
described. The neutrophils accounted for the majority of
infiltrating inflammatory cells in the nasal polyps, accompanied by
increased macrophages and enhanced expression of Th1- and
Th17-related cytokines. Therefore, this model is consistent with
the immune histopathologic features of major nasal polyps that may
be typically exhibited in those of Asian descent (1,3).
Nasal polyp formation results from a combination of
individual susceptibility and environmental factors. Viruses,
bacteria, fungi, allergens and alternative factors all have an
important role in nasal tissue remodeling and the eventual
formation of nasal polyps (2).
Eosinophilic nasal polyps are primarily related to allergens
(1,14) and widely used endoscopic nasal
surgery and long-term postoperative use of glucocorticoids have
significantly increased the cure rate of eosinophilic nasal polyps
(15). However, the pathogenesis of
the neutrophilic nasal polyps is more complex and is typically
combined with the concurrence of a variety of factors, which
contribute to the difficulty in treating polyps (16). For this reason, it is considered
important to establish animal models of neutrophilic nasal polyps.
It is worth mentioning that bacterial infections associated with
polyp formation are typically mixed infections (17) and the results of several studies have
identified that the Gram-negative bacterial infections in
rhinosinusitis were closely associated with drug resistance and
postoperative recurrence (11,12,18,19).
Furthermore, it has been reported that following effective drug
treatment, the LPS released by Gram-negative bacteria may persist
for up to 3 months (6).
LPS is an abundant component that may be present
in vivo or in vitro and particularly low doses of LPS
may cause a bioactive effect, including poisoning of the body or
provocation of an immune reaction (10). Long-term respiratory exposure to a
sufficient concentration of LPS from the environment may result in
sinusitis and pneumonia (10). LPS
mediates biological effects through interactions with several
different proteins, including LPS-binding protein (LBP), CD14 and
TLR4. LBP initially binds directly to LPS and combines with CD14 to
form a complex recognized and bound by TLR4 (20–23).
Once internalized, LPS is able to activate several intracellular
signaling pathways that include the IκB kinase-nuclear factor-κB
pathway and three mitogen-activated protein kinase (MAPK) pathways,
as well as initiate the production of a variety of inflammatory
cytokines (20–22). LPS/TLR4 signaling pathways may be
divided into MyD88-dependent and non-dependent pathways, the former
mediating the release of pro-inflammatory cytokines, including
IL-1β, IL-6, IL-8 and TNF-α. The latter pathway mediates the
secretion of type I IFN (22,23). The
present study indicated that the expression levels of TLR4, TNF-α
and IFN-γ in the nasal mucosa were significantly upregulated
following repeated intranasal LPS instillation, suggesting that
both MyD88-dependent and non-dependent pathways may have been
involved in biological effects of LPS. The present findings also
revealed that IL-17 expression was significantly increased in the
model groups when compared with the control group. Consistent with
previous findings, LPS may regulate the secretion of IL-17 in a
variety of cell types through TLR4 receptors (24–26). In
addition, LPS may i) increase secretions of adhesion molecules,
such as E-selectin in endothelial cells (10,27); ii)
induce the cellular proliferation, prostaglandin E2 production, and
expression of cyclooxygenase-2 and matrix metalloproteinase-9 in
peripheral blood mononuclear cells (20); iii) and promote the adhesion and
migration of inflammatory cells into peripheral tissues. The
expression of a variety of inflammatory cytokines may result in
nasal tissue remodeling and the formation of nasal polyps,
including goblet cell metaplasia, inflammatory cell infiltration,
tissue fibrosis and tissue edema (1,2). Nasal
polyps may cause mechanical obstruction, including ventilation and
drainage obstacles such as nasal congestion, runny nose, dizziness
and olfactory dysfunction (1,3).
The present results demonstrated that the expression
of CD68 in nasal mucosa was increased following intranasal
administration of 5 or 10 µg of LPS in mice. However, it was not
further distinguished whether the increased numbers of cells were
M1 or M2 macrophages, although previous studies have indicated that
LPS predominantly induces an increase in M1 macrophages (28,29).
Therefore, the increased CD68+ cells observed in the
present study may be primarily M1 macrophages. Previous studies
have suggested that very low doses of LPS significantly promoted
ovalbumin-induced allergic inflammation, whereas larger doses of
LPS inhibited ovalbumin-induced allergic inflammation (30,31). In
the present study, the levels of IL-4 protein present in nasal
lavage liquids were measured and the results demonstrated that the
expression levels of IL-4 in the nasal cavities were significantly
increased following intranasal administration of 5 µg of LPS;
however, no statistically significant difference was observed in
the 10 µg LPS group compared with the NS group. This suggests that
the current LPS dose of 5 or 10 µg was large enough to induce
inflammation consisting primarily of neutrophils, which was further
administrated by increased CD68+ macrophages,
MPO+ cells, IFN-γ, TNF-α and IL-17, which is consistent
with results from previous studies (26,30,31). LPS
commonly induces allergic reactions with the help of allergens,
such as ovalbumin. Different doses of LPS may alter the type or
extent of inflammation (26,30,31).
However, to the best of our knowledge, LPS alone has not been
demonstrated to induce an allergic reaction. In the present study,
LPS without allergens was used to establish a model of nasal
polyps, and therefore the polyps were not attributable to
allergens; however, allergens may induce the formation of the
polyps in other cases. The results of the present study suggest
that different doses of LPS lead to inconsistent types of
inflammation, a result similar to those of previous studies
(26,30,31).
Kim et al (6)
established a mouse model of rhinosinusitis with LPS and indicated
marked thickening of the sinus mucosa without obvious polyp
formation. These results may have been attributable to a modeling
time that was too short as the study was completed in 3 days. The
present study extended the modeling time to 3 months and
successfully established a mouse model of neutrophilic nasal
polyps. Based on an analysis of the morphological characteristics
of the nasal polyps, it was revealed that the formation of nasal
polyps in mice was primarily achieved by two approaches. One
approach was through nasal tissue remodeling to form nasal polyps.
Under the influence of inflammatory stimulation, secretion of the
extracellular matrix increased and parts of the sinus mucosa became
damaged and fell off into the sinus cavities. This prompted the
sinus mucosa and inflammatory cells in the sinus cavities to form a
tissue mass that was wrapped annularly by the proliferated basement
membrane, causing hypoxia inside the tissue mass. Under the action
of the multiple factors, such as hypoxia-inducible factors and
inflammatory factors, angiogenesis was enhanced, the tissues were
remodeled, and the structure of a typical nasal polyp formed.
Another approach involved the proliferation and uplifting of local
tissues. Affected by repeated inflammatory stimulation, the sinus
mucosa became part of the substrate and local hyperplasia of the
mucosa was formed into small upheavals, which subsequently turned
into nasal polyps.
In the present study, LPS was used by the way of
intranasal instillation, not by intravenous injection, which
avoided systematic toxic or side effects resulting from the
intravenous injection on the vital organs of the experimental
animals. Since LPS is produced by various gram-negative bacteria
and tends to form aggregates of varying sizes, various types of
LPS, which may produce different inflammatory responses, require
further investigation. In the present study, only LPS selected from
E. coli was investigated as E. coli are common
Gram-negative bacteria associated with nasal polyps (17). It is possible to obtain the same
result after colonizing the nose with LPS-producing bacteria.
Producing LPS is one of the important mechanisms of pathogenicity
of Gram-negative pathogenic bacteria (10). Released LPS may be maintained for 3
months in local tissue to stimulate the releasing of inflammatory
mediators, even in the instance that Gram-negative bacteria did not
survive (6). However, no mouse of
nasal polyps has been established by implanting Gram-negative
bacteria into the nasal cavity, which requires further
investigation.
Toll-like receptors (TLRs) include TLR1-9 subtypes.
Various receptors are able to specifically bind to different
ligands, such as TLR4 and LPS, TLR3 and viral ds-RNA, TLR2 and
lipoproteins, and fat polypeptides and yeast polysaccharides, all
of which may have roles in neutrophilic nasal polyps (32–34).
However, it has not been reported whether administering TLR2 or
TLR3 ligands may result in the formation of neutrophilic nasal
polyps in an animal model. In the present study, TLR4 was chosen as
the research subject based on the role of Gram-negative bacteria in
the pathogenesis of chronic rhinosinusitis and nasal instillation
of LPS in rats inducing significant inflammatory inflammation
(6,17). Nasal polyps were the result of tissue
remodeling of the nasal mucosa during chronic inflammatory
stimulation and would persist even if stimulation was not required.
Nasal polyps did not automatically disappear without the treatment
of drugs or surgery when they formed (2). Therefore, in the present study, the
time was not extended for experiments when the animals developed
significant nasal polyps after 90 days.
In conclusion, the current study established a mouse
model of nasal polyps using LPS and provided a preliminary analysis
of the characteristics of nasal polyps using histopathological
methods. The predominant inflammatory cells identified in the nasal
polyps were neutrophils, macrophages and higher levels of Th1- and
Th17-related cytokines. Furthermore, the established model met with
the histological features of nasal polyps that primarily consist of
neutrophils, which are typically observed in Asian patients. The
present study revealed that LPS may have a role in the process of
nasal polyp formation through the TLR4 receptor pathway.
Acknowledgements
The present study was supported by the Hubei
Province Health and Family Planning Scientific Research Project
(grant no. WJ2015Z088) and the National Nature Science Foundation
of China (grant no. 81372880 and 81300813).
References
|
1
|
Cao PP, Li HB, Wang BF, Wang SB, You XJ,
Cui YH, Wang DY, Desrosiers M and Liu Z: Distinct immunopathologic
characteristics of various types of chronic rhinosinusitis in adult
Chinese. J Allergy Clin Immunol. 124(478–484): 484.e1–e2. 2009.
|
|
2
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European position paper on rhinosinusitis and nasal polyps
2012. Rhinol Suppl. 23(3): p preceding table of contents. 1–298.
2012.
|
|
3
|
Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia
W, Luo Q, Zheng J, Wang H, Li Z, et al: Increased neutrophilia in
nasal polyps reduces the response to oral corticosteroid therapy. J
Allergy Clin Immunol. 129:1522–1528.e5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith KA and Rudmik L: Impact of continued
medical therapy in patients with refractory chronic rhinosinusitis.
Int Forum Allergy Rhinol. 4:34–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DW, Khalmuratova R, Hur DG, Jeon SY,
Kim SW, Shin HW, Lee CH and Rhee CS: Staphylococcus aureus
enterotoxin B contributes to induction of nasal polypoid lesions in
an allergic rhinosinusitis murine model. Am J Rhinol Allergy.
25:e255–e261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DH, Jeon EJ, Park SN, Park KH, Park YS
and Yeo SW: Effects of a tumor necrosis factor-a antagonist on
experimentally induced rhinosinusitis. J Biomed Biotechnol.
2011:3604572011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Lu X, Cao PP, Chu Y, Long XB,
Zhang XH, You XJ, Cui YH and Liu Z: Histological and immunological
observations of bacterial and allergic chronic rhinosinusitis in
the mouse. Am J Rhinol. 22:343–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin M, Gu Z, Bian Z, Yang J, Cao Z, Yu X
and Guo G: Developing a mouse model of acute bacterial
rhinosinusitis. Eur Arch Otorhinolaryngol. 268:857–861. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SW, Kim JH, Jung MH, Hur DG, Lee HK,
Jeon SY and Kim DW: Periostin may play a protective role in the
development of eosinophilic chronic rhinosinusitis with nasal
polyps in a mouse model. Laryngoscope. 123:1075–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rylander R: Endotoxin in the
environment-exposure and effects. J Endotoxin Res. 8:241–252. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grindler D, Thomas C, Hall GS and Batra
PS: The role of Stenotrophomonas maltophilia in refractory chronic
rhinosinusitis. Am J Rhinol Allergy. 24:200–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rombaux P, Collet S, Hamoir M, Eloy P,
Bertrand B, Jamart F and Gigi J: The role of nasal cavity
disinfection in the bacteriology of chronic sinusitis. Rhinology.
43:125–129. 2005.PubMed/NCBI
|
|
13
|
Wang SB, Deng YQ, Ren J, Xiao BK, Liu Z
and Tao ZZ: Exogenous interleukin-10 alleviates allergic
inflammation but inhibits local interleukin-10 expression in a
mouse allergic rhinitis model. BMC Immunol. 15:92014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peterson S, Poposki JA, Nagarkar DR,
Chustz RT, Peters AT, Suh LA, Carter R, Norton J, Harris KE,
Grammer LC, et al: Increased expression of CC chemokine ligand 18
in patients with chronic rhinosinusitis with nasal polyps. J
Allergy Clin Immunol. 129(119–127): e1–e9. 2012.
|
|
15
|
Potter PC and Pawankar R: Indications,
efficacy, and safety of intranasal corticosteriods in
rhinosinusitis. World Allergy Organ J. 5 Suppl 1:S14–S17. 2012.
View Article : Google Scholar
|
|
16
|
Mitroi M, Căpitănescu A, Georgescu CV,
Mogoantă CA, Popescu C, Georgescu M, Mitroi G and Ioniţă E:
Expression pattern of CK7 and CK20 in nasal polyps, at patients
with chronic rhinosinusitis with nasal polyposis. Rom J Morphol
Embryol. 52 3 Suppl:S1051–S1057. 2011.
|
|
17
|
Dlugaszewska J, Leszczynska M, Lenkowski
M, Tatarska A, Pastusiak T and Szyfter W: The pathophysiological
role of bacterial biofilms in chronic sinusitis. Eur Arch
Otorhinolaryngol. 273:1989–1994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rom D, Snidvongs K, Sacks PL, Dalgorf D,
Pratt E, Earls P, Sacks R and Harvey RJ: The impact of culturable
bacterial community on histopathology in chronic rhinosinusitis.
Int Forum Allergy Rhinol. 4:29–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharyya N and Kepnes LJ: The
microbiology of recurrent rhinosinusitis after endoscopic sinus
surgery. Arch Otolaryngol Head Neck Surg. 125:1117–1120. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Curran CS, Demick KP and Mansfield JM:
Lactoferrin activates macrophages via TLR4-dependent and
-independent signaling pathways. Cell Immunol. 242:23–30. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inubushi T, Kawazoe A, Miyauchi M, Kudo Y,
Ao M, Ishikado A, Makino T and Takata T: Molecular mechanisms of
the inhibitory effects of bovine lactoferrin on
lipopolysaccharide-mediated osteoclastogenesis. J Biol Chem.
287:23527–23536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukamoto H, Fukudome K, Takao S,
Tsuneyoshi N and Kimoto M: Lipopolysaccharide-binding
protein-mediated Toll-like receptor 4 dimerization enables rapid
signal transduction against lipopolysaccharide stimulation on
membrane-associated CD14-expressing cells. Int Immunol. 22:271–280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanno D, Akahori Y, Toyama M, Sato K, Kudo
D, Abe Y, Miyasaka T, Yamamoto H, Ishii K, Kanno E, et al:
Involvement of Gr-1 dull+ cells in the production of TNF-α and
IL-17 and exacerbated systemic inflammatory response caused by
lipopolysaccharide. Inflammation. 37:186–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao AT, Yao S, Stefka AT, Liu Z, Qin H,
Liu H, Evans-Marin HL, Elson CO, Nagler CR and Cong Y: TLR4
regulates IFN-γ and IL-17 production by both thymic and induced
Foxp3+ Tregs during intestinal inflammation. J Leukoc Biol.
96:895–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barboza R, Câmara NO, Gomes E, Sá-Nunes A,
Florsheim E, Mirotti L, Labrada A, Alcântara-Neves NM and Russo M:
Endotoxin exposure during sensitization to blomia tropicalis
allergens shifts TH2 immunity towards a TH17-mediated airway
neutrophilic inflammation: Role of TLR4 and TLR2. PLoS One.
8:e671152013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elass-Rochard E, Legrand D, Salmon V,
Roseanu A, Trif M, Tobias PS, Mazurier J and Spik G: Lactoferrin
inhibits the endotoxin interaction with CD14 by competition with
the lipopolysaccharide-binding protein. Infect Immun. 66:486–491.
1998.PubMed/NCBI
|
|
28
|
Qin H, Holdbrooks AT, Liu Y, Reynolds SL,
Yanagisawa LL and Benveniste EN: SOCS3 deficiency promotes M1
macrophage polarization and inflammation. J Immunol. 189:3439–3448.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al Faraj A, Sultana Shaik A, Pureza MA,
Alnafea M and Halwani R: Preferential macrophage recruitment and
polarization in LPS-induced animal model for COPD: Noninvasive
tracking using MRI. PLoS One. 9:e908292014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong L, Li H, Wang S and Li Y: Different
doses of lipopolysaccharides regulate the lung inflammation of
asthmatic mice via TLR4 pathway in alveolar macrophages. J Asthma.
46:229–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YK, Oh SY, Jeon SG, Park HW, Lee SY,
Chun EY, Bang B, Lee HS, Oh MH, Kim YS, et al: Airway exposure
levels of lipopolysaccharide determine type 1 versus type 2
experimental asthma. J Immunol. 178:5375–5382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Hwang HJ and Kim Y: Modeling the
role of TGF-β in regulation of the Th17 phenotype in the LPS-driven
immune system. Bull Math Biol. 76:1045–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Wang CS, Han DM, Sy C, Huang Q,
Sun Y, Fan EZ, Li Y and Zhou B: Differential expression of
Toll-like receptor pathway genes in chronic rhinosinusitis with or
without nasal polyps. Acta Otolaryngol. 133:165–173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tengroth L, Millrud CR, Kvarnhammar AM,
Kumlien Georén S, Latif L and Cardell LO: Functional effects of
Toll-like receptor (TLR)3, 7, 9, RIG-I and MDA-5 stimulation in
nasal epithelial cells. PLoS One. 9:e982392014. View Article : Google Scholar : PubMed/NCBI
|