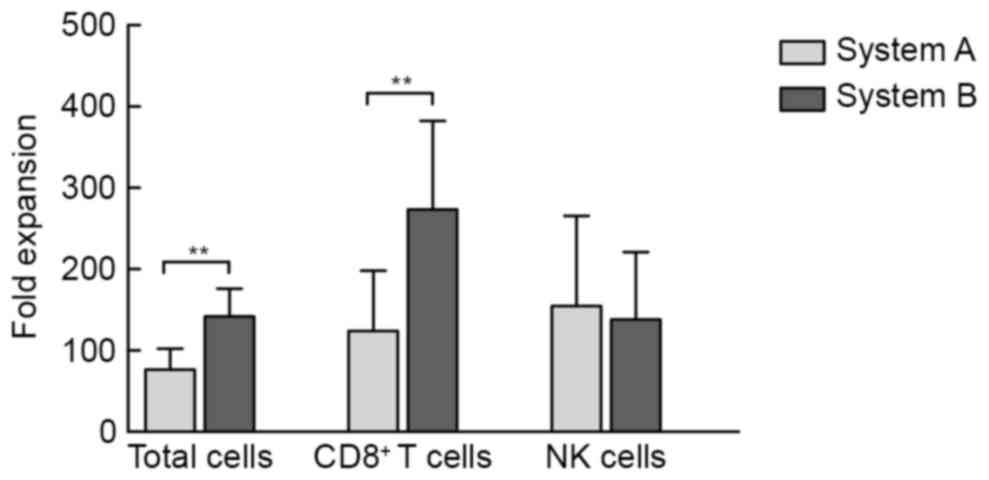
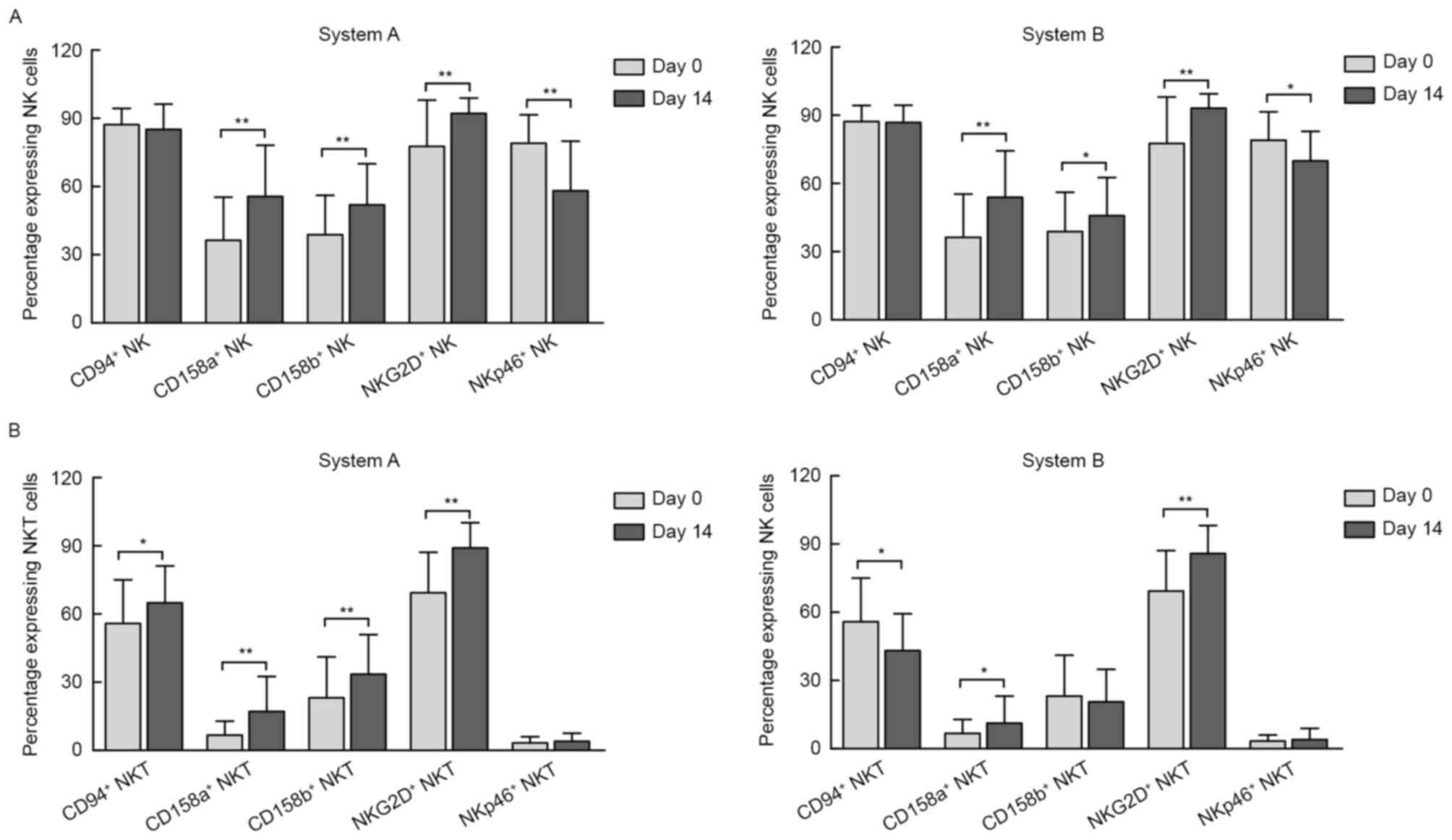
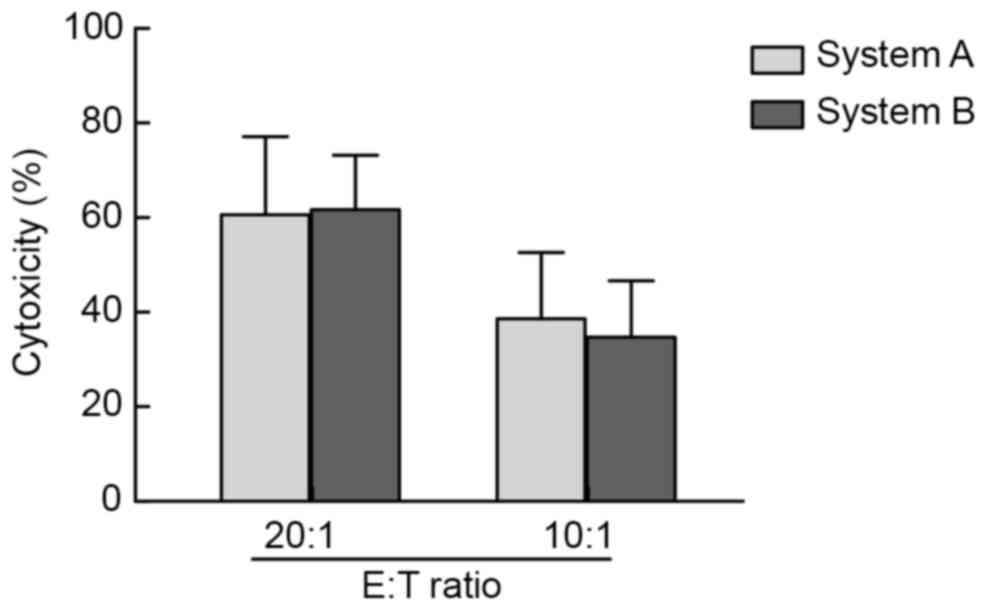
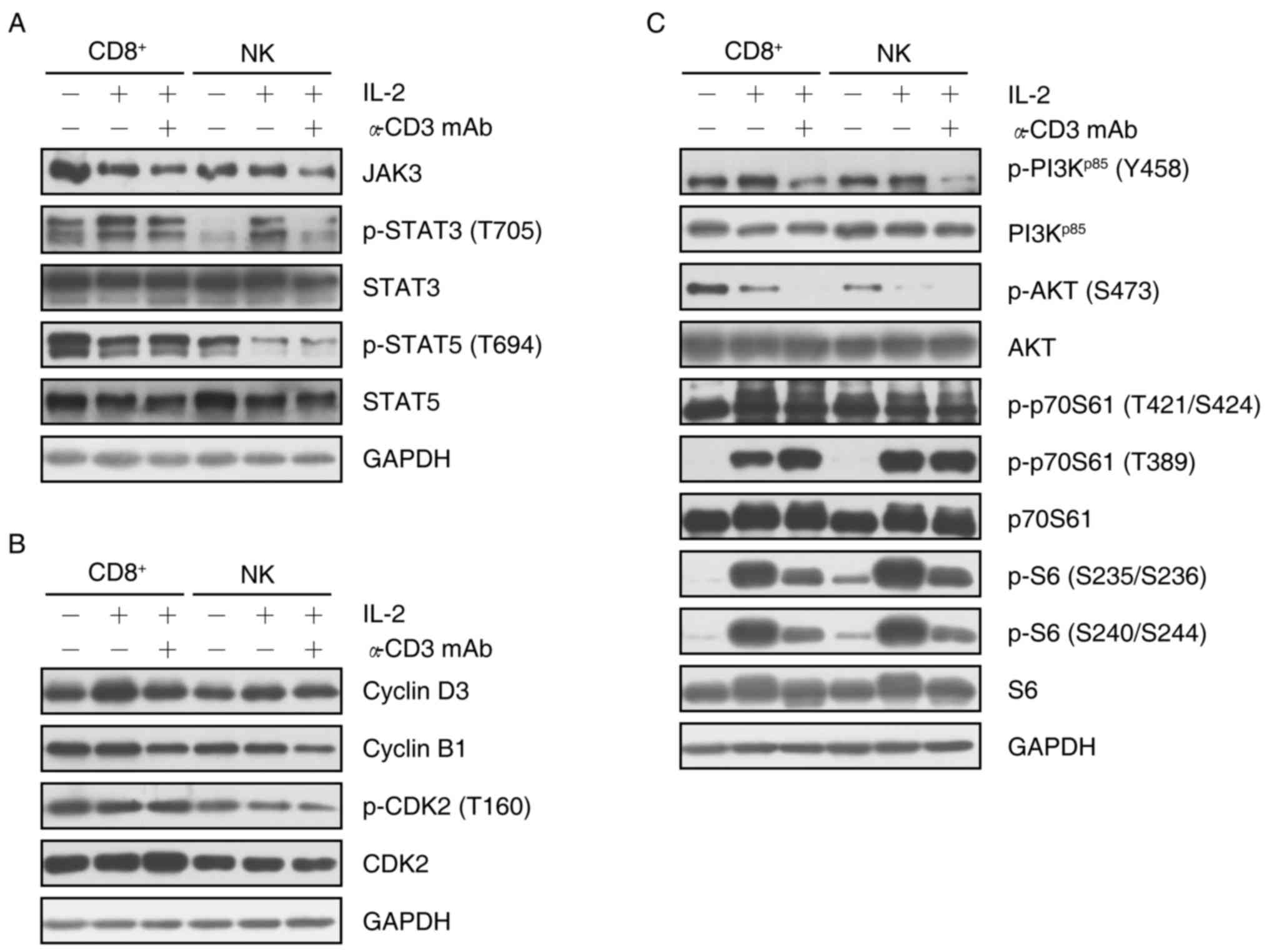
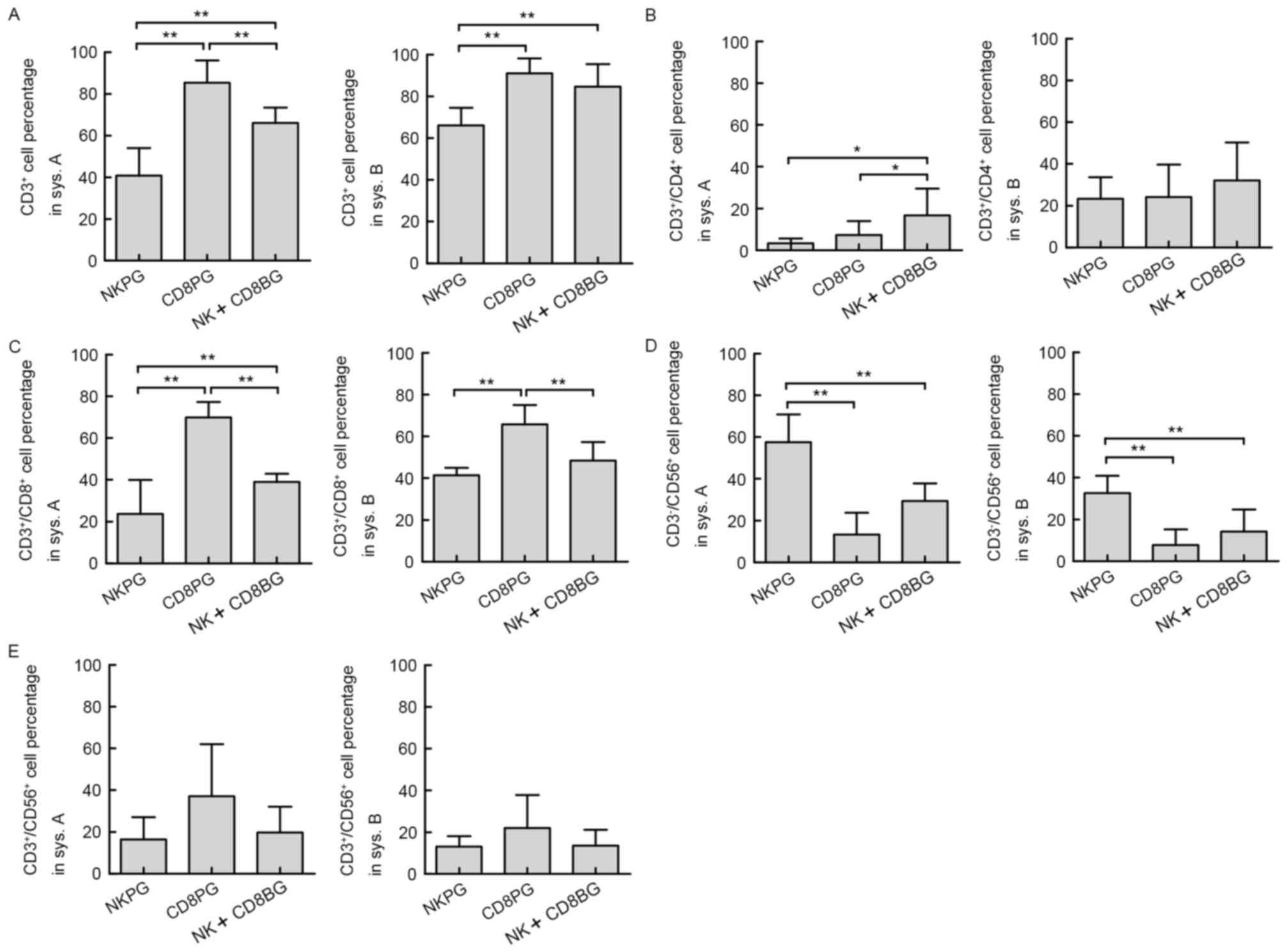
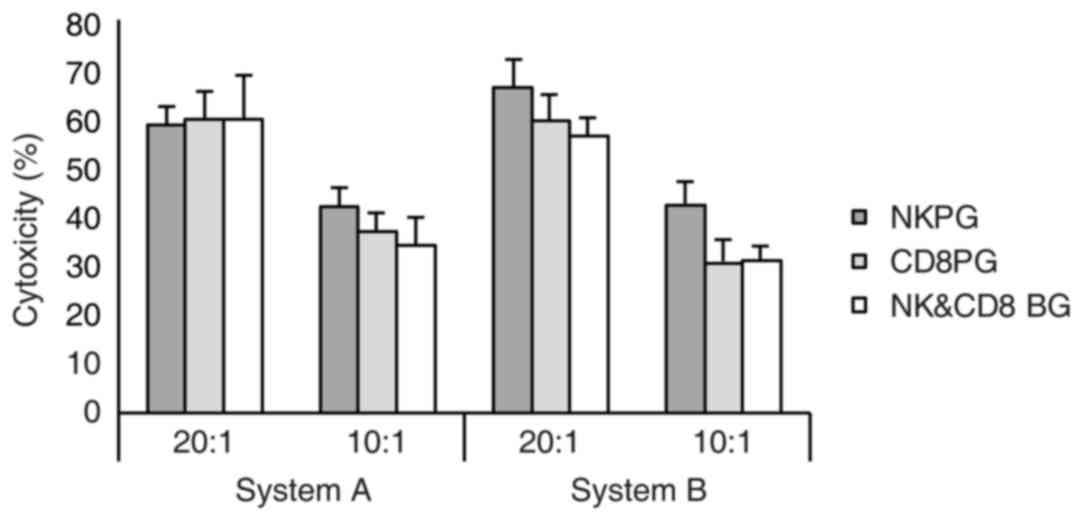
|
1
|
Ruella M and Kalos M: Adoptive
immunotherapy for cancer. Immunol Rev. 257:14–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimasaki N, Coustan-Smith E, Kamiya T and
Campana D: Expanded and armed natural killer cells for cancer
treatment. Cytotherapy. 18:1422–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Introna M: CIK as therapeutic agents
against tumors. J Autoimmun. Jul 2–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bollino D and Webb TJ: Chimeric antigen
receptor-engineered natural killer and natural killer T cells for
cancer immunotherapy. Transl Res. 187:32–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ping Y, Liu C and Zhang Y: T-cell
receptor-engineered T cells for cancer treatment: Current status
and future directions. Protein Cell. Jan 20–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang BL, Qin DY, Mo ZM, Li Y, Wei W, Wang
YS, Wang W and Wei YQ: Hurdles of CAR-T cell-based cancer
immunotherapy directed against solid tumors. Sci China Life Sci.
59:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi FD, Ljunggren HG, La Cava A and Van
Kaer L: Organ-specific features of natural killer cells. Nat Rev
Immunol. 11:658–671. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Konjević G, Mirjacić Martinović K, Vuletić
A, Jurisić V and Spuzić I: Distribution of several activating and
inhibitory receptors on CD3-CD16+ NK cells and their correlation
with NK cell function in healthy individuals. J Membr Biol.
230:113–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caligiuri MA: Human natural killer cells.
Blood. 112:461–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang J, Wu C and Lu B: Cytokine-induced
killer cells promote antitumor immunity. J Transl Med. 11:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt-Wolf IG, Lefterova P, Mehta BA,
Fernandez LP, Huhn D, Blume KG, Weissman IL and Negrin RS:
Phenotypic characterization and identification of effector cells
involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
12
|
Lu PH and Negrin RS: A novel population of
expanded human CD3+CD56+ cells derived from T cells with potent in
vivo antitumor activity in mice with severe combined
immunodeficiency. J Immunol. 153:1687–1696. 1994.PubMed/NCBI
|
|
13
|
Maniar A, Zhang X, Lin W, Gastman BR,
Pauza CD, Strome SE and Chapoval AI: Human gammadelta T lymphocytes
induce robust NK cell-mediated antitumor cytotoxicity through CD137
engagement. Blood. 116:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pittari G, Filippini P, Gentilcore G,
Grivel JC and Rutella S: Revving up natural killer cells and
cytokine-induced killer cells against hematological Malignancies.
Front Immunol. 6:2302015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berg M, Lundqvist A, McCoy P Jr..Samsel L,
Fan Y, Tawab A and Childs R: Clinical-grade ex vivo-expanded human
natural killer cells up-regulate activating receptors and death
receptor ligands and have enhanced cytolytic activity against tumor
cells. Cytotherapy. 11:341–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujisaki H, Kakuda H, Shimasaki N, Imai C,
Ma J, Lockey T, Eldridge P, Leung WH and Campana D: Expansion of
highly cytotoxic human natural killer cells for cancer cell
therapy. Cancer Res. 69:4010–4017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siegler U, Meyer-Monard S, Jörger S, Stern
M, Tichelli A, Gratwohl A, Wodnar-Filipowicz A and Kalberer CP:
Good manufacturing practice-compliant cell sorting and large-scale
expansion of single KIR-positive alloreactive human natural killer
cells for multiple infusions to leukemia patients. Cytotherapy.
12:750–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong W, Xiao W, Hu M, Weng X, Qian L, Pan
X and Ji M: Ex vivo expansion of natural killer cells with high
cytotoxicity by K562 cells modified to co-express major
histocompatibility complex class I chain-related protein A, 4–1BB
ligand and interleukin-15. Tissue Antigens. 76:467–475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Childs RW and Berg M: Bringing natural
killer cells to the clinic: Ex vivo manipulation. Hematology Am Soc
Hematol Educ Program. 2013:234–246. 2013.PubMed/NCBI
|
|
20
|
Cobleigh MA, Vogel CL, Tripathy D, Robert
NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman
G and Slamon DJ: Multinational study of the efficacy and safety of
humanized anti-HER2 monoclonal antibody in women who have
HER2-overexpressing metastatic breast cancer that has progressed
after chemotherapy for metastatic disease. J Clin Oncol.
17:2639–2648. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albanell J, Bellmunt J, Molina R, García
M, Caragol I, Bermejo B, Ribas A, Carulla J, Gallego OS, Español T
and Solé Calvo LA: Node-negative breast cancers with
p53(−)/HER2-neu(−) status may identify women with very good
prognosis. Anticancer Res. 16:1027–1032. 1996.PubMed/NCBI
|
|
22
|
Steplewski Z, Lubeck MD and Koprowski H:
Human macrophages armed with murine immunoglobulin G2a antibodies
to tumors destroy human cancer cells. Science. 221:865–867. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shields RL, Namenuk AK, Hong K, Meng YG,
Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al: High
resolution mapping of the binding site on human IgG1 for Fc gamma
RI, Fc gamma RII, Fc gamma RIII and FcRn and design of IgG1
variants with improved binding to the Fc gamma R. J Biol Chem.
276:6591–6604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa S, Matsuoka Y, Ichihara H,
Yoshida H, Yoshida K and Ueoka R: New cancer immunotherapy using
autologous lymphocytes activated with trastuzumab. Biol Pharm Bull.
35:1213–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnouin K, Fredersdorf S, Eddaoudi A,
Mittnacht S, Pan LX, Du MQ and Lu X: Antiproliferative function of
p27kip1 is frequently inhibited in highly malignant Burkitt's
lymphoma cells. Oncogene. 18:6388–6397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Whenham N, D'Hondt V and Piccart MJ:
HER2-positive breast cancer: From trastuzumab to innovatory
anti-HER2 strategies. Clin Breast Cancer. 8:38–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baselga J, Gianni L, Geyer C, Perez EA,
Riva A and Jackisch C: Future options with trastuzumab for primary
systemic and adjuvant therapy. Semin Oncol. 31 5 Suppl 10:S51–S57.
2004. View Article : Google Scholar
|
|
28
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nimmerjahn F and Ravetch JV: Antibodies,
Fc receptors and cancer. Curr Opin Immunol. 19:239–245. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beano A, Signorino E, Evangelista A, Brusa
D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L and
Matera L: Correlation between NK function and response to
trastuzumab in metastatic breast cancer patients. J Transl Med.
6:252008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huijskens MJ, Walczak M, Sarkar S, Atrafi
F, Senden-Gijsbers BL, Tilanus MG, Bos GM, Wieten L and Germeraad
WT: Ascorbic acid promotes proliferation of natural killer cell
populations in culture systems applicable for natural killer cell
therapy. Cytotherapy. 17:613–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malek TR: The biology of interleukin-2.
Annu Rev Immunol. 26:453–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Groh V, Rhinehart R, Secrist H, Bauer S,
Grabstein KH and Spies T: Broad tumor-associated expression and
recognition by tumor-derived gamma delta T cells of MICA and MICB.
Proc Natl Acad Sci USA. 96:pp. 6879–6884. 1999, View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vivier E, Tomasello E and Paul P:
Lymphocyte activation via NKG2D: Towards a new paradigm in immune
recognition? Curr Opin Immunol. 14:306–311. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Groh V, Rhinehart R, Randolph-Habecker J,
Topp MS, Riddell SR and Spies T: Costimulation of CD8alphabeta T
cells by NKG2D via engagement by MIC induced on virus-infected
cells. Nat Immunol. 2:255–260. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Yang D, Xu W, Wang Y, Ruan Z, Zhao
T, Han J and Wu Y: Tumor-derived soluble MICs impair CD3(+)CD56(+)
NKT-like cell cytotoxicity in cancer patients. Immunol Lett.
120:65–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diefenbach A, Jamieson AM, Liu SD, Shastri
N and Raulet DH: Ligands for the murine NKG2D receptor: Expression
by tumor cells and activation of NK cells and macrophages. Nat
Immunol. 1:119–126. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meyer A, Carapito R, Ott L, Radosavljevic
M, Georgel P, Adams EJ, Parham P, Bontrop RE, Blancher A and Bahram
S: High diversity of MIC genes in non-human primates.
Immunogenetics. 66:581–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pende D, Rivera P, Marcenaro S, Chang CC,
Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L and
Moretta A: Major histocompatibility complex class I-related chain A
and UL16-binding protein expression on tumor cell lines of
different histotypes: Analysis of tumor susceptibility to
NKG2D-dependent natural killer cell cytotoxicity. Cancer Res.
62:6178–6186. 2002.PubMed/NCBI
|
|
41
|
Friese MA, Platten M, Lutz SZ, Naumann U,
Aulwurm S, Bischof F, Bühring HJ, Dichgans J, Rammensee HG, Steinle
A and Weller M: MICA/NKG2D-mediated immunogene therapy of
experimental gliomas. Cancer Res. 63:8996–9006. 2003.PubMed/NCBI
|
|
42
|
Salih HR, Antropius H, Gieseke F, Lutz SZ,
Kanz L, Rammensee HG and Steinle A: Functional expression and
release of ligands for the activating immunoreceptor NKG2D in
leukemia. Blood. 102:1389–1396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Watson NF, Spendlove I, Madjd Z, McGilvray
R, Green AR, Ellis IO, Scholefield JH and Durrant LG: Expression of
the stress-related MHC class I chain-related protein MICA is an
indicator of good prognosis in colorectal cancer patients. Int J
Cancer. 118:1445–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vetter CS, Groh V, Thor Straten P, Spies
T, Brocker EB and Becker JC: Expression of stress-induced MHC class
I related chain molecules on human melanoma. J Invest Dermatol.
118:600–605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Assarsson E, Kambayashi T, Schatzle JD,
Cramer SO, von Bonin A, Jensen PE, Ljunggren HG and Chambers BJ: NK
cells stimulate proliferation of T and NK cells through 2B4/CD48
interactions. J Immunol. 173:174–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martin-Fontecha A, Thomsen LL, Brett S,
Gerard C, Lipp M, Lanzavecchia A and Sallusto F: Induced
recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1
priming. Nat Immunol. 5:1260–1265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan K, Wang QJ, Liu Q, Zheng HX, Li YQ,
Weng DS, Li JJ, Huang LX, He J, Chen SP, et al: The phenotype of ex
vivo generated cytokine-induced killer cells is associated with
overall survival in patients with cancer. Tumour Biol. 35:701–707.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meyer-Monard S, Passweg J, Siegler U,
Kalberer C, Koehl U, Rovó A, Halter J, Stern M, Heim D, Gratwohl
Alois JR and Tichelli A: Clinical-grade purification of natural
killer cells in haploidentical hematopoietic stem cell
transplantation. Transfusion. 49:362–371. 2009. View Article : Google Scholar : PubMed/NCBI
|