Introduction
Mesenchymal stem cells (MSCs) are currently widely
used in stem cell therapy for chronic inflammatory disorders,
autoimmune diseases, graft-vs.-host disease and allograft rejection
(1–3). MSCs are widely distributed in
vivo and are found in the bone marrow, adipose tissue, brain,
heart, skin, umbilical cord and peripheral blood (4). These cells can be differentiated into
numerous tissue phenotypes, including neural, pancreatic and
hepatocytic phenotypes, in specific microenvironments (5). The lack or low expression of
co-stimulatory factors, such as CD80, CD86, CD40, CD34 and human
leukocyte antigen-II, largely benefit the MSC-based therapy
(6). Another advantage of MSCs in
clinical therapy is their immunosuppressive effect. MSCs are able
to inhibit the immune responses by blocking the functions of T-,
B-, natural killer (NK) and dendritic cells (7–9).
The mechanism underlying the effect of MSCs on the
in vivo biological functions remains unknown (10). In spite of the known
immunosuppressive effect of MSCs, several studies have reported
that MSCs induced immune responses, resulting in the injury and
rejection of MSCs, and eventually leading to the failure of
clinical therapy (11–15). Implanted MSCs are recognized by the
immune system of recipients and are eliminated (12). Previous studies have confirmed this
notion, since MSCs activated NK cell-mediated immune rejection and
induced memory T cell response (13,14).
Evidence also indicated the injury of MSCs was mediated by the
complement system (15).
Toll-like receptors (TLRs) are a family of
pathogen-associated molecular patterns, which serve important roles
in the activation of immune responses. There are 11 members in the
human TLRs family, which bind to distinct components from bacteria,
viruses, fungi, as well as circulating endogenous ligands (16). TLR6 is located on the surface of cell
membrane and recognizes diacylated lipoprotein of bacteria and
conserved regions of dengue virus (17). However, to the best of our knowledge,
no previous study has elucidated the role of TLR6 in regulating the
immune status of MSCs isolated from the umbilical cord. In the
present study, the TLR6 pathway was stimulated by a TLR6 agonist,
namely macrophage-activated lipopeptide-2 (MALP-2), and the effects
of TLR6 activation on the immune status of umbilical cord MSCs
(UCMSCs) was investigated.
Materials and methods
Culture and stimulation of UCMSCs
Human UCMSCs were provided by the Sichuan Umbilical
Cord Blood Stem Cell Bank (Chengdu, China). The UCMSCs were
isolated by the cell bank according to a previously described
method (18). Briefly, following
disinfection in 75% ethanol for 10–20 min, the umbilical cord was
diced and then placed into 25 mm2 plates. The culture
medium was added when tissues were firmly attached to the plates
(usually 15–20 h). Next, the mesenchymal tissues were removed 5–8
days later, and then dissociated MSCs were collected. The UCMSCs
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and then stored in liquid nitrogen (−160°C) for
future use. The present study was approved by the Ethics Committee
of the West China Second Hospital, Sichuan University (Chengdu,
China). The TLR6 agonist MALP-2 was purchased from Novus
Biologicals LLC (Littleton, CO, USA) and prepared as recommended by
the instruction manual.
Leukocyte proliferation and cell
injury assay
Peripheral blood leukocytes (PBLs) were isolated
from blood samples of healthy volunteers (subsequent to obtaining
written informed consent) by centrifugation gradient (1,200 × g for
5 min and 10°C). PBLs were then labeled with carboxyfluorescein
diacetate succinimidyl ester (CFSE) at a final concentration of 10
µM, and cold complete DMEM was added for 10 min to stop the
reaction. CFSE-labeled PBLs were then washed three times by cold
phosphate buffered saline and centrifuged at 1,200 × g for 5 min at
10°C to remove the unlabeled CFSE. Subsequently, the cells were
co-cultured with UCMSCs (co-culture ratio, 10:1) in the presence of
MALP-2 (100 µg/ml) and the MALP-2 untreated co-culture group was
used as control. UCMSCs treated with 5% Triton X-100 was used as
the positive control, which acted as the maximum release group.
Cells were collected at 72 h after stimulation for detection of the
PBL proliferation by fluorescence-activated cell sorting,
(increased PBL proliferation dilutes the labeled fluorescence). The
supernatants from the two PBL-UCMSC co-culture groups (untreated
control and MALP-2-treated group) were collected by centrifugation
at 3,000 × g for 20 min at 4°C, and the lactate dehydrogenase (LDH)
levels were measured by a cytotoxicity detection kit (Roche Applied
Science, Indianapolis, IN, USA) according to the manufacturer's
instructions. The lysis percentage was calculated using the
following formula: Lysis (%) = (E-M)/(T-M) × 100, where E is the
experimental release, M is the spontaneous release in the presence
of media alone, and T is the maximum release in the presence of 5%
Triton X-100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for quantification
detection
The mRNA expression levels of interleukin (IL)-1β,
IL-6, IL-8, IL-9, IL-10, chemokine (C-C motif) ligand 1 (CCL1),
CCL2, CCL4, CCL26, Kruppel-like factor 4 (Klf4), Lin28, Nanog,
SRY-box 17 (Sox 17) and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were determined by RT-qPCR. Briefly, total RNA was
extracted from the treated and untreated UCMSCs, and reverse
transcription was performed using a ReverTra Ace qPCR RT kit
(FSQ-101; Toyobo Co., Ltd., Kagoshima, Japan) to synthesize cDNA,
using the following conditions: 65°C for 5 min, 37°C for 15 min and
98°C for 5 min. Next, qPCR was conducted with the SYBR Green
RealMaster Mix (FP202; Tiangen Biotech Co., Ltd., Beijing, China)
under the following thermal cycling conditions: One cycle at 95°C
for 30 sec, 40 cycles at 95°C for 30 sec, 58°C for 30 sec and 72°C
for 30 sec, followed by a melt curve from 55 to 95°C in 0.5°C
increments and 10 sec intervals. The primers used for qPCR assay
are listed in Table I. GAPDH was
used as an internal control. Quantitative data were analyzed using
Bio-Rad iQ5 software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The figures were completed using GraphPad Prism5 (GraphPad
Software, Inc., La Jolla, CA, USA).
 | Table I.List of oligonucleotides used in
quantitative polymerase chain reaction analysis. |
Table I.
List of oligonucleotides used in
quantitative polymerase chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | GenBank no. |
|---|
| IL-1β |
ACGAATCTCCGACCACCACT |
CCATGGCCACAACAACTGAC | M15330 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-8 |
CTGGCCGTGGCTCTCTTG |
CCTTGGCAAAACTGCACCTT | NM_000584 |
| IL-9 |
CTCTGTTTGGGCATTCCCTCT |
GGGTATCTTGTTTGCATGGTGG | M30134 |
| IL-10 |
GGTGATGCCCCAAGCTGA |
TCCCCCAGGGAGTTCACA | U16720 |
| CCL1 |
GCAGATCATCACCACAGCC |
GTCCACATCTTCCGGCCA | NM_002981 |
| CCL2 |
CTGCTCTCCAGCGCTCTCA |
GTAAGAAAAGCAGCAGGCGG | NM_002984 |
| CCL4 |
CAGTGCTTCTGTGCCTGCTG |
TGCATCTGGCTGAGCAAGTC | NM_005408 |
| CCL26 |
CCAAGACCTGCTGCTTCCAA |
GAATTCATAGCTTCGCACCCA | NM_006072 |
| Klf4 |
GTCATCAGCGTCAGCAAAGG |
CCCTGCTGCTCAGCACTT | NM_004235 |
| Lin28 |
TGCTGTCGAGGGATGGATA |
CCACCCAATGCGTTCTATTG | NM_024674 |
| Nanog |
CCAAAGGCAAACAACCCACTT |
CGGGACCTTGTCTTCCTTTTT | NM_001297 |
| Sox 17 |
TGGCGCAGCAGAATCCA |
CGACTTGCCCAGCATCTTG | NM_022454 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
Detection of surface markers
MALP-2-treated and untreated UCMSCs were harvested
at 72 h post-stimulation. The UCMSCs were stained with different
antibodies to detect the expression levels of surface molecules,
including co-stimulators and stem cell markers. The antibodies used
in this assay were the following: CD40 (cat. no. 11-0809), CD59
(cat. no. 11-0596), CD86 (cat. no. 12-0869) and CD90 (cat. no.
45-0909; all from eBioscience, Thermo Fisher Scientific, Inc.), all
were diluted to 1:200 with serum-free DMEM. Flow cytometry was then
conducted and analyzed using CXP flow cytometry software (Beckman
Coulter, Brea, CA, USA). Positive expression was gated according to
the fluorescence intensity of the negative control group.
UCMSC differentiation
UCMSCs were seeded into 6-well plate with a density
of 1.5×105 cells/well. Conditioned mediums for
chondrocyte induction (A10071-01; Gibco; Thermo Fisher Scientific,
Inc.), adipocyte induction (A10070-01; Gibco; Thermo Fisher
Scientific, Inc.) and osteocyte induction (A10072-01; Gibco; Thermo
Fisher Scientific, Inc.) were added in each well with or without
MALP-2 treatment. The results of differentiation were collected at
10- and 15-days post-induction, and analyzed using oil-red staining
for adipocytes, alizarin red staining for osteocytes and safranin
staining for chondrocytes (eBioscience; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean using SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA). Significant differences of cytotoxicity percentage between
MALP-2 treated and untreated groups were analyzed using a Student's
t-test. Significant differences between untreated UCMSCs and
different time points of MALP-2 treated UCMSCs were analyzed by
analysis of variance and post hoc test (Tukey's) after homogeneity
test of variances. Values of P<0.05 and P<0.01 were
considered to indicate statistically significant differences
compared with the control group.
Results
Activation of TLR6 in UCMSCs enhances
the proliferation of PBLs
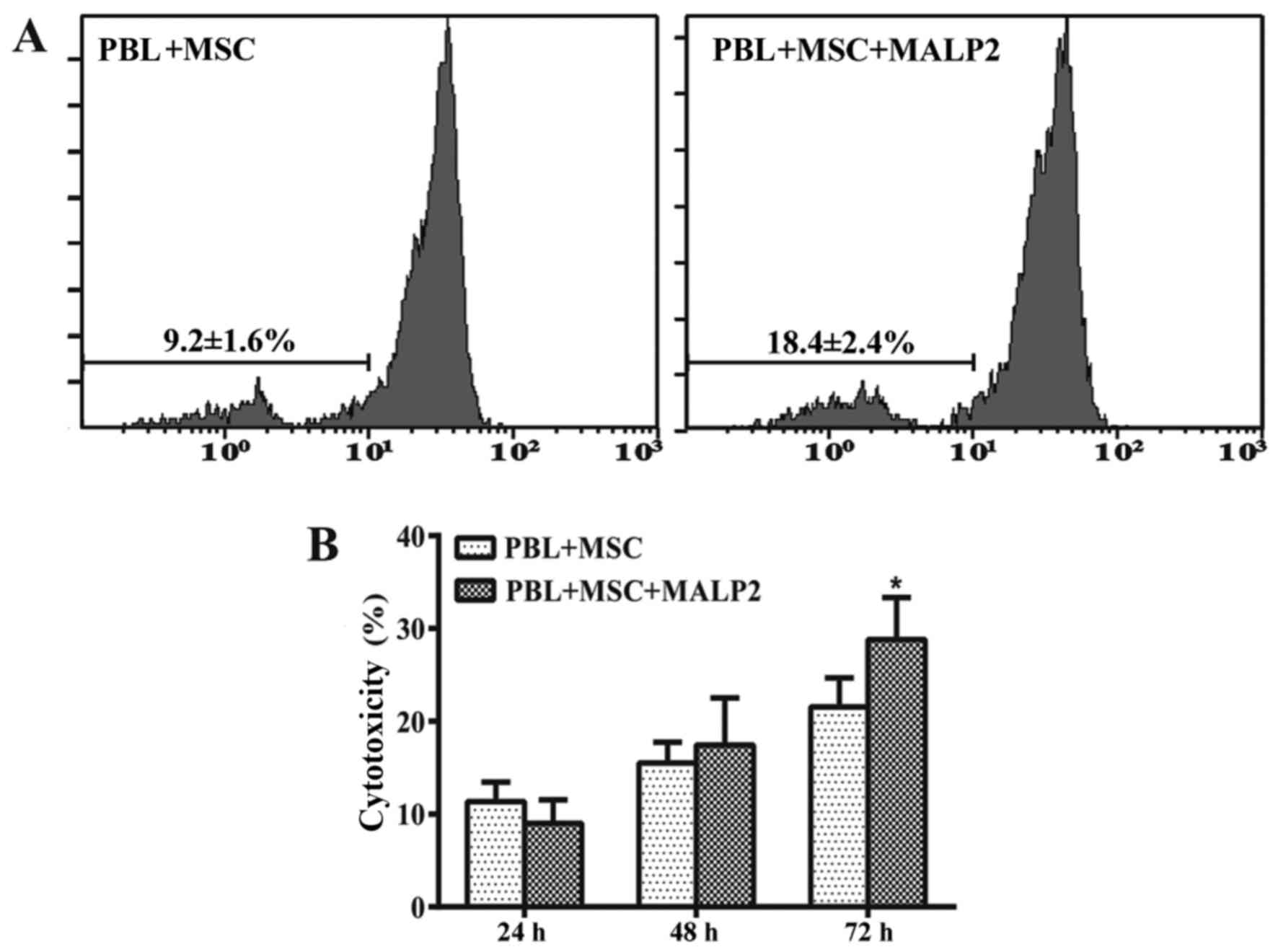
The proliferation of CFSE-labeled PBLs is widely
used to detect an increased immune attack. In the present study,
CFSE-labeled PBLs were co-cultured with UCMSCs in the presence of
MALP-2. PBLs were harvested at 72-h after the co-culture, and the
proliferation of PBLs was then detected by flow cytometry. The
results indicated that the proliferation of PBLs in the group
treated with MALP-2 was increased (18.4%) as compared with that in
the untreated co-culture group (9.2%), which suggested that TLR6
activation was able to increase the immune response of PBLs against
UCMSCs (Fig. 1A).
The damage of cells is frequently detected on the
basis of LDH release from injured cells into the culture
supernatant. The results of the current study indicated that
cytotoxicity percentage were not significantly different between
the MALP-2 treated and control groups following 24 and 48 h of
co-culture (Fig. 1B). However, the
cytotoxicity percentage was significantly increased in the
TLR6-activated group compared with the untreated group after 72 h
(P<0.05; Fig. 1B). These results
suggested that the activation of TLR6 stimulated the immune attack
of PBLs against UCMSCs.
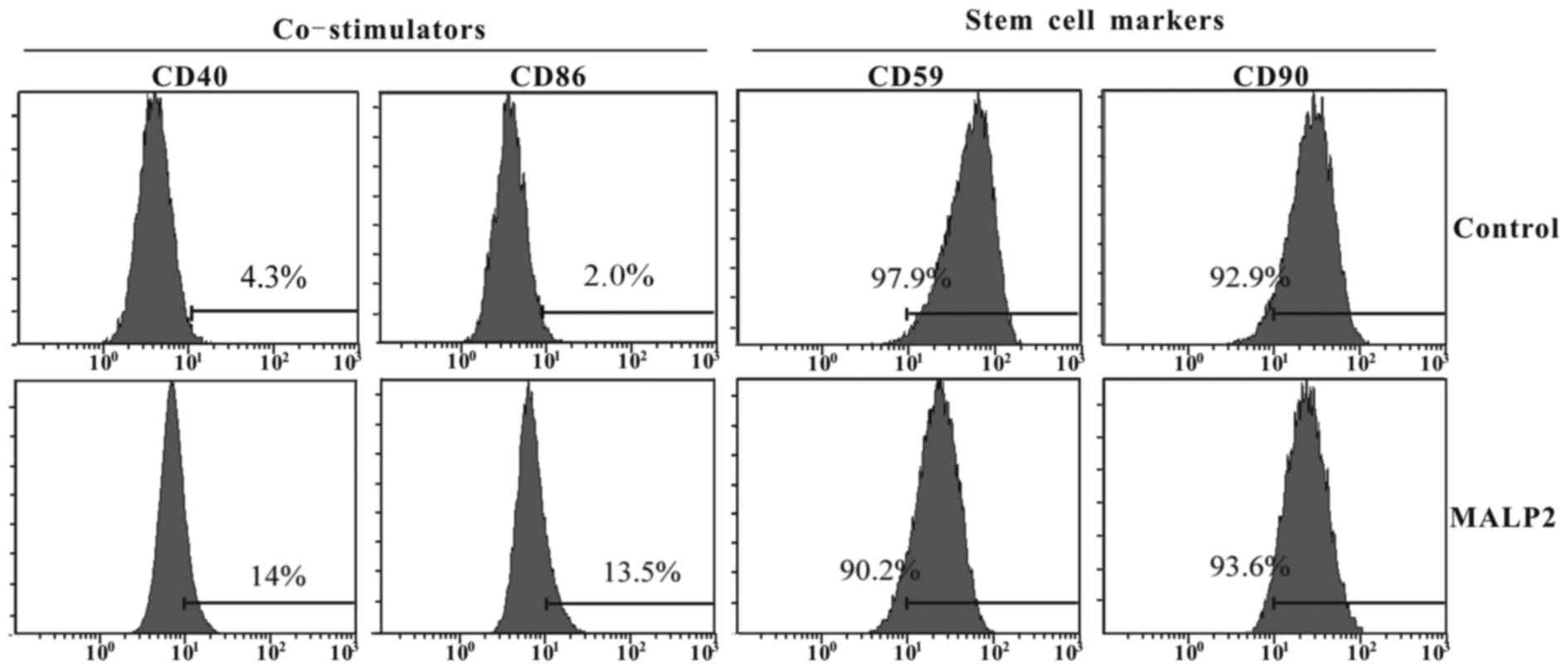
TLR6 activation increases the
expression of surface co-stimulatory molecules in UCMSCs
Given the aforementioned results stating that TLR6
activation increased the proliferation and immune attack in the
co-culture of PBLs and UMCSCs, the present study also investigated
whether MALP-2 treatment influences the expression of surface
co-stimulatory factors and stem cell surface markers on UCMSCs. The
UCMSCs were treated in the presence or absence of MALP-2, and the
surface markers were measured after 72-h incubation by flow
cytometry, including measurement of the stimulatory molecules CD40
and CD86, as well as of the stem cell specific markers CD59 and
CD90. The results indicated that TLR6 activation enhanced the
expression levels of CD40 (15 vs. 4.3%) and CD86 (13.5 vs. 2.0%) as
compared with the untreated group (Fig.
2). By contrast, the surface stem cell marker CD90 presented no
evident variation, while the expression of CD59 was slightly
inhibited in MALP-2 treated UCMSCs (97.9 vs. 90.2%; Fig. 2).
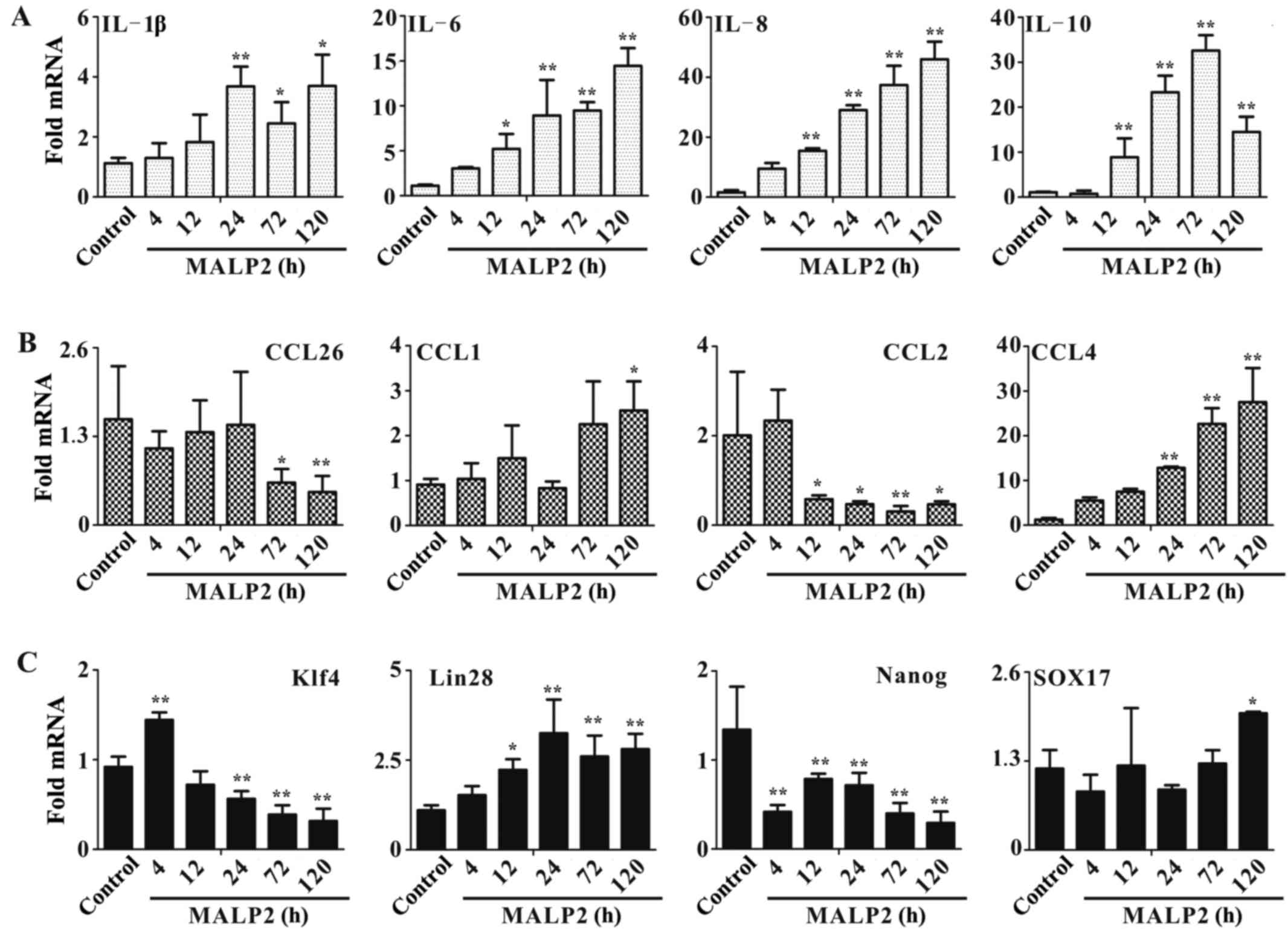
Treatment with MALP-2 promotes the
expression of cytokines and chemokines
RT-qPCR was conducted to detect the expression
levels of important cytokines, chemokines and stem cell markers.
UCMSCs were stimulated with MALP-2, and total RNA was extracted at
4, 12, 24, 72 and 120 h post stimulation. qPCR was performed to
assay the expression of these important immune system-associated
molecules. In cytokine detection, the expression level of IL-10 was
significantly induced after treatment for 12–120 h (all P<0.01;
Fig. 3A), while IL-1β expression
only increased after 24–120 h (24 h, P<0.01; 72–120 h,
P<0.05). IL-6 (12 h, P<0.05; 24–120 h, P<0.01) and IL-8
(all P<0.01) expression increased 12–120 h post stimulation. In
chemokine assessment, CCL4 was significantly induced from 24–120 h
of treatment (all P<0.01; Fig.
3B), and CCL1 expression increased after 120 h of stimulation
(P<0.05). However, the other two chemokines, CCL26 (72 h,
P<0.05; 120 h, P<0.01) and CCL2 (12–24 h, P<0.05; 72 h,
P<0.01; 120 h, P<0.05), presented inhibited expression,
possibly due to the complex biological functions of chemokines
in vivo.
To confirm whether activation of TLR6 influences the
stemness of UCMSCs, expression levels of stem cell markers,
including Klf4, Lin28, Nanog and Sox17, were detected in the
present study. The Nanog expression significantly decreased from
4–120 h of treatment (all P<0.01; Fig. 3C), while Klf4 expression increased at
4 h (P<0.01), but was then significantly inhibited from 24–120 h
of stimulation with MALP-2 (all P<0.0l; Fig. 3C). Furthermore, the expression of
Lin28 was significantly increased after 12 h of MALP-2 stimulation
(12 h, P<0.05; 24–120 h, P<0.01), and Sox17 expression was
only induced after 120 h of treatment with MALP-2 (P<0.05;
Fig. 3C).
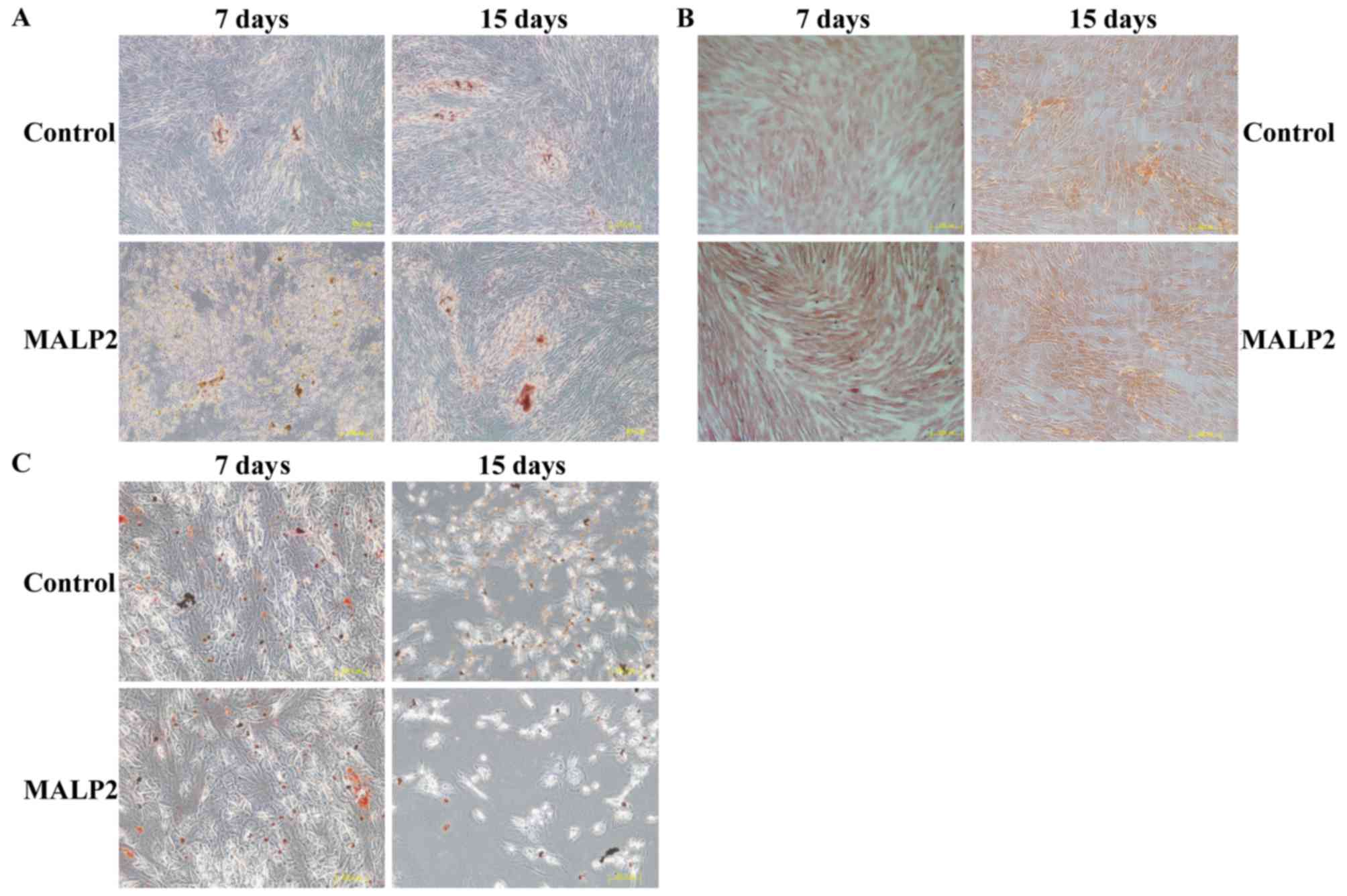
Stimulation with a TLR6 agonist has no
effect on the differentiation ability of UCMSCs
The differentiation ability of UCMSCs was also
assayed to confirm whether it was regulated by the activation of
the TLR6 pathway. Conditional media for adipocytes, osteoblasts and
chondrocytes were used to induce UCMSC differentiation. The
specific cell phenotypes were identified by different staining
methods, more specifically alizarin red for osteoblasts (Fig. 4A), safranin for chondrocytes
(Fig. 4B) and oil red O for
adipocytes (Fig. 4C), in order to
detect the differentiation status of UCMSCs at 7 and 15 days post
MALP-2 treatment. The results indicated that there were no
differences between the control and TLR6-activated groups, which
suggested that the TLR6 pathway had no role in regulating the
differentiation of UCMSCs (Fig.
4A-C).
Discussion
The characteristics of MSCs that result in their
important role in cell-based therapy include multipotent
differentiation ability, lack of co-stimulatory molecule expression
and an immunosuppressive effect on the immune system (19). However, recent clinical trials,
including those using MSCs to treat graft versus host disease
(GVHD), myocardial infarction and liver cirrhosis have not achieved
encouraging results due to incomplete understanding of the
engrafted MSCs (11,12). Human and animal studies have
demonstrated the recognition and elimination of the MSCs within a
few days of infusion by modifying the low immune status of MSCs in
specific in vivo microenvironments, which eventually
resulted in enhanced immune responses.
Proteins of the TLR family are important in bridging
innate and adaptive immune response by recognizing conserved
components of invasive pathogens, as well as circulating endogenous
ligands (20). As UCMSCs is
currently becoming more important in cell therapy, the role of TLRs
in regulating the biological functions of UCMSCs is critical for
improving the controlled and desirable clinical outcomes.
Previously, it was confirmed that TLRs are important in regulating
the differentiation capacity and immunosuppressive effect, as well
as serving a role in MSC survival (21). However, the role of TLRs in the
alteration of immunogenicity has seldom been reported. For
instance, the study by Zhang et al reported that a TLR7
agonist increased the immunogenicity of UCMSCs (22), while the activation of TLR3, TLR4 and
TLR9 had no effect on the immune status of UCMSCs (23,24).
In the present study, the role of TLR6 in the immune
status of UCMSCs was assessed by stimulation with the TLR6 agonist,
MALP-2. The results indicated that activation of TLR6 increased the
proliferation of PBLs and enhanced the release of LDH in the
co-culture system, which demonstrated the existence of immune
response against UCMSCs. In addition, surface molecule detection
revealed increased expression of CD40 and CD86 following
stimulation, which are well known co-stimulatory factors in
mediating immune response. Furthermore, the expression levels of
proinflammatory cytokines (IL-1β, IL-6, IL-8 and IL-10) were
significantly induced upon MALP-2 stimulation, as well as the
levels of two chemokines (CCL1 and CCL4). Huang et al has
suggested that differentiation of MSCs into cardiomyocytes
increased the immunogenicity of MSCs (25), while Zhang et al also
confirmed that activation of TLR7 increased the immune status of
UCMSCs by enhancing their differentiation ability (22). Thus, the differentiation capacity of
UCMSCs was detected by adding conditional media in the present
study, and the results revealed no marked difference between the
control and TLR6 agonist-stimulated groups, which suggested that
TLR6 had no effect on the differentiation of UCMSCs.
In conclusion, the present study demonstrated that
activation of TLR6 resulted in alteration of the immune status of
UCMSCs. In clinical trials, the infused UCMSCs will encounter a
number of endogenous ligands that may function as agonists to
activate different TLR pathways. Thus, future studies are required
to confirm the mechanisms underlying the effect of immune
response-associated factors in regulating the biological functions
of UCMSCs via the TLR6 pathway.
References
|
1
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vojtassak J, Danisovic L, Kubes M, Bakos
D, Jarabek L, Ulicna M and Blasko M: Autologous biograft and
mesenchymal stem cells in treatment of the diabetic foot. Neuro
Endocrinol Lett. 27 Suppl 2:S134–S137. 2006.
|
|
3
|
Guiducci S, Porta F, Saccardi R, Guidi S,
Ibba-Manneschi L, Manetti M, Mazzanti B, DalPozzo S, Milia AF,
Bellando-Randone S, et al: Autologus mesenchymal stem cells foster
revascularization of ischemic limbs in systemic sclerosis: A case
report. Ann Intern Med. 153:650–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bordignon C, Carlo-Stella C, Colombo MP,
De Vincentiis A, Lanata L, Lemoli RM, Locatelli F, Olivieri A,
Rondelli D, Zanon P and Tura S: Cell therapy: Achievements and
perspectives. Haematologica. 84:1110–1149. 2011.
|
|
5
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Poll D, Parekkadan B, Cho CH,
Berthiaume F, Nahmias Y, Tilles AW and Yarmuch ML: Mesenchymal stem
cell-derived molecules directly modulate hepatocellular death and
regeneration in vitro and in vivo. Hepatology. 47:1634–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom
HJ, Kim MG, Oh KH, Ahn C and Yang J: Immunosuppressive mechanisms
of embryonic stem cells and mesenchymal stem cells in alloimmune
response. Transpl Immunol. 25:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bassi E, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cells. 3:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui J, Wahl RL, Shen T, Fisher SJ, Recker
E, Ginsburg D and Long MW: Bone marrow cell trafficking following
intraveneous administration. Br J Haematol. 107:895–902. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ankrum J and Karp JM: Mesenchymal stem
cell therapy: Two steps forward, one step back. Trends Mol Med.
16:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allison M: Genzyme back Osiris, despite
Prochymal flop. Nat Biotechnol. 27:966–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spaggiari GM, Capobianco A, Becchetti S,
Mingari MC and Moretta L: Mesenchymal stem cell-natural killer cell
interactions: Evidence that activated NK cells are capable of
killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell
proliferation. Blood. 107:1484–1490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nauta AJ, Westerhuis G, Kruisselbrink AB,
Lurvink EG, Willemze R and Fibbe WE: Donor-derived mesenchymal stem
cells are immunogenic in an allogeneic host and stimulate donor
graft rejection in a nonmyeloablative setting. Blood.
108:2114–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y and Lin F: Mesenchymal stem cells are
injured by complement after their contact with serum. Blood.
120:3436–3443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JC, Ng MM and Chu JJ: Activation of
TLR2 and TLR6 by Dengue NS1 protein and its implications in the
immunopathogenesis of Dengue virus infection. PLoS Pathog.
11:e10050532015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM,
Chou SC, Shih YH, Ko MH and Sung MS: Conversion of human umbilical
cord mesenchymal stem cells in Wharton's jelly to dopaminergic
neurons in vitro: Potential therapeutic application for
parkinsonism. Stem Cells. 24:115–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tolar J, Le Blanc K, Keating A and Blazar
BR: Concise review: Hitting the right spot with mesenchymal stromal
cells. Stem Cells. 28:1446–1455. 2011. View
Article : Google Scholar
|
|
20
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Liu D, Pu D, Wang YW, Li L, He
YQ, Li YL, Li L and Li WM: The TLR7 agonist Imiquimod promote the
immunogenicity of mesenchymal stem cessl. Biol Res. 48:62015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Liu D, Pu D, Wang YW, Li L, He Y,
Li YL, Li L, Qiu ZC, Zhao S and Li WM: The role of Toll-like
receptor 3 and 4 in regulating the function of mesenchymal stem
cells isolated from umbilical cord. Int J Mol Med. 35:1003–1010.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Wang Y, Li L, Bao J, Chen F and
Zhang L: Toll-like receptor 9 agonist stimulation enables
osteogenic differentiation without altering the immune status of
umbilical cord mesenchymal stem cells. Mol Med Rep. 12:8077–8084.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang XP, Sun Z, Miyagi Y, McDonald
Kinkaid H, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesenchymal stem cells induces immunogenicity and limits
their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|