Introduction
Atherosclerosis is the primary underlying cause of
myocardial infarction, stroke, unstable angina and sudden cardiac
death, which account for the leading cause of death in the world
(1,2). In westernized societies, it has been
reported that atherosclerosis is responsible for ~50% of all deaths
and the incidence is continuing to rise (1). Epidemiological studies have revealed
that several important environmental and genetic risk factors
associated with development and progression of atherosclerosis
(3,4). However, progress in defining the
cellular and molecular interactions involved in atherosclerosis
outbreak has been hindered by the disease's etiological complexity
(3,4). Lifestyle modifications are the
first-line treatment, while medicines are usually the next step in
treating atherosclerosis (5).
Nevertheless, the current medicine does not sufficiently ameliorate
atherosclerosis progression in a number of susceptible individuals
(6,7). Therefore, it is important to develop
novel therapeutic strategies for atherosclerosis.
Atherosclerosis occurs in the subendothelial space
of medium-sized arteries at regions of disturbed blood flow, and is
triggered by an interplay between endothelial dysfunction and
subendothelial lipoprotein retention, particularly low-density
lipoprotein (LDL) (8). While in the
arterial intima formation, LDL particles can undergo oxidation,
becoming a putative promoter of atherogenesis (9). Once oxidized, LDL particles can induce
endothelial and smooth muscle cell activation, secretion of
inflammatory mediators and expression of adhesion molecules, a
sequence of steps that culminates inflammatory cells especially
monocyte in subendothelial space (9). Recruited monocytes can enhance the
oxidation of LDL particles, leading to a vicious local loop
(10). Once in the intima, monocytes
become tissue macrophages, which can avidly internalize
oxidized-LDL via scavenger receptors (10). This process generates macrophages
loaded with lipids, also known as foam cells, which are a prominent
feature of atherosclerotic plaque (11). By capturing these lipid particles,
intimal macrophages can promote local vascular damage through
various secreted mediators, such as proinflammatory cytokines and
chemokines (11). Over time, this
process stimulates inflammatory response that can cause intimal
destruction, arterial thrombosis, and end-organ ischemia (12).
Sicyos angulatus (SA) is a summer annual vine
belonging to the gourd family Cucurbitaceae originated from
north-eastern USA (13,14). SA is widely distributed throughout
both Europe and Asia, and has been recognized as a noxious invasive
alien species (13,15). SA is a problem weed because of its
aggressive habit in some summer crops, such as maize, soybean and
pumpkin (13). SA is also common
along water-courses and in open spaces, where it suppresses native
vegetation (16). However, up to
date, none of study has reported the usefulness and the beneficial
roles of noxious plant SA in medicine field.
In the present study, we evaluated the effects of SA
on atherosclerosis using RAW 264.7 cells, a murine macrophage cell
line, and apolipoprotein E-deficient (apoE−/−) mice.
Materials and methods
Preparation of SA extract
SA was purchased from Gangwon Herbs (Gangwon, Korea)
on August, 2015. The voucher specimen (SNU2015-0008) was identified
by Professor Won-Keun Oh, and was deposited at the College of
Pharmacy, Seoul National University (Seoul, Korea). The SA extract
was prepared and supplied by the Korea Bioactive Natural Material
Bank (Seoul, Korea). Briefly, the dried aerial parts of SA were
extracted with 70% ethanol three times for 6 h at room temperature.
After that, the 70% ethanol-soluble extract was filtered and
exhaustively concentrated to produce a 70% ethanolic extract under
reduced pressure. The yield of SA extraction is 11%. This SA
extract was suspended in 0.5% carboxymethyl cellulose (CMC) at a
concentration of 50 mg/ml as a stock solution, and the working
solution of SA was adjusted to the intended concentrations for use
in the in vitro and in vivo experiments in the
present study.
Animal experiments
Male 8-week-old apoE−/− mice were
obtained from Jackson Laboratory (Bar Harbor, ME, USA). Prior to
experiments, mice were acclimatized to a 12-h light/dark cycle at
22±2°C for 2 weeks with unlimited food and water in a specific
pathogen-free facility. The mice were randomly divided into three
groups: Those fed i) an atherogenic diet (no. 102571; Dyets Inc.,
Bethlehem, PA, USA) plus 0.5% carboxymethyl cellulose as the
vehicle group (n=13); ii) an atherogenic diet plus 300 mg/kg of SA
as the SA300 group (n=4); and iii) an atherogenic diet plus 500
mg/kg of SA as the SA500 group (n=12). For en face Oil-red O
staining of aorta, 9, 4 or 7 mice were used in the vehicle, the
SA300 or the SA500 group, respectively. For aortic total RNA
extraction, 4 or 5 mice were used in the vehicle or the SA500
group, respectively. We used The dose of SA were determined by
preliminary tests. SA was administered daily by oral gavage for 8
weeks and changes of body weight were measured each week. All mice
were euthanized by CO2 asphyxiation. All animal
experiments were approved by the Institutional Animal Use and Care
Committee of the Korea Research Institute of Bioscience and
Biotechnology and were performed in accordance with the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health.
Cell culture and SA treatment
The RAW 264.7 cells, a murine macrophage cell line,
were purchased from the American Type Cell Culture (ATCC; Manassas,
VA, USA) and were cultured in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum, 100 U/ml penicillin and
100 µg/ml streptomycin in a humidified atmosphere (5%
CO2/95% air) at 37°C. The cells were pre-treated with
different concentrations of SA (100, 200 or 300 µg/ml) for 1 h, and
then stimulated with lipopolysaccharide (LPS, 1 µg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or vehicle for 24 h.
The doses of SA were determined by preliminary tests using various
concentration without cell cytotoxicity to examine the
anti-inflammatory effects.
Quantitative real-time polymerase
chain reaction
The total RNA was isolated from whole aortas or RAW
264.7 cells using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), and reverse transcribed using the
iScript™ cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The resulting cDNA was subjected to qPCR using
the StepOnePlus™ Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA) with AccuPower® 2X
Greenstar qPCR Master Mix (Bioneer, Daejeon, Korea) according to
the manufacturers' instructions. Relative gene expression levels
were analyzed using the 2−ΔΔCt method and normalized
against the expression of 18S rRNA. The primer sequences used in
the experiments are listed in Table
I.
 | Table I.Sequences of PCR primers used in this
study. |
Table I.
Sequences of PCR primers used in this
study.
| Gene | GenBank accession
number | Primer
sequence |
|---|
| Tnfα | NM_013693.3 | Forward:
5′-TGGCCTCCCTCTCATCAGTT-3′ |
|
|
| Reverse:
5′-CCTCCACTTGGTGGTTTGCT-3′ |
| Il6 | NM_031168.2 | Forward:
5′-TTCCATCCAGTTGCCTTCTTG-3′ |
|
|
| Reverse:
5′-GGGAGTGGTATCCTCTGTGAAGTC-3′ |
| Il1β | NM_008361.4 | Forward:
5′-CTACAGGCTCCGAGATGAACAAC-3′ |
|
|
| Reverse:
5′-TCCATTGAGGTGGAGAGCTTTC-3′ |
| Il10 | NM_010548.2 | Forward:
5′-GGGTTGCCAAGCCTTATCG-3′ |
|
|
| Reverse:
5′-TCTCACCCAGGGAATTCAAATG-3′ |
| Vcam1 | NM_011693.3 | Forward:
5′-TGACTCCATGGCCCTCACTT-3′ |
|
|
| Reverse:
5′-CGTCCTCACCTTCGCGTTTA-3′ |
| Icam1 | NM_010493.3 | Forward:
5′-TCACCAGGAATGTGTACCTGACA-3′ |
|
|
| Reverse:
5′-ATCACGAGGCCCACAATGAC-3′ |
Blood analysis
At the end of the experimental period, blood samples
were collected from the retro-orbital venous plexus of mice. Plasma
was prepared by centrifugation of blood at 10,000 × g for 10 min at
4°C and subsequently storing it at −80°C. Plasma alanine
aminotransferase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN), creatine kinase (CK), triglyceride, total
cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and
low density lipoprotein cholesterol (LDL-C) were determined with an
automated blood chemistry analyzer (Hitachi 7150; Hitachi, Ltd.,
Tokyo, Japan).
Analysis of atherosclerotic lesion
formation in the aorta of ApoE−/− mice
The whole aortas were isolated and the adventitial
tissue were removed. After fixation in 10% neutral buffered
formalin, the whole aortas were longitudinally dissected, and
pinned flat on a rubber plate. Lipid plaques in the whole aorta
were stained with Oil-red O (Sigma-Aldrich; Merck KGaA), and en
face images were captured using a digital camera (Canon, Tokyo,
Japan). The whole aorta surface and stained plaque areas were
analyzed by digital image analysis software (Image Inside; GS
Media, Daejeon, Korea).
Analysis of atherosclerotic lesion
formation in the aortic sinus of ApoE−/− mice
The aortic sinuses were isolated, and fixed in 10%
neutral buffered formalin. After fixation, the aortic sinuses were
embedded in a Tissue-Tek optimal cutting temperature (OCT) compound
(Sakura Finetek, Tokyo, Japan) and sectioned at a thickness of 8 µm
using a cryotome (Sakura Finetek). Cryostat sections of the aortic
sinus were stained with Oil-red O, and images were obtained using a
light microscope (BX51; Olympus, Tokyo, Japan). The atheroma areas
of the aortic sinus were quantified using digital image analysis
software (image inside).
Analysis of monocyte/macrophage
infiltration in the aortic sinus of ApoE−/− mice
The infiltration of monocytes and macrophages were
detected using anti-MOMA-2 antibody (Abcam, Cambridge, UK). The
aortic sinuses were embedded in a Tissue-Tek OCT compound, and then
sections of the aortic sinus (8 µm thickness) were incubated with
anti-MOMA-2 antibody (1:100), followed by Alexa Fluor 488 nm goat
anti-rat IgG (1:200; Invitrogen Life Technologies) for
visualization. Images of the aortic sinus were captured under
confocal laser scanning microscopy (Carl Zeiss AG, Oberkochen,
Germany).
Statistical analysis
Numerical data are presented as the mean ± SEM.
Comparisons two groups were performed using a two-tailed Student's
t-test. Comparisons multiple groups were performed using
Tukey-Kramer HSD test after the one-way ANOVA. The threshold of
significance was set at P<0.05.
Results
SA reduces formation of
atherosclerotic lesions in the aorta of apoE−/−
mice
We examined the effect of SA on the formation of
atherosclerotic lesions in vivo. SA was administered to
apoE−/− mice under an atherogenic western diet for 8
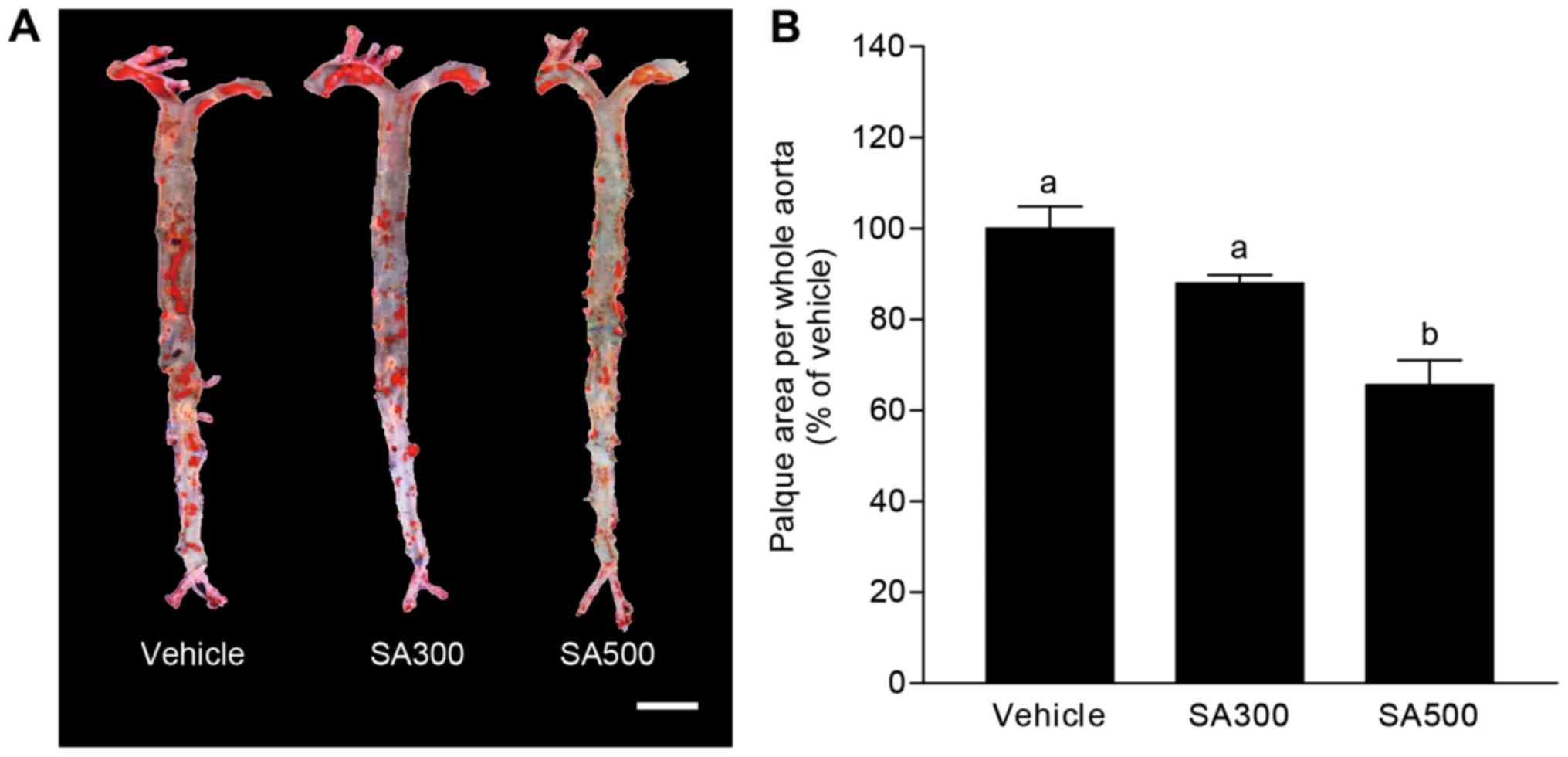
weeks and the whole aorta was obtained. Fig. 1A shows aorta en face stained by
Oil-red O, and the area of atherosclerotic plaque was evaluated as
shown in Fig. 1B. The percentage of
aortic surface area covered by atherosclerotic lesions was tended
to decrease in SA300 group (87.9±1.3%) compared with vehicle group
(Fig. 1). Interestingly, SA500 group
(65.6±2.4%) showed significantly reduced atherosclerotic lesion
area in aorta en face compared with vehicle group (Fig. 1). These results could suggest that SA
has inhibitory effects on atherogenic lesion formation in
apoE−/− mice.
SA diminishes atherosclerotic area in
the aortic sinus of apoE−/− mice
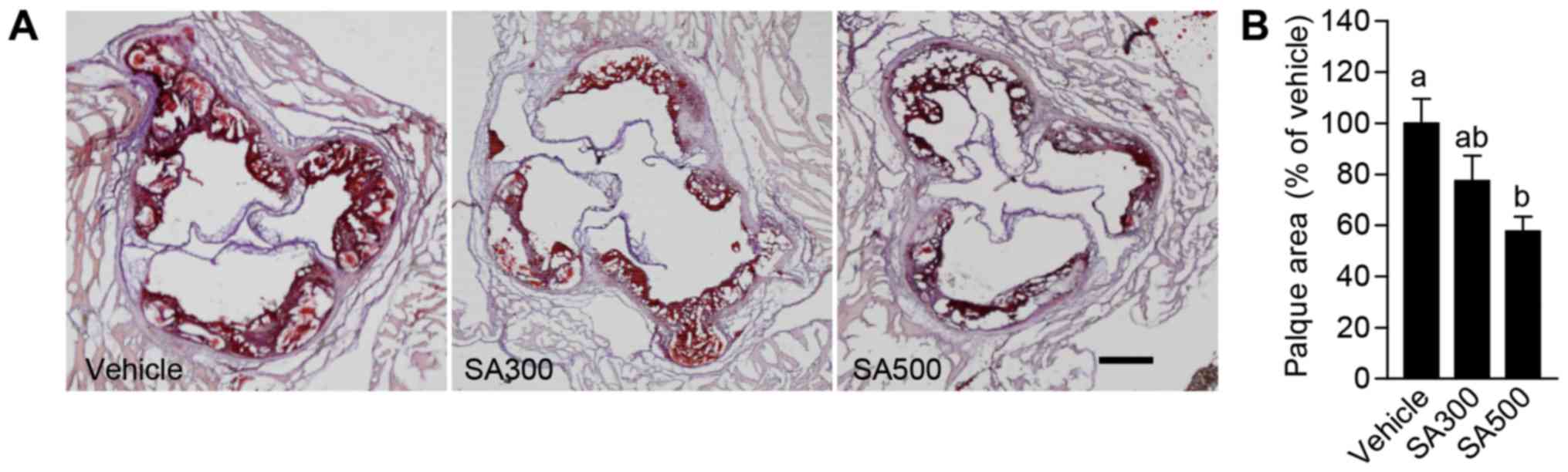
To confirm the anti-atherogenic effects of SA in
aorta, atherosclerotic lesions were analyzed in aortic sinus.
Aortic sinus stained by Oil-red O (Fig.
2A), and the area of atherosclerotic lesions was analyzed
(Fig. 2B). SA300 group (77.4±6.6%)
showed slightly reduced atherosclerotic lesion area in aortic sinus
compared with vehicle group (Fig.
2). However, the percentage of aortic sinus covered by
atherosclerotic plaque was significantly decreased in SA500 group
(57.6±2.3%) compared with vehicle group (Fig. 2). From these results, it is
demonstrated that SA can ameliorate atherosclerosis in
apoE−/− mice.
Hyperlipidemia in apoE-/- mice is not
affected by SA administration
Hyperlipidemia is an elevation of plasma lipids,
such as cholesterol or triglyceride, in the blood, and has been
known as potential risk factor for atherosclerosis (11,17).
Therefore, we firstly evaluated whether the anti-atherogenic
effects of SA is associated with plasma lipid modulation in
atherogenic diet-fed apoE−/− mice. Plasma triglyceride
levels were not significantly different, and were tended to rather
increase in both SA300 group and SA500 group compared with vehicle
group (Table II). In addition,
hypercholesterolemia shown by high plasma TC, HDL-C and LDL-C
levels in vehicle group was comparable with both SA300 group and
SA500 group (Table II). These
results show that the SA may reduce atherogenic lesions independent
of hyperlipidemia regulation in apoE−/− mice.
 | Table II.Effects of SA on plasma biomarkers in
ApoE KO mice. |
Table II.
Effects of SA on plasma biomarkers in
ApoE KO mice.
| Groups | ALT (IU/l) | AST (IU/l) | BUN (mg/dl) | CK (IU/l) | TG (mg/dl) | TC (mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) |
|---|
| Vehicle | 17.8±1.2 | 95.4±2.2 | 28.8±0.4 | 192.0±7.9 | 120.0±1.4 | 2819.2±25.4 | 93.1±1.1 | 2506.2±21.5 |
| SA300 | 17.0±0.9 | 85.5±2.1 | 29.5±0.6 | 159.5±8.5 | 147.5±14.5 | 2645.0±142.2 | 100.0±6.1 | 2302.5±143.5 |
| SA500 | 13.7±0.6 | 88.7±2.8 | 29.5±0.3 | 144.5±6.4 | 129.2±2.5 | 2778.3±33.2 | 100.0±1.6 | 2438.3±29.5 |
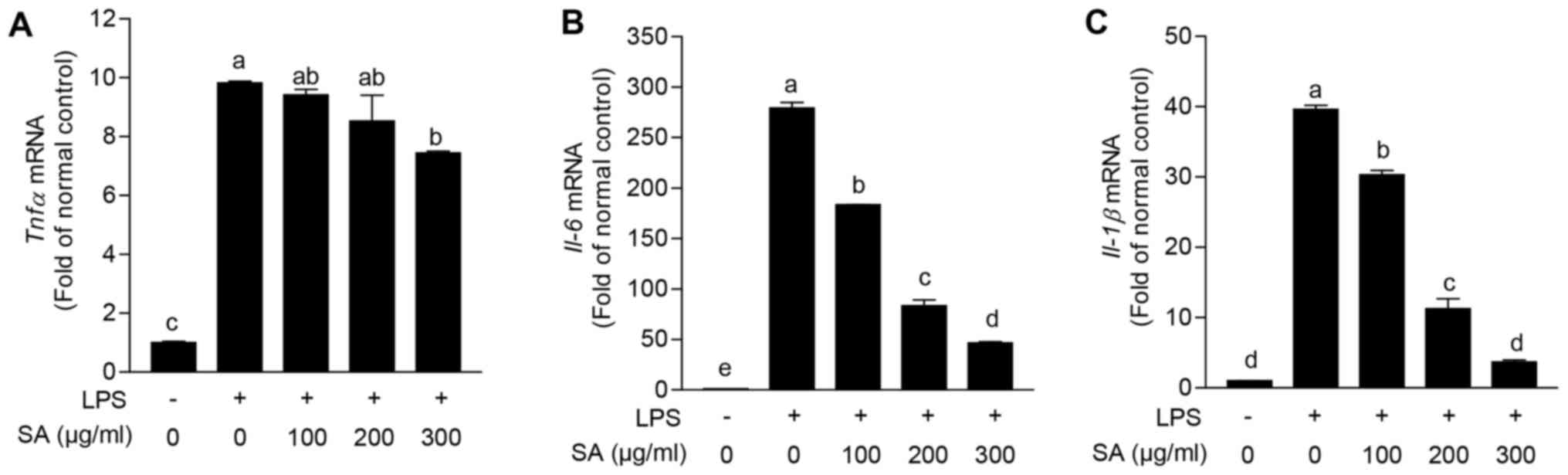
SA inhibits the gene expression of
proinflammatory cytokines in LPS-stimulated RAW 264.7 cells
The pathogenesis of atherosclerosis is inseparably
related with proinflammatory cytokines released by various immune
cells including macrophage (18,19), and
we identified whether SA can regulate proinflammatory reaction of
immune cell in in vitro. RAW 264.7 cells, mouse macrophage
cell line, were stimulated with LPS, and gene expression levels of
proinflammatory cytokines were evaluated. LPS stimulation highly
increased the expression levels of Tnfα (9.8±0.1-fold),
Il-6 (279.2±8.1-fold) and Il-1β (39.6±0.8-fold)
compared with vehicle treated group. Intriguingly, co-treatment of
SA eminently reduced all of these elevated proinflammatory cytokine
expression levels in a dose dependent manner (Fig. 3). From these results, it is suggested
that SA can diminish proinflammatory reaction in macrophage.
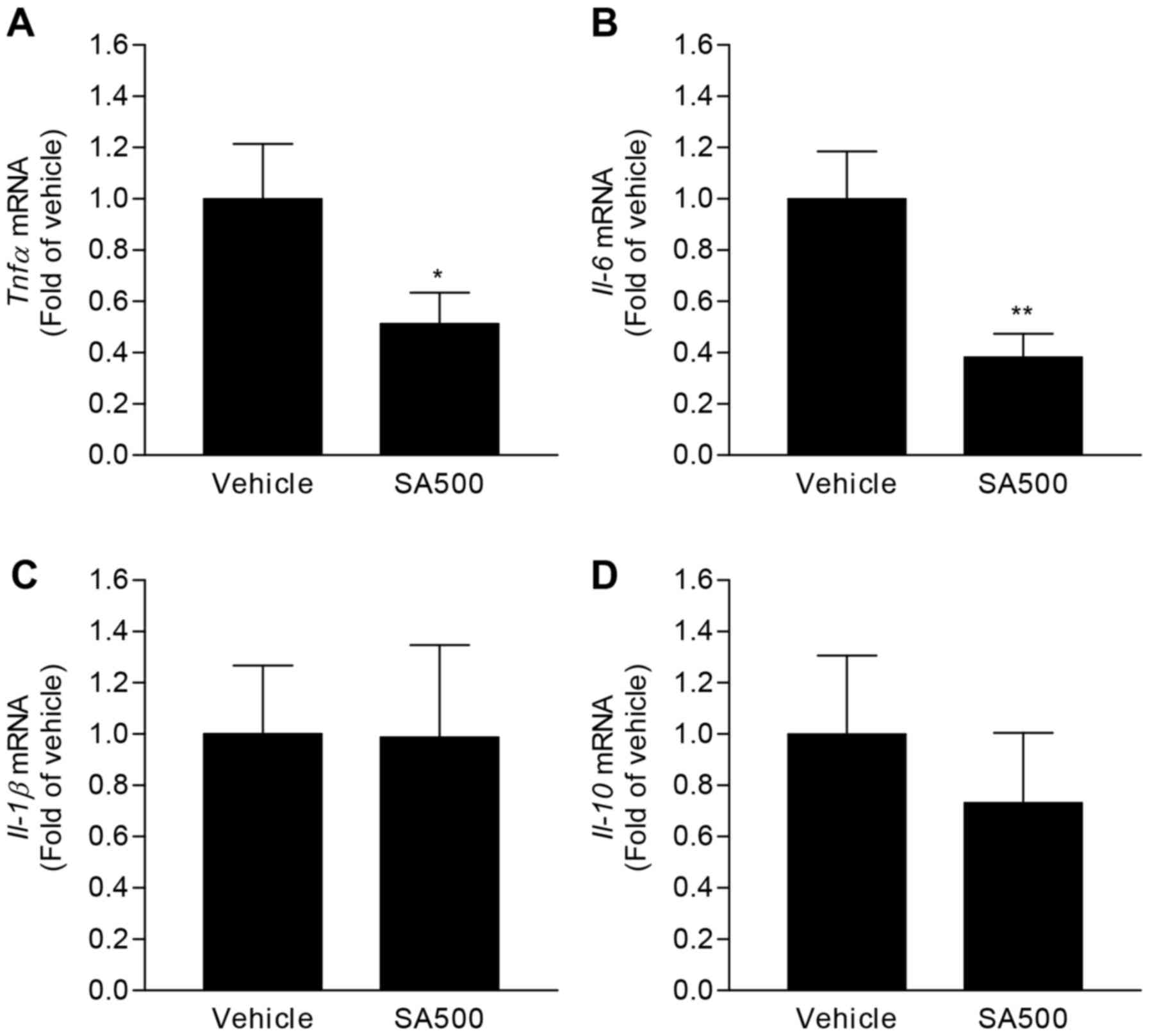
Proinflammatory cytokine expressions
are reduced by SA treatment in aorta of apoE−/−
mice
To examine anti-inflammatory effects of SA shown in
in vitro, aortic expression levels of cytokines were
measured in SA-treated apoE−/− mice. Because significant
anti-atherogenic effects were only found in SA500 group, we focused
on molecular biological changes in SA500 group. In accordance with
in vitro results, gene expression levels of Tnfα
(0.51±0.05-fold) and Il-6 (0.38±0.03-fold) were
significantly decreased in aorta of SA500 group compared with
vehicle group (Fig. 4A and B).
However, Il-1β and Il-10 expression levels were comparable between
vehicle group and SA500 group (Fig. 4C
and D). These results would demonstrate that SA reduces
proinflammatory responses in aorta of atherogenic diet-fed
apoE−/− mice.
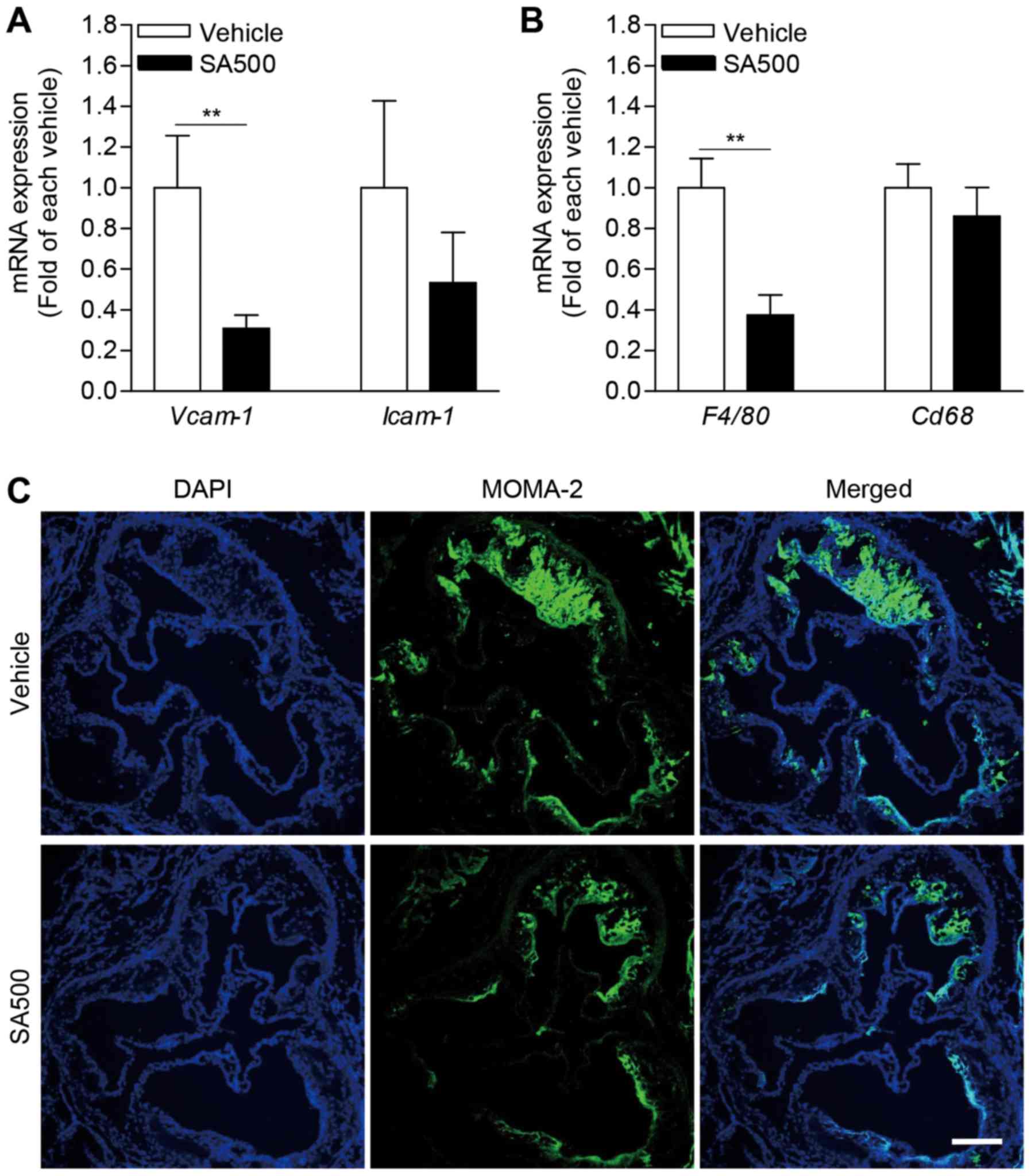
SA inhibits aortic expression of
adhesion molecules and infiltration of monocyte/macrophage in
apoE−/− mice
We further evaluated aortic adhesion molecules,
which is important for immune cell migration in pathogenesis of
atherosclerosis (20).
Interestingly, Vcam-1 expression level was significantly
diminished in aorta of SA500 group (0.31±0.03-fold) compared with
vehicle group (Fig. 5A). SA500
treatment also tended to reduce aortic Icam-1 expression
(0.53±0.14-fold) (Fig. 5A). In line
with decreased aortic expression of adhesion molecules, expression
of F4/80, a marker of murine macrophage, was significantly
reduced in aorta of SA500 group (0.37±0.04-fold) compared with
vehicle group (Fig. 5B). Aortic
Cd68 expression was also tended to decrease by SA
administration in apoE−/− mice (Fig. 5B). Based on aortic expression of
macrophage marker, next, we confirmed macrophages infiltration
using immunofluorescence staining for MOMA-2 in atherosclerotic
plaque of aortic sinus (Fig. 5C). In
accordance with aortic F4/80 and Cd68 expression
levels, the areas stained by MOMA-2 were prominently diminished in
SA500 group compared with vehicle group. Taken together, these
findings suggest that SA treatment can inhibits aortic adhesion
molecule expression followed by reduced monocyte/macrophage
infiltration.
SA administration does not show
toxicological phenotype in apoE−/− mice
To evaluate the toxicity of SA in in vivo,
toxicological markers found in blood were determined in plasma of
8-week-SA-treated apoE−/− mice. Plasma ALT, AST, BUN and
CK levels were comparable among vehicle group, SA300 group and
SA500 group (Table II). Moreover,
the changes in body weight were not decreased but rather increased
in both SA300 group and SA500 group compared with vehicle group
(data not shown). Based on these results, it is suggested that
long-term treatment of SA, at least for 8 weeks, does not evoke
significant toxic effects in apoE−/− mice.
Discussion
The present study demonstrated a novel beneficial
effects and useful roles of noxious plant SA in atherosclerosis,
and provided the insight into anti-inflammatory potency of SA. When
SA was administered to apoE−/− mice under an atherogenic
western diet for 8 weeks, atherosclerosis was prominently
ameliorated, as revealed by smaller atherosclerotic lesion area,
reduced expression of proinflammatory cytokines and adhesion
molecules, and decreased monocyte/macrophage infiltration in whole
aorta or aortic sinus.
Atherosclerosis is a complex disease in which many
processes contribute to lesion development (17). Until now, it is well accepted that
hypercholesterolemia, especially high level of plasma LDL-C, play a
key role in the initiation and progression of atherosclerosis
(11,17). In healthy conditions, the endothelium
maintains the homeostasis of vascular wall via control of vascular
tone (21). Nitric oxice (NO) plays
a central atheroprotective role through the regulation of this
vascular tone (22). Whereas, the
exposure to high-LDL-C levels shows to decrease NO bioavailability,
and causes endothelial dysfunction resulting in the preceding step
to progression of atherosclerosis via LDL-C entry within the
arterial intima (23). In clinical
approach, lipid-lowering therapy with statins can lower LDL-C and
effective in prevention of primary stroke by atherosclerosis
(24). In contrary to LDL-C, HDL-C
are generally inversely associated with the risk for the
development of atherosclerosis (17). The major anti-atherosclerotic effect
of HDL-C is reverse cholesterol transport, which scavenge
cholesterol from the peripheral vasculature with transport to the
liver where is it excreted in the biliary system (17,25). In
the present study, however, the levels of LDL-C, HDL-C and other
lipids were not changed by SA administration in plasma of
atherogenic diet-fed apoE−/− mice. These results
demonstrate that protective effect of SA is not related with
modulation of plasma lipid profile in the context of
atherogenesis.
Atherosclerosis is a well-known progressive chronic
inflammatory disease, and many cytokines are expressed in
atherosclerotic plaques (10–12,18).
Various cells involved in atherosclerosis are capable of producing
cytokines and responding to them, and these cytokines can modulate
atherogenesis (18,26). Among cytokines, it has been
demonstrated that TNFα plays a key role in development of
atherosclerosis (27). TNFα cause
reorganization of the actin and tubulin cytoskeletons in
endothelial cells, thereby opening up gaps between adjacent cells
(28). Moreover, TNF-deficient
apoE−/− mice shows significantly smaller atherosclerotic
lesion size in the aortic sinus than that of control mice (29). In accordance with these previous
studies, anti-atherogenic effects of SA was correlated with reduced
Tnfα expression in aorta of atherogenic diet-fed mice and
macrophage cells. Meanwhile, in the present study, SA also reduced
expression of Il-6 in aorta of apoE−/− mice and
macrophage cells. IL-6 has been suggested as proatherogenic
cytokine, as evidenced by increased atherogenic lesion in IL-6
treated apoE−/− mice and destabilized plaques by
letivirus-induced IL-6 overexpression in mice (30,31).
Inhibition of IL-6 trans-signaling reduces atherosclerosis
by decreasing endothelial cell activation and recruitment of
monocytes (32). However, old
IL-6-deficient apoE−/− and LDLr−/− mice show
enhanced atherosclerotic plaque formation (33,34).
Although the role of IL-6 in atherogenesis still appears
ambivalent, it could be considered that modulation of IL-6 is
related with regulatory roles of SA in atherosclerosis in
apoE−/− mice. In regards to IL-1β, mouse models of
atherosclerosis have confirmed the proatherogenic properties of
IL-1β, associated with upregulation of endothelial adhesion
molecules and activation of macrophages and vascular cells
(35,36). Bone marrow transplantation study in
IL-1 receptor and apoE double knockout mice have shown that
selective loss of IL-1 in the vessel wall reduces plaque burden
rather than immune cells (37).
Intriguingly, SA reduced Il-1β expression not in aorta of
apoE−/− mice but only in LPS-stimulated macrophage
cells, and it could be supposed that anti-atherogenic effects of SA
may not be dependent on IL-1β. Collectively, SA may reduce
atherosclerotic lesions through regulation of several
proatherogenic cytokines in apoE−/− mice.
It has been well-known that recruitment of
circulating immune cells, particularly monocytes, is crucial for
initiation and progression of atherosclerosis (38,39). A
triggering event for this process is induction of proatherogenic
factors including cytokines, which stimulate the overlying
endothelial cells to produce adhesion molecules (1). In this regard, TNF-deficiency in
apoE−/− mice shows decreased atherosclerotic lesion in
the aortic sinus, which is associated with decreased expression of
VCAM-1 and ICAM-1 (29). In line
with theses previous studies, SA significantly reduced Tnfα
expression followed by declined expression of Vcam-1 and
Icam-1 in aorta, and ameliorated atherosclerosis in
atherogenic diet-fed apoE−/− mice. Moreover, decreased
expression of adhesion molecules may be accompanied by reduced
monocyte/macrophage infiltration in aortic sinus of atherogenic
diet-fed apoE−/− mice. These findings suggest that SA
improve atherosclerosis, in part, by downregulation of adhesion
molecule expressions.
Previous studies on chemical constituents from SA
reported that some sterols and flavonoids were consisted in this
plant (40,41). In the present study, we have not
found the key compound for the anti-atherogenic effects of SA, but
we are going to try to isolate chemical constituents by
activity-guided fractionations with in immune cell lines. As we
isolate active constituents with this in vitro cell line
system, the biological activities of the isolated compounds will be
tested and confirmed with atherogenic diet-fed apoE−/−
mice. Further studies are needed and will be continued for the
confirmation of key compound for the anti-atherogenic property of
SA.
In conclusion, this is the first study to
demonstrate that SA suppresses the development of atherosclerosis
by inhibiting the expression of proatherogenic factors including
inflammatory cytokines and adhesion molecules, which is followed by
reduction of atherogenic plaque formation and immune cell
infiltration in aorta of apoE−/− mice. The present study
provides new insight into the usefulness and the beneficial effects
of noxious plant SA in medicine field, and identifies the
potentiality of SA as a therapeutically effective novel natural
product for preventing atherosclerosis.
Acknowledgements
We appreciate In-Bok Lee, Young-Keun Choi, Jung-Hyun
Choi and Yun-Jeong Seo for technical assistance. This study was
supported by a grant from the National Research Foundation of Korea
(NRF) and the Korean government (MSIP) (2016R1A2A1A05004858), KRIBB
Research Initiative Program of the Republic of Korea, and the
Development of Platform Technology for Innovative Medical
Measurements Program from Korea Research Institute of Standards and
Science (KRISS-2017-GP2017-0020).
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart Disease and Stroke Statistics-2017 Update: A Report
From the American Heart Association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vargas JD, Manichaikul A, Wang XQ, Rich
SS, Rotter JI, Post WS, Polak JF, Budoff MJ and Bluemke DA: Common
genetic variants and subclinical atherosclerosis: The Multi-Ethnic
Study of Atherosclerosis (MESA). Atherosclerosis. 245:230–236.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hicken MT, Adar SD, Hajat A, Kershaw KN,
Do DP, Barr RG, Kaufman JD and Diez Roux AV: Air pollution,
cardiovascular outcomes and social disadvantage: The Multi-ethnic
Study of Atherosclerosis. Epidemiology. 27:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spring B, Moller AC, Colangelo LA,
Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S and
Liu K: Healthy lifestyle change and subclinical atherosclerosis in
young adults: Coronary Artery Risk Development in Young Adults
(CARDIA) study. Circulation. 130:10–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian J, Gu X, Sun Y, Ban X, Xiao Y, Hu S
and Yu B: Effect of statin therapy on the progression of coronary
atherosclerosis. BMC Cardiovasc Disord. 12:702012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoffmann H, Frieler K, Schlattmann P, Hamm
B and Dewey M: Influence of statin treatment on coronary
atherosclerosis visualised using multidetector computed tomography.
Eur Radiol. 20:2824–2833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabas I, García-Cardeña G and Owens GK:
Recent insights into the cellular biology of atherosclerosis. J
Cell Biol. 209:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witztum JL and Steinberg D: Role of
oxidized low density lipoprotein in atherogenesis. J Clin Invest.
88:1785–1792. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rocha VZ and Libby P: Obesity,
inflammation, and atherosclerosis. Nat Rev Cardiol. 6:399–409.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansson GK and Libby P: The immune
response in atherosclerosis: A double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi H, Kurokawa S and Ikeda K:
Dairyland populations of bur cucumber (Sicyos angulatus) as
a possible seed source for riverbank populations along the Abukuma
River, Japan. Weed Biol Manag. 12:147–155. 2012. View Article : Google Scholar
|
|
14
|
Lee SM, Radhakrishnan R, Kang SM, Kim JH,
Lee IY, Moon BK, Yoon BW and Lee IJ: Phytotoxic mechanisms of bur
cucumber seed extracts on lettuce with special reference to
analysis of chloroplast proteins, phytohormones and nutritional
elements. Ecotoxicol Environ Saf. 122:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hulina N: New dangerous weed in Croatia:
Sicyos angulatus L. (Cucurbitaceae). Poljopr Znan Smotra.
61:259–264. 1996.
|
|
16
|
Watanabe O, Kurokawa S, Sasaki H, Nishida
T, Onoue T and Yoshimura Y: Geographic scale distribution and
occurrence pattern of invasive weeds. Grassl Sci. 48:440–450.
2002.
|
|
17
|
Badimon L and Vilahur G: LDL-cholesterol
versus HDL-cholesterol in the atherosclerotic plaque: Inflammatory
resolution versus thrombotic chaos. Ann N Y Acad Sci. 1254:18–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ait-Oufella H, Taleb S, Mallat Z and
Tedgui A: Recent advances on the role of cytokines in
atherosclerosis. Arterioscler Thromb Vasc Biol. 31:969–979. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramji DP and Davies TS: Cytokines in
atherosclerosis: Key players in all stages of disease and promising
therapeutic targets. Cytokine Growth Factor Rev. 26:673–685. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blankenberg S, Barbaux S and Tiret L:
Adhesion molecules and atherosclerosis. Atherosclerosis.
170:191–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Merritt WT: Nitric oxide: An important
bioregulator. Transplant Proc. 25:2014–2016. 1993.PubMed/NCBI
|
|
22
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vidal F, Colomé C, Martínez-González J and
Badimon L: Atherogenic concentrations of native low-density
lipoproteins down-regulate nitric-oxide-synthase mRNA and protein
levels in endothelial cells. Eur J Biochem. 252:378–384. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Law MR, Wald NJ and Rudnicka AR:
Quantifying effect of statins on low density lipoprotein
cholesterol, ischaemic heart disease, and stroke: Systematic review
and meta-analysis. BMJ. 326:14232003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trigatti BL, Krieger M and Rigotti A:
Influence of the HDL receptor SR-BI on lipoprotein metabolism and
atherosclerosis. Arterioscler Thromb Vasc Biol. 23:1732–1738. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McLaren JE, Michael DR, Ashlin TG and
Ramji DP: Cytokines, macrophage lipid metabolism and foam cells:
Implications for cardiovascular disease therapy. Prog Lipid Res.
50:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKellar GE, McCarey DW, Sattar N and
McInnes IB: Role for TNF in atherosclerosis? Lessons from
autoimmune disease. Nat Rev Cardiol. 6:410–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohta H, Wada H, Niwa T, Kirii H, Iwamoto
N, Fujii H, Saito K, Sekikawa K and Seishima M: Disruption of tumor
necrosis factor-alpha gene diminishes the development of
atherosclerosis in ApoE-deficient mice. Atherosclerosis. 180:11–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huber SA, Sakkinen P, Conze D, Hardin N
and Tracy R: Interleukin-6 exacerbates early atherosclerosis in
mice. Arterioscler Thromb Vasc Biol. 19:2364–2367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang K, Huang XZ, Li XN, Feng M, Li L,
Cai XJ, Zhang C, Liu XL, Zhang MX, Zhang Y, et al: Interleukin 6
destabilizes atherosclerotic plaques by downregulating
prolyl-4-hydroxylase alpha1 via a mitogen-activated protein kinase
and c-Jun pathway. Arch Biochem Biophys. 528:127–133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schuett H, Oestreich R, Waetzig GH, Annema
W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D,
Kempf T, et al: Transsignaling of interleukin-6 crucially
contributes to atherosclerosis in mice. Arterioscler Thromb Vasc
Biol. 32:281–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schieffer B, Selle T, Hilfiker A,
Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M,
Schmittkamp C, Heeneman S, et al: Impact of interleukin-6 on plaque
development and morphology in experimental atherosclerosis.
Circulation. 110:3493–3500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song L and Schindler C: IL-6 and the acute
phase response in murine atherosclerosis. Atherosclerosis.
177:43–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirii H, Niwa T, Yamada Y, Wada H, Saito
K, Iwakura Y, Asano M, Moriwaki H and Seishima M: Lack of
interleukin-1beta decreases the severity of atherosclerosis in
ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 23:656–660.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clarke MC, Talib S, Figg NL and Bennett
MR: Vascular smooth muscle cell apoptosis induces
interleukin-1-directed inflammation: Effects of
hyperlipidemia-mediated inhibition of phagocytosis. Circ Res.
106:363–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shemesh S, Kamari Y, Shaish A, Olteanu S,
Kandel-Kfir M, Almog T, Grosskopf I, Apte RN and Harats D:
Interleukin-1 receptor type-1 in non-hematopoietic cells is the
target for the pro-atherogenic effects of interleukin-1 in
apoE-deficient mice. Atherosclerosis. 222:329–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weber C, Zernecke A and Libby P: The
multifaceted contributions of leukocyte subsets to atherosclerosis:
Lessons from mouse models. Nat Rev Immunol. 8:802–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moore KJ, Sheedy FJ and Fisher EA:
Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol.
13:709–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akihisa T, Tamura T and Matsumoto T:
24-Methylene-25-methyllathosterol: A sterol from Sicyos
angulatus. Phytochemistry. 26:575–577. 1987. View Article : Google Scholar
|
|
41
|
Na CS, Lee YH, Murai Y, Iwashina T, Kim TW
and Hong SH: Flavonol 3,7-diglycosides from the aerial parts of
Sicyos angulatus (Cucurbitaceae) in Korea and Japan. Biochem
Syst Ecol. 48:235–237. 2013. View Article : Google Scholar
|