Introduction
Herpes Zoster (HZ), which is a form of recurrent
infection of varicella zoster virus (VZV), typically occurs decades
after an instance of childhood VZV attack (1). It occurs most commonly in otherwise
healthy, elderly individuals and immunocompromised hosts, including
those with acquired immune deficiency syndrome, malignancies and
systemic lupus erythematosus (SLE), amongst other conditions
(2). The annual incidence of HZ is
1.5–3.0 per 1,000 persons in the general population in the United
States and markedly increased to 6.4–32.5 per 1,000 person-years in
patients with SLE (3–6). Furthermore, compared with healthy
individuals, patients with SLE and HZ have a greater tendency to
develop cutaneous and visceral dissemination of lesions (7), which are significantly associated with
a poor prognosis (7,8). Furthermore, those with HZ are also more
likely to develop bacterial superinfection during their disease
course (9). At present, the most
effective way to reduce the incidence and severity of HZ is zoster
vaccination (10,11). Despite the overall efficacy of the
zoster vaccine, this is uncertain in SLE patients and has a high
financial cost (12,13). The zoster vaccine is currently not
available in China, therefore patients who require treatment have
to go abroad. Consequently, it is essential for clinicians to
identify the risk factors that may predispose patients with SLE to
the development of HZ. Risk factors predisposing SLE patients to HZ
infection are not well established. A number of previous studies
have attempted to identify risk factors for HZ in SLE; however, the
findings of these studies were inconsistent (9,14,15).
Furthermore, whereas the role of leucopenia has been extensively
analyzed (15), lymphopenia as a
risk factor for HZ is seldom discussed. However, incidence rates,
patient history and risk factors differ markedly by geographical
location and among different ethnic groups (3–6),
possibly due to genetic and environmental factors. Little is known
about the clinical features of SLE when complicated with HZ in
Southern China.
It was hypothesized that a study of HZ in patients
with SLE in Southern China may help increase the awareness of the
extent and natural history of this disease in the region, assess
therapeutic strategies on higher risk patients with HZ, and
identify any subgroup(s) of patients who may benefit the most from
the new zoster vaccine. In these regards, a retrospective study was
performed to systematically examine the prevalence, nature and
complications of HZ in patients with SLE in a single rheumatology
center. Clinical and laboratory features, as well as administered
therapies were reviewed to determine their association with the
occurrence of HZ as well as the complications of HZ.
Patients and methods
Study design and patients
A retrospective study was conducted using clinical
records from the First Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China) from January 2009-January 2013. A
total of 1,265 patients recruited from the rheumatology outpatient
clinics and inpatients department were diagnosed with SLE and
fulfilled ≥4 of the American College of Rheumatology revised
classification criteria for SLE (1997) (16). A diagnosis of HZ was clinically
established by the presence of a typical vesicular eruption
developing in a dermatomal distribution (14). Viral isolation by culture or serology
was not done for confirmation of HZ. For the present study, only
those patients with a history of HZ following the diagnosis of SLE
were included. Patients with a history of HZ onset prior to SLE
were excluded. Among these SLE patients, 46 were diagnosed with HZ,
and another 48 age- and gender-matched SLE patients without history
of HZ were randomly selected as control.
Review of the clinical files of these 94 SLE
patients was performed and data was extracted. From the initial
diagnosis of SLE, patients were subsequently followed-up until the
occurrence of HZ. Follow-up also ended in instances of death or if
there was no episode of HZ prior to the last documented visit.
Disease definitions
Complications of HZ were defined as the occurrence
of one or more of the following conditions: i) Ocular, visceral or
neurological involvements caused by VZV (17); ii) chronic (lasting for >30 days)
atypical skin lesions; iii) postherpetic neuralgia (defined as pain
persisting for >6 weeks following initial appearance of the
rash) (8); iv) cutaneous
dissemination (defined as vesicular lesions outside the primary and
adjacent dermatomes) (7); and v)
infections associated with HZ that required treatment with
antibiotics.
Leucopenia was defined as a total white blood cell
count <4.0×109/l. Neutropenia was defined as a total
neutrophil count <1.5×109/l. Lymphopenia was defined
as a total lymphocyte count <1.0×109/l. Monocytosis
was defined as a total monocyte count >1.0×109/l.
Demographic, clinical, laboratory data
and therapeutic variables
The following data were collected from medical
records: Demographic information including gender and age at SLE
diagnosis; duration between the onset of SLE and HZ; lupus disease
activity; complications of HZ; clinical symptoms and signs; and
laboratory findings such as blood routine test, various
autoantibodies, erythrocyte sedimentation rate and C-reactive
protein. The use of therapeutic agents including glucocorticoids
(GC) and other immunosuppressive agents (ISA) within 1 month of HZ
onset was also recorded. The dosage of GC was defined as none,
low-dose (<30 mg prednisone or equivalent per day) or high-dose
(≥30 mg prednisone or equivalent per day). The SLE Disease Activity
Index (SLEDAI) was used to evaluate SLE activity during HZ, and
patients were defined as active SLE if the SLEDAI score was ≥6
(18).
Statistical analysis
Data was analyzed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Continuous data are presented as the mean ±
standard deviation. Categorical variables were presented as the
absolute value and percentage. The Mann-Whitney U test was used to
compare differences in continuous variables, and the chi-squared
test was used for categorical variables. Variables that
demonstrated significant associations with dependent variables in
univariate analysis were included in a stepwise multivariate
logistic regression analysis. Odds ratios (ORs) and 95% confidence
intervals (CIs) were also calculated. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic data
In the present retrospective analysis, from a total
of 1,265 patients with SLE, 48 instances of HZ were recorded in 46
patients. In total, HZ patients accounted for a prevalence of 3.6%.
Of these 48 patients, 2 experienced multiple episodes (2 each) of
HZ, whereas the remaining 44 patients experienced only one episode
during the follow-up period.
All subjects were of Chinese Han ethnicity. As
presented in Table I, the majority
of patients with HZ were female (95.7%) and mean age at the
diagnosis of SLE was 35.0±14.0 years (range, 18–75 years). The mean
duration between the diagnosis of SLE and HZ was 9.1±1.3 months,
with a range from 0.5–36.0 months. Following SLE diagnosis, the
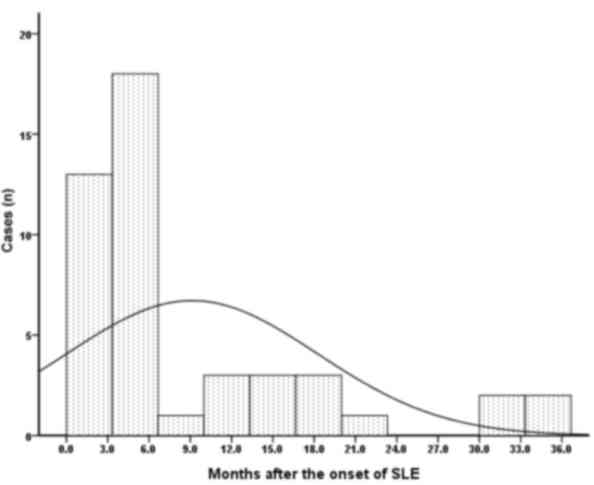
risk of HZ was highest within 3–6 months and reduced thereafter
(Fig. 1). The majority (31/46;
67.4%) of patients experienced HZ in this period, whereas the
remaining 15 patients developed HZ in the chronic stage of SLE
(>6 months).
 | Table I.Comparison between SLE patients with
and without HZ. |
Table I.
Comparison between SLE patients with
and without HZ.
| Characteristic | With HZ (n=46) | Without HZ
(n=48) | P-value |
|---|
| Demographic
characteristics |
|
|
|
| Sex,
male:female | 2:44 | 7:41 | 0.2 |
| Age at
SLE diagnosis, years | 35.0±14.0 | 33.8±14.4 | 0.7 |
| Laboratory data |
|
|
|
| ANA
positive, n (%) | 45 (97.8) | 43 (89.6) | 0.2 |
|
Anti-dsDNA positive, n
(%) | 27 (58.7) | 24 (50.0) | 0.4 |
| Anti-Sm
antibody positive, n (%) | 3 (6.5) | 8
(16.7) | 0.2 |
| ACL
positive, n (%) | 3 (6.5) | 9
(18.8) | 0.1 |
|
Leucopenia, n (%) | 7
(15.2) | 12 (25.0) | 0.3 |
|
Neutropenia, n (%) | 6
(13.0) | 8
(16.7) | 0.8 |
|
Lymphopenia, n (%) | 17 (37.0) | 6
(12.5) | 0.008 |
|
Monocytosis, n (%) | 18 (39.1) | 14 (29.2) | 0.4 |
| Anemia, n
(%) | 29 (63.0) | 24 (50.0) | 0.2 |
|
Hypoproteinemia, n (%) | 26 (56.5) | 23 (47.9) | 0.4 |
| ESR,
mm/h | 36.8±30.7 | 44.1±23.9 | 0.2 |
| CRP,
mg/l | 24.3±61.7 | 6.7±12.0 | 0.08 |
| Clinical
features |
|
|
|
| Renal
involvement, n (%) | 36 (78.3) | 27 (56.2) | 0.03 |
|
Neuro-psychiatric
manifestations, n (%) | 3 (6.5) | 3 (6.2) | 1 |
|
Articular, n (%) | 20 (43.5) | 23 (47.9) | 0.7 |
|
Mucocutaneous involvement, n
(%) | 21 (45.7) | 26 (51.0) | 0.6 |
|
Serositis, n (%) | 5
(10.9) | 7
(14.6) | 0.7 |
|
SLEDAI | 9.1±7.4 | 5.4±4.2 | 0.004 |
| Active
lupus, n (%) | 25 (54.3) | 14 (29.2) | 0.02 |
| Number
of organs involved | 2.4±1.3 | 2.3±1.2 | 0.6 |
| Treatments prior to
HZ onset |
|
|
|
|
Cyclophosphamide, n (%) | 20 (43.5) | 17 (35.4) | 0.4 |
|
Mycophenolate motifile, n
(%) | 8
(17.4) | 9
(18.8) | 0.9 |
|
Methotrexate, n (%) | 6
(13.0) | 7
(16.7) | 0.4 |
|
Hydroxychloroquine, n (%) | 38 (82.6) | 32 (66.7) | 0.08 |
|
Glucocorticoid, n (%) | 42 (91.3) | 42 (87.5) | 0.7 |
| Dosage,
mg/day | 34.8±38.4 | 13.9±11.7 | <0.001 |
|
Low-dosea, n (%) | 22 (47.8) | 34 (70.8) | 0.01 |
|
High-doseb, n (%) | 20 (43.5) | 8
(16.7) | 0.004 |
Clinical characteristics of SLE
patients with HZ
HZ skin lesions were most commonly located in the
thoracic region (37/48; 77.1%), followed by the lumbar segment
(6/48; 12.5%) and sacral area (3/48; 6.3%). The dermatome occurred
in trigeminal regions in 2 instances (2/48, 4.2%), 1 in the inner
corner of the eyelids and 1 on the tip of the ear.
A total of 35 patients exhibited typical skin
lesions without complications. Of the remaining 11 patients who had
complications in their disease course, 4 (8.7%) had superimposed
infections that required antibiotic therapy (2 with pneumonia, 2
with sepsis and 1 with cellulitis), and 7 (15.2%) had postherpetic
neuralgia. Only 2 of these 7 patients with postherpetic neuralgia
had a course longer than 12 months. No patients in the present
cohort experienced other serious complications, such as ocular,
visceral or neurological involvement, chronic atypical skin
lesions, and cutaneous dissemination.
Outcome and treatment
All patients received antiviral treatment with oral
acyclovir or ganciclovir. A total of 10 patients (21.7%) were
ultimately treated with intravenous ganciclovir due to clinical
resistance to acyclovir. All cases had complete resolution of rash
with no cutaneous scarring. There were no deaths attributed to HZ
in the present cohort.
In total, 42 of the 46 patients (91.3%) were
undergoing GC treatment at the time of HZ onset, at a mean
equivalent dose of 34.8±38.4 mg (range, 5–200 mg) prednisone. A
total of 20 patients (43.5%) received high-dose GC therapy, and the
remaining 22 patients received low-dose GC therapy. Additional ISA
were also administrated in 40 patients prior to the onset of HZ,
including 20 with cyclophosphamide (CYC), 8 with mycophenolate
mofetil (MMF), 6 with methotrexate (MTX) and 38 with
hydroxychloroquine (HCQ).
A total of 5 patients (10.9%) continued treatment
with a previous dose of GC and the remaining 37 patients were
treated with a decreased dose of GC (5.0–15.0 mg/day). Following
the onset of HZ, HCQ was continuously prescribed in 33 patients and
discontinued in the remaining 5 patients. CYC and MMF were
discontinued in all patients throughout their HZ episodes. MTX
treatment was discontinued in 4 patients and continued in the
remaining 2 patients.
Comparison of study variables between
SLE patients with and without HZ
Demographic data, clinical characteristics and
administered treatments were compared between SLE patients with and
without HZ (Table I). SLE patients
with HZ presented with a significantly higher SLEDAI score (9.1±7.4
vs. 5.4±4.2; P=0.004), a significantly higher proportion of active
lupus (54.3 vs. 29.2%; P=0.02), a significantly higher frequency of
lymphopenia (37.0 vs. 12.5%; P=0.008), and a significantly higher
percentage of renal involvement (78.3 vs. 56.2%; P=0.03) compared
with SLE patients without HZ. Focusing on immunosuppressive
treatment administered prior to the onset of HZ infection, the mean
oral GC dose was significantly higher in SLE patients with HZ
compared with non-HZ controls (34.8±38.4 vs. 13.9±11.7 mg/day;
P=<0.001). Instances of high-dose GC therapy prescription was
significantly increased in HZ patients compared with non-HZ
patients (43.5 vs. 16.7%; P=0.004). The frequency of patients who
received ISA therapy was not significantly different between the
two groups.
Factors associated with the occurrence
of HZ in SLE patients
The risk of developing HZ was calculated for
patients with SLE with various predisposing factors. All variables
with a significant association with HZ in univariate analysis
(Table II) were further included in
a stepwise multivariate logistic regression analysis. The following
variables were found to be risk factors for the development of HZ
in patients with SLE in multiple analysis: Lymphopenia (OR=4.6; 95%
CI=1.5–13.8; P=0.006) and high-dose GC therapy (OR=4.3; 95%
CI=1.6–11.7; P=0.004). Therefore, these factors may act as
predictors for the occurrence of HZ in patients with SLE.
 | Table II.Significant variables associated with
occurrence of herpes zoster on univariate logistic regression. |
Table II.
Significant variables associated with
occurrence of herpes zoster on univariate logistic regression.
| Variable | Crude OR
(95%CI) | P-value |
|---|
| Lymphopenia | 4.1 (1.4–11.7) | 0.008 |
| Renal
involvement | 2.8 (1.1–6.9) | 0.03 |
| Active
lupusa | 2.9 (1.2–6.8) | 0.01 |
| High-dose
GCb | 3.8 (1.5–10.0) | 0.006 |
Comparison of study variables between
HZ patients with and without complications
Of the 46 SLE patients with HZ, 11 developed
complications and 35 did not. The comparison of the study variables
between these two groups is presented in Table III. Patients with complications of
HZ exhibited significantly higher SLEDAI scores than patients
without complications (13.0±8.5 vs. 7.9±6.8; P=0.04), which
indicated that the disease was more active in patients with
complications. Compared with patients without complications,
patients with complications of HZ demonstrated a significantly
higher percentage of lymphopenia (63.6 vs. 28.6%; P=0.04) and more
abnormal hematologic findings (100 vs. 28.6%; P<0.001). The mean
oral prednisone dose and the percentage of patients who received
ISA therapy were similar between these two groups.
 | Table III.Comparison of SLE patients with
complicated and uncomplicated HZ. |
Table III.
Comparison of SLE patients with
complicated and uncomplicated HZ.
| Characteristic | Complicated HZ
(n=11) | Uncomplicated HZ
(n=35) | P-value |
|---|
| Demographic
characteristics |
|
|
|
| Sex,
male:female | 0:11 | 2:32 | 1.0 |
| Age,
years | 32.1±13.4 | 35.8±14.5 | 0.5 |
|
Duration of SLE, years | 3.4±5.1 | 2.9±4.4 | 0.8 |
| Laboratory
data |
|
|
|
| ANA
positive, n (%) | 11 (100) | 34
(97.14) | 1.0 |
|
Anti-dsDNA positive, n
(%) | 7 (63.6) | 20 (57.1) | 0.7 |
| Anti-Sm
antibody positive, n (%) | 1 (9.1) | 2 (5.7) | 0.7 |
| ACL, n
(%) | 1 (9.1) | 2 (5.7) | 0.7 |
|
Leucopenia, n (%) | 2
(18.2) | 5
(14.3) | 0.8 |
|
Neutropenia, n (%) | 3
(27.3) | 3 (8.6) | 0.1 |
|
Lymphopenia, n (%) | 7
(63.6) | 10 (28.6) | 0.04 |
|
Monocytosis, n (%) | 7
(63.6) | 11 (31.4) | 0.06 |
| Anemia,
n (%) | 8
(72.7) | 21 (60.0) | 0.5 |
|
Hypoproteinemia, n (%) | 8
(72.7) | 18 (51.4) | 0.2 |
| ESR,
mm/h | 32.5±25.8 | 38.4±32.5 | 0.6 |
| CRP,
mg/l | 13.9±23.0 | 28.3±71.0 | 0.4 |
| Clinical
features |
|
|
|
| Renal
involvement, n (%) | 8
(72.7) | 28 (80.0) | 0.7 |
|
Neuro-psychiatric
manifestations, n (%) | 2
(18.2) | 1 (2.9) | 0.1 |
|
Hematological involvement, n
(%) | 11 (100) | 10 (28.6) | <0.001 |
|
Articular, n (%) | 7
(63.6) | 13 (37.1) | 0.2 |
|
Mucocutaneous involvement, n
(%) | 7
(63.6) | 14 (40.0) | 0.2 |
|
Serositis, n (%) | 1 (9.1) | 4
(11.4) | 1.0 |
|
SLEDAI | 13.0±8.5 | 7.9±6.8 | 0.04 |
| Lupus
active, n (%) | 8
(72.7) | 17 (48.6) | 0.2 |
| Treatments prior to
HZ onset |
|
|
|
|
Methotrexate, n (%) | 2
(18.2) | 4
(11.4) | 0.6 |
|
Cyclophosphamide, n (%) | 6
(54.5) | 14 (40.0) | 0.5 |
|
Mycophenolate motifile, n
(%) | 3
(27.3) | 5
(13.5) | 0.4 |
|
Hydroxychloroquine, n (%) | 8
(72.7) | 30 (85.7) | 0.4 |
|
Glucocorticoid, n (%) | 10 (90.9) | 32 (91.4) | 1.0 |
| Dosage,
mg/day | 43.9±28.9 | 46.7±44.3 | 0.7 |
|
High-dosea, n (%) | 5
(45.5) | 15 (42.9) | 1.0 |
Factors associated with complications
of HZ in SLE patients
Variables associated with the onset of HZ
complications, i.e., lymphopenia and SLEDAI scores, were included
in the multivariate logistic regression model. Lymphopenia was the
only predictor of complicated HZ in the final model with an OR of
15.2 (95% CI=2.7–85.1; P=0.002). This finding indicated that SLE
patients with lymphopenia were 15.2 times more likely to develop
complications of HZ when compared with patients with normal
lymphocyte count.
Discussion
VZV typically remains latent in cranial or spinal
ganglia following resolution of a systemic infection (1). Reactivation, which tends to occur in
elderly persons and immune compromised patients, induces a
vesicular skin eruption accompanied by pruritus and dysesthesias
(19). Minimal information is
available in the literature describing the characteristics of HZ
infection. To the best of our knowledge, the present study is the
first from Southern China to focus on SLE patients with HZ
infection. Of note was the lower prevalence than that reported from
other Asian regions. In a previous study from Saudi Arabia by
Sayeeda et al (7), a total of
32 cases of HZ infection were identified among 624 SLE patients
with a prevalence of 5.1%. Ishikawa et al (6) previously reported that Japanese
patients with SLE were vulnerable to HZ with an incidence of 46.6%.
There are two possible explanations for the lower prevalence in the
present cohort. Firstly, the retrospective nature of the present
study may have led to an underestimation of the incidence caused by
mild HZ cases. Secondly, it may be due to the large number of
patients who received HCQ, which had a wide range of antimicrobial
effects. There are two previous studies that suggested that
antimalarials may be protective against infections in patients with
SLE (20,21).
The present study also indicated that most instances
of HZ occurred early in the course of the disease, with the peak
occurrence of HZ at 3–6 months following SLE diagnosis. This time
period was earlier than other reports. Nagasawa et al
(22) previously summarized that
almost half of the Japanese adult patients developed HZ in the
first year following SLE diagnosis. Borba et al (4) observed that HZ was typically a late-SLE
complication, as >50% of HZ events occurred over 5 years
following SLE diagnosis and only 7.9% within the first 2 years. In
the present study, 2/3 of HZ was developed within 6 months after
SLE diagnosis, which may be associated with activity of lupus or
drugs. Therefore, the present findings suggested that clinicians
must pay close attention to the latent vesicular skin eruption,
particularly within the initial 3–6 months following SLE
diagnosis.
Another notable finding was the higher prevalence of
complications of HZ among the present cohort than that based on
general population. The most common complication was postherpetic
neuralgia, followed by superimposed bacterial infection. In the
general population infected with HZ, postherpetic neuralgia occurs
in 5–9% of people and is significantly more common in persons over
age 60 (23,24). In the present study, the rate of
postherpetic neuralgia was in accordance with previous studies in
SLE patients (4,25), which was more frequent than that in
the general population. Conversely, superimposed bacterial skin
infection was observed in 8.7% of the present cohort, which was
higher than the 1.4% reported in a previous general
population-based study (3). All HZ
patients in the present study exhibited full recovery and none of
them experienced severe complications or succumbed to mortality.
Therefore, despite a higher frequency of complications,
complications with HZ infections in SLE patients were relatively
benign.
Only a small number of studies have explored
predictors for developing HZ in patients with SLE (9,14,15),
which have yielded inconsistent results. Furthermore, previous
studies have rarely investigated the association between
lymphopenia and HZ. The present study demonstrated that lymphopenia
led to a risk of HZ infection and serious complications concomitant
with HZ in patients with SLE. This finding was in accordance with
two previous reports. Ng et al (26) demonstrated that HZ was more likely to
develop in SLE patients with lymphopenia, and Hu et al
(15) also suggested that in
patients with SLE who developed HZ, the frequency of lymphopenia
was increased compared with those without HZ. Although the exact
mechanisms underlying the association between lymphopenia and VZV
reactivation remain to be elucidated, it was hypothesized that
decreased cell-mediated immunity serves a key role, as lymphopenia
was a more specific reflection of defective cell-mediated immunity
(CMI). The main defense against VZV reactivation in general
populations was CMI rather than humoral immunity, as recurrent VZV
infections occurred in patients with antibodies against VZV. A
number of previous studies have demonstrated that CMI in patients
with SLE may increase the risk of HZ. Nagasawa et al
(22) suggested that the high
incidence of HZ in patients with SLE was likely due to defects in
CMI. Park et al (27)
revealed that patients with lupus exhibited significantly lower
VZV-specific cluster of differentiation T cell frequencies than
rheumatoid arthritis patients and healthy controls. VZV-specific T
cells have an important role in maintaining the equilibrium between
the host and the virus during latency. A decline in the frequency
of VZV-specific T cells has been demonstrated to be associated with
an increased risk of VZV reactivation, leading to HZ (28). Furthermore, VZV itself has been
reported to induce lymphopenia once reactivated, which may also
have contributed to poor patient outcomes such as cutaneous
dissemination, prolonged atypical skin lesions, ocular
complications, and CNS involvement (29).
The present study demonstrated that high-dose GC
(≥30 mg prednisone or equivalent per day) was an independent risk
factor for infection with HZ in patients with SLE. Similar results
were previously reported by Wu et al (14) and Manzi et al (25), who noted that steroid usage increased
the risk of HZ in a dose-dependent manner. Consistent with findings
from Wu et al (14) and
Sayeeda et al (7), the
present study also demonstrated that treatment with additional ISA,
including CYC, MMF and MTX, did not appear to confer higher risk.
The present results highlighted the relevance of GC as the most
important immunosuppressive drug in terms of risk of HZ infections
in SLE. This suggests that careful monitoring of HZ occurrence is
warranted in patients with SLE taking high-dose GC, and that
discontinuing ISA therapy may be unnecessary during instances of HZ
in patients with SLE. A prospective study with a larger sample size
may be required to confirm the validity of this therapeutic
strategy by following up HZ-infected patients.
In conclusion, this is, to the best of our knowledge
the first cohort in Southern Chinese patients with SLE to determine
that HZ has features including a relatively low prevalence and more
common complications with a relatively benign course. In addition,
HZ was demonstrated to be an early complication of SLE, with the
highest risk of HZ within 3–6 months following SLE diagnosis. The
present data supported the role of lymphopenia and high-dose GC
therapy as risk factors for the occurrence HZ. Furthermore,
lymphopenia was considered as an independent risk factor for severe
HZ in patients with SLE. Therefore, these findings suggest that
careful monitoring of HZ occurrence was warranted in SLE patients
with risk factors. In addition, those with lymphopenia may benefit
most from vaccination for HZ.
Acknowledgements
The present study was supported by grants from the
Guangdong Technology Project (grant nos. 2014A020221009, and
2016A020215043) and a grant of National Natural Science Foundation
of China (grant no. 81603435).
References
|
1
|
Mahalingam R, Wellish M, Wolf W, Dueland
AN, Cohrs R, Vafai A and Gilden D: Latent varicella-zoster viral
DNA in human trigeminal and thoracic ganglia. N Engl J Med.
323:627–631. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pope JE, Krizova A, Ouimet JM, Goodwin JL
and Lankin M: Close association of herpes zoster reactivation and
systemic lupus erythematosus (SLE) diagnosis: Case-control study of
patients with SLE or noninflammatory nusculoskeletal disorders. J
Rheumatol. 31:274–279. 2004.PubMed/NCBI
|
|
3
|
Yawn BP, Saddier P, Wollan PC, St Sauver
JL, Kurland MJ and Sy LS: A population-based study of the incidence
and complication rates of herpes zoster before zoster vaccine
introduction. Mayo Clin Proc. 82:1341–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borba EF, Ribeiro AC, Martin P, Costa LP,
Guedes LK and Bonfá E: Incidence, risk factors, and outcome of
Herpes zoster in systemic lupus erythematosus. J Clin Rheumatol.
16:119–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chakravarty EF, Michaud K, Katz R and
Wolfe F: Increased incidence of herpes zoster among patients with
systemic lupus erythematosus. Lupus. 22:238–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishikawa O, Abe M and Miyachi Y: Herpes
zoster in Japanese patients with systemic lupus erythematosus. Clin
Exp Dermatol. 24:327–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sayeeda A, Al Arfaj H, Khalil N and Al
Arfaj AS: Herpes Zoster infections in SLE in a university hospital
in Saudi Arabia: Risk factors and outcomes. Autoimmune Dis.
2010:1748912010.
|
|
8
|
Oomatia A, Fang H, Petri M and Birnbaum J:
Peripheral neuropathies in systemic lupus erythematosus (SLE):
Clinical features, disease associations, and immunological
characteristics evaluated over a 25-year study period. Arthritis
Rheumatol. 66:1000–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee PP, Lee TL, Ho MH, Wong WH and Lau YL:
Herpes zoster in juvenile-onset systemic lupus erythematosus:
Incidence, clinical characteristics and risk factors. Pediatr
Infect Dis J. 25:728–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guthridge JM, Cogman A, Merrill JT,
Macwana S, Bean KM, Powe T, Roberts V, James JA and Chakravarty EF:
Herpes zoster vaccination in SLE: A pilot study of immunogenicity.
J Rheumatol. 40:1875–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimberlin DW and Whitley RJ:
Varicella-zoster vaccine for the prevention of herpes zoster. N
Engl J Med. 356:1338–1343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh JA, Furst DE, Bharat A, Curtis JR,
Kavanaugh AF, Kremer JM, Moreland LW, O'Dell J, Winthrop KL,
Beukelman T, et al: 2012 update of the 2008 American College of
Rheumatology recommendations for the use of disease-modifying
antirheumatic drugs and biologic agents in the treatment of
rheumatoid arthritis. Arthritis Care Res (Hoboken). 64:625–639.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papadopoulou D and Sipsas NV: Comparison
of national clinical practice guidelines and recommendations on
vaccination of adult patients with autoimmune rheumatic diseases.
Rheumatol Int. 34:151–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu SA, Yeh KW, Yao TC and Huang JL:
Association of herpes zoster infection with clinical
characteristics and MBL2 gene polymorphisms in Chinese children
with systemic lupus erythematosus. Pediatr Infect Dis J.
30:656–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu SC, Lin CL, Lu YW, Chen GS, Yu HS, Wu
CS and Lan CC: Lymphopaenia, anti-Ro/anti-RNP autoantibodies, renal
involvement and cyclophosphamide use correlate with increased risk
of herpes zoster in patients with systemic lupus erythematosus.
Acta Derm Venereol. 93:314–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eilertsen GØ, Becker-Merok A and Nossent
JC: The influence of the 1997 updated classification criteria for
systemic lupus erythematosus: Epidemiology, disease presentation,
and patient management. J Rheumatol. 36:552–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen JI: Clinical practice: Herpes
zoster. N Engl J Med. 369:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bombardier C, Gladman DD, Urowitz MB,
Caron D and Chang CH: Derivation of the SLEDAI. A disease activity
index for lupus patients. The committee on prognosis studies in
SLE. Arthritis Rheum. 35:630–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brisson M, Edmunds WJ, Law B, Gay NJ,
Walld R, Brownell M, Roos LL and De Serres G: Epidemiology of
varicella zoster virus infection in Canada and the United Kingdom.
Epidemiol Infect. 127:305–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sisó A, Ramos-Casals M, Bové A,
Brito-Zerón P, Soria N, Muñoz S, Testi A, Plaza J, Sentís J and
Coca A: Previous antimalarial therapy in patients diagnosed with
lupus nephritis: Influence on outcomes and survival. Lupus.
17:281–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruiz-Irastorza G, Olivares N, Ruiz-Arruza
I, Martinez-Berriotxoa A, Egurbide MV and Aguirre C: Predictors of
major infections in systemic lupus erythematosus. Arthritis Res
Ther. 11:R1092009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagasawa K, Yamauchi Y, Tada Y, Kusaba T,
Niho Y and Yoshikawa H: High incidence of herpes zoster in patients
with systemic lupus erythematosus: An immunological analysis. Ann
Rheum Dis. 49:630–633. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Straus SE, Ostrove JM, Inchauspé G, Felser
JM, Freifeld A, Croen KD and Sawyer MH: NIH conference.
Varicella-zoster virus infections. Biology, natural history,
treatment, and prevention. Ann Intern Med. 108:221–237. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carroll S, Gater A, Abetz-Webb L, Smith F,
Demuth D and Mannan A: Challenges in quantifying the
patient-reported burden of herpes zoster and post-herpetic
neuralgia in the UK: Learnings from the Zoster Quality of Life
(ZQOL) study. BMC Res Notes. 6:4862013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manzi S, Kuller LH, Kutzer J, Pazin GJ,
Sinacore J, Medsger TA Jr and Ramsey-Goldman R: Herpes zoster in
systemic lupus erythematosus. J Rheumatol. 22:1254–1258.
1995.PubMed/NCBI
|
|
26
|
Ng WL, Chu CM, Wu AK, Cheng VC and Yuen
KY: Lymphopenia at presentation is associated with increased risk
of infections in patients with systemic lupus erythematosus. QJM.
99:37–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HB, Kim KC, Park JH, Kang TY, Lee HS,
Kim TH, Jun JB, Bae SC, Yoo DH, Craft J and Jung S: Association of
reduced CD4 T cell responses specific to varicella zoster virus
with high incidence of herpes zoster in patients with systemic
lupus erythematosus. J Rheumatol. 31:2151–2155. 2004.PubMed/NCBI
|
|
28
|
Cohen EJ: Prevention of herpes zoster: We
need to do better. JAMA Ophthalmol. 131:396–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levin MJ, Smith JG, Kaufhold RM, Barber D,
Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, et al:
Decline in varicella-zoster virus (VZV)-specific cell-mediated
immunity with increasing age and boosting with a high-dose VZV
vaccine. J Infect Dis. 188:1336–1344. 2003. View Article : Google Scholar : PubMed/NCBI
|