Introduction
Laryngeal cancer is the most common type of head and
neck cancer and the morbidity and mortality rates from laryngeal
cancer are increasing worldwide (1).
There is currently no established method of diagnosing laryngeal
cancer. Furthermore, patients with laryngeal cancer have a high
rate of recurrence and often develop resistance to chemotherapy or
radiotherapy; therefore, the clinical outcome for patients with
advanced laryngeal cancer remains poor (2,3). Thus,
more effective diagnostic and therapeutic targets are required.
Annexin family proteins promote invasion and
metastasis in several types of cancer, including breast cancer,
esophageal carcinoma, liver cancer and nasopharyngeal carcinoma
(4). However, different members of
the Annexin family proteins are either up- or downregulated in
cancer tissue, so different Annexins may perform different roles in
specific types of cancer (5,6). It has been demonstrated that Annexin A1
is associated with esophageal cancer and is overexpressed in
laryngeal carcinoma cells; it may therefore progressively migrate
from the nucleus towards the membrane during laryngeal
tumorigenesis (7). By contrast,
Annexin A2 is associated with breast cancer (8) and knockdown of Annexin A2 by RNA
interference decreases the proliferation and invasion of breast
cancer cells (9). Additionally,
Annexin A2 is associated with prostate cancer, invasive cervical
carcinoma and lung cancer (10).
Similarly, Annexins A4 and A5 are highly expressed in laryngeal
carcinoma, indicating that they may contribute to its onset and
development (11). The
aforementioned results indicate that Annexin A2 expression may be
important in determining the invasion and metastasis of various
types of cancer. However, it remains unknown whether Annexin A2 is
associated with the development of laryngeal carcinoma and whether
it may act as a prognostic biomarker.
In the present study, differential proteomics
analysis indicated that Annexin A2 was highly expressed in
laryngeal carcinoma and its increased expression was confirmed
using immunohistochemistry in tissues taken from patients with
laryngeal carcinoma. The predictive value of Annexin A2 in the
prognosis of patients with laryngeal carcinoma was subsequently
evaluated.
Materials and methods
Study population
A total of 209 laryngeal cancer tissue samples and
88 adjacent tissues were collected from patients undergoing surgery
at the Department of Otorhinolaryngology Head and Neck Surgery,
Xiangya Hospital of Central South University (Changsha, China)
between February 2010 and December 2011. The patients included 120
males and 89 females, aged 38–88 years old. The clinical
characteristics of the patients with laryngeal cancer are
summarized in Table I. All the
tissues were fixed with formalin for 4 h at 4°C, embedded in
paraffin and stored at 4°C prior to usage. An additional 5
laryngeal cancer tissue samples and 5 matched adjacent tissue
samples were immediately frozen at −150°C and used for proteomics
analysis. Prior to sample collection, no participants received any
therapy. The present study was approved by the Ethics Committee of
Xiangya Hospital of Central South University and all the
participants provided written informed consent for inclusion in the
current study.
 | Table I.Association between annexin A2
expression and clinicopathological variables in patients with
laryngeal cancer. |
Table I.
Association between annexin A2
expression and clinicopathological variables in patients with
laryngeal cancer.
|
| Annexin A2 |
|
|---|
|
|
|
|
|---|
| Variables | Low expression
(n=89) | High expression
(n=120) | P-values |
|---|
| Age (years) |
|
|
|
| <60 | 45 | 65 | 0.674 |
| ≥60 | 44 | 55 |
|
| Sex |
|
|
|
| Male | 50 | 70 | 0.779 |
|
Female | 39 | 50 |
|
| Tumor size (cm) |
|
|
|
|
<5 | 60 | 47 |
<0.001a |
| ≥5 | 29 | 73 |
|
| Histological
grade |
|
|
|
| I | 57 | 83 | 0.460 |
|
II–III | 32 | 37 |
|
| Histology type |
|
|
|
|
Adenocarcinoma | 11 | 24 | 0.881 |
|
Squamous | 68 | 96 |
|
| Lymph node
metastasis |
|
|
|
| No | 68 | 64 | 0.001a |
| Yes | 21 | 56 |
|
| Distant
metastasis |
|
|
|
| No | 76 | 85 | 0.019a |
| Yes | 13 | 35 |
|
| Clinical stage |
|
|
|
| I–II | 74 | 81 | 0.011a |
|
III–IV | 15 | 39 |
|
Proteomics analysis
Total proteins were extracted and purified from
fresh tissues using a ReadyPrep™ Protein Extraction kit
(GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) following the
manufacturer's instructions. A 2-D Quant kit (GE Healthcare
Bio-Sciences) was used to determine the concentration of the total
protein. Proteomics analysis was performed as previously described
(12). Proteins (300 µg) were
separated using 12.5% two-dimensional polyacrylamide gel
electrophoresis (2-DE). The 20-cm immobilized pH gradient strips
(pH 3–10) were loaded with samples and subjected to rehydration
overnight. Samples containing 150 µg protein for analytical gels
and unlabeled samples containing 1 mg protein were diluted to 450
µl with rehydration solution (Promega Corp., Madison, WI, USA) and
used for isoelectric focusing (IEF). Proteins (60 µg) were
separated by 12% SDS-PAGE, and then each gel was stained with
Coomassie Brilliant Blue dye and scanned with UMAXpowerlook 1120
(Umax Data Systems, Inc., Taipei, Taiwan). The DeCyder software
version 6.5 (GE Healthcare, Chicago, IL, USA) was used to
spot-detect and determine the quantity, intergel matching and
statistics. The statistical significance was assessed for each
change in abundance using a one-way analysis of variance with a
post hoc Tukey's test. Protein spots for which the mean ratio was
>1.5-fold or <-1.5-fold were selected.
Immunohistochemical (IHC) staining
assay
The expression of Annexin A2 was evaluated using IHC
staining. IHC staining was performed as previously described
(13). Briefly, the tissue sections
(4 µm thick) were deparaffinized and hydrated and then retrieved
using citrate buffer in boiling water for 15 min. Subsequently,
sections were incubated with primary antibodies (mouse monoclonal
anti-Annexin A2; cat. no. WH0000302M1; 1:200; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) overnight at 4°C. Sections were then
incubated with horseradish peroxidase conjugated-polymer anti-mouse
secondary antibody (cat. no. KIT-5902; 1:200; Maxim Biotech, Inc.,
Rockville, MD, USA) for 60 min at 37°C. Sections were then
visualized using diaminobenzidine, counterstained with hematoxylin
for 5 min at room temperature and observed under a light microscope
(magnification, ×100).
To measure the expression of Annexin A2 in tissues,
the extent and intensity of staining were assessed in each section.
All tissue sections were analyzed and scored independently by three
experienced pathologists who were blinded to the experiments
designed. Annexin A2 staining intensity was scored as 0 (negative,
-), 1 (positive, weak, +), 2 (positive, moderate, ++) and 3
(positive, strong, +++). The extent of staining was scored as 0–1.0
(0–100%). The final staining score (0–3) was calculated as the
multiplication of the intensity score and extent score. The
expression of Annexin A2 was determined to be high when the score
was ≥1 and low when the score was <1. To compare the expression
of Annexin A2 between adjacent and tumor tissues, Annexin A2
expression was normalized to the average score in normal
tissues.
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. Student's t test was used to analyze
the differential expression of Annexin A2 between laryngeal cancer
tissue and adjacent normal tissue. A χ2 test was used to
analyze the association between Annexin A2 expression and the
clinicopathological parameters of patients with laryngeal cancer.
Kaplan-Meier analysis with a log-rank test was used to examine the
association between serum levels of Annexin A2 and overall survival
(OS). The overall survival (OS) was defined as the time from
diagnosis to the date of death or the date last known alive. The
patients were followed up every two months for five years. All the
patients completed the follow-up. Finally, the cox proportional
hazard regression model was used to estimate the independent
predicators for the prognosis of patients with laryngeal cancer.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The expression of Annexin A2 is higher
in laryngeal cancer tissues than in adjacent healthy tissue
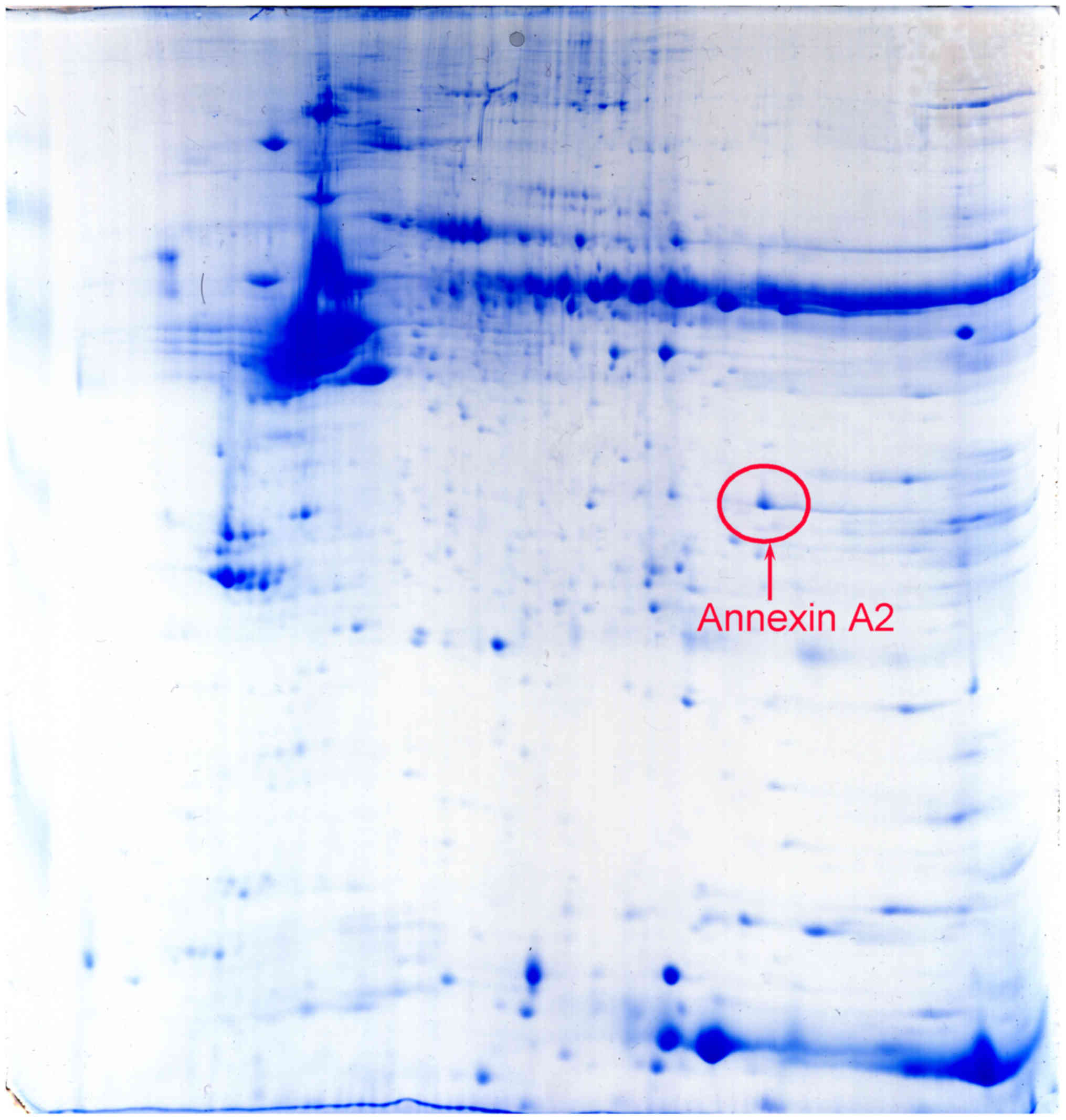
Proteins from 5 human laryngeal cancer tissues and 5
matched adjacent normal tissues were run in duplicate using
difference gel electrophoresis together with an internal pool
sample on each gel. DeCyder software determined that the positional
deviation of the protein spot was 1.67±0.24 mm for IEF and
1.25±0.13 mm for 2-DE. Fully automated spot detection and
quantification were also conducted using the Decyder software
(>1.5 fold). Among the differential proteins detected, it was
identified that Annexin A2 was overexpressed in cancer tissues
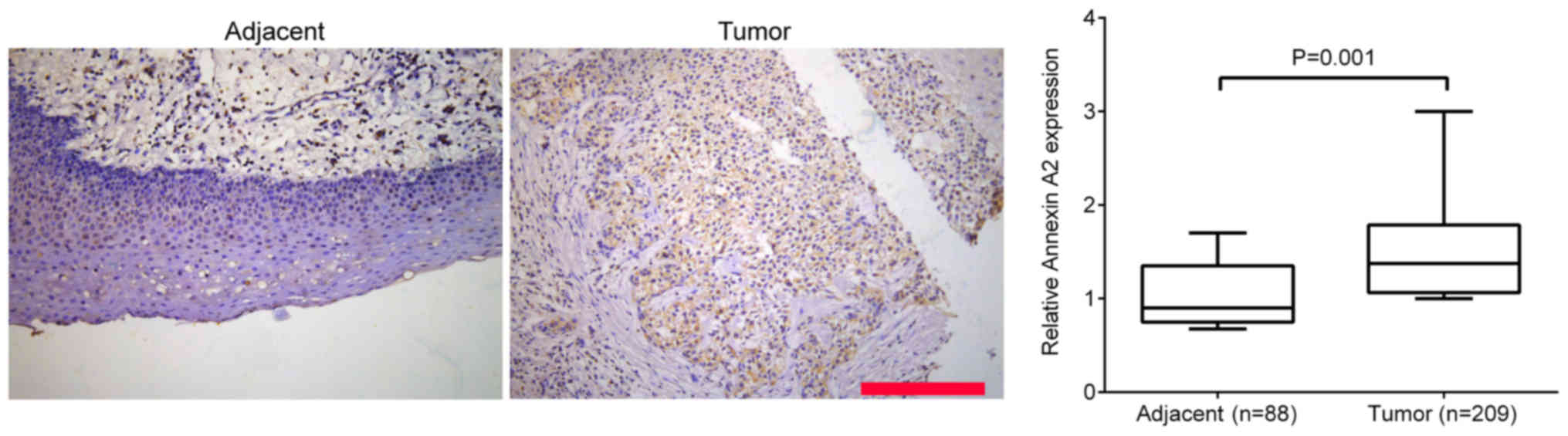
(Fig. 1). Additionally, IHC staining
was performed to measure the expression of Annexin A2 in the 209
laryngeal cancer tissues and 88 adjacent tissues. It was
demonstrated that Annexin A2 expression was significantly higher in
laryngeal cancer tissue compared with adjacent healthy tissues
(P=0.001; Fig. 2).
Expression of Annexin A2 is
significantly associated with tumor size, clinical stage and lymph
node metastasis in laryngeal cancer
The association between Annexin A2 expression and
the clinical characteristics of patients with laryngeal cancer was
further investigated. All patients with laryngeal cancer were
divided into two groups: A high Annexin A2 expression group (score
≥1) and a low Annexin A2 expression (score <1) group, according
to the final staining score. As presented in Table I, it was demonstrated that there were
no associations between Annexin A2 expression and age (P=0.674),
sex (P=0.779), histological grade (P=0.460) or histology type
(P=0.881). However, high Annexin 2 expression was significantly
associated with tumor size (P<0.001), lymph node metastasis
(P=0.001), distant metastasis (P=0.019) and clinical stage
(P=0.011). These results indicate that Annexin A2 expression may be
used as a prognostic biomarker in the evaluation of the malignant
progression of laryngeal cancer.
High Annexin A2 expression is
associated with poor 5-year survival rates in patients with
laryngeal cancer
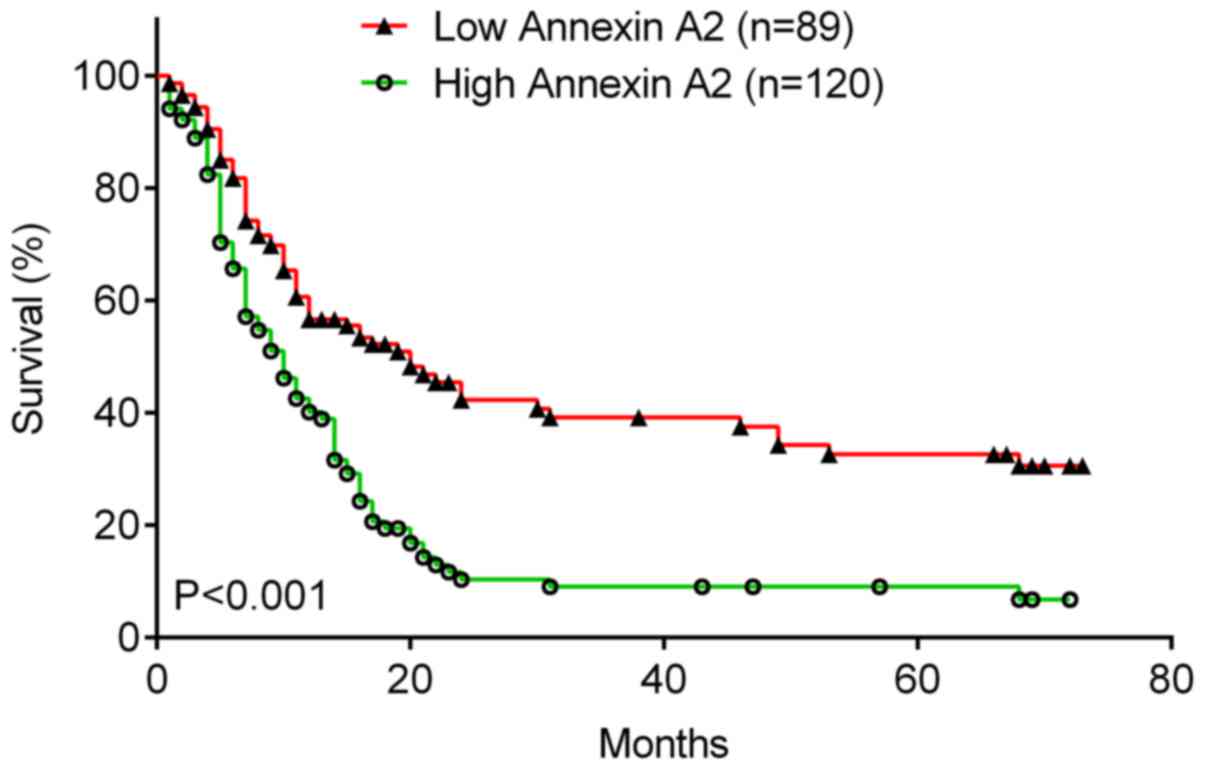
The association between Annexin A2 expression and
the survival rate was investigated in patients with laryngeal
cancer using the Kaplan-Meier method. It was demonstrated that the
5-year OS rate of patients with laryngeal cancer with high Annexin
A2 expression was significantly lower (Fig. 3; P<0.001) than that of patients
with low Annexin A2 levels. Therefore, high Annexin A2 expression
is associated with poor prognosis in patients with laryngeal
cancer.
In addition, the factors that could predicate the
prognosis of laryngeal cancer patients were investigated using
univariate and multivariate analyses. The results of the univariate
analysis indicated that Annexin A2 expression (P=0.018), as well as
tumor size (P=0.014), lymph node metastasis (P=0.005), distant
metastasis (P=0.011) and clinical stage (P=0.009) were
significantly associated with the survival of patients (Table II). Furthermore, Annexin A2
expression (P=0.001), tumor size (P=0.037), lymph node metastasis
(P=0.013), distant metastasis (P=0.002) and clinical stage
(P=0.018) were identified as independent factors for predicating
the prognosis of patients with laryngeal cancer (Table III).
 | Table II.Univariate analysis of prognostic
factors of laryngeal cancer. |
Table II.
Univariate analysis of prognostic
factors of laryngeal cancer.
| Variables | Hazard ratio | P-values |
|---|
| Age (≥60/<60
years) | 1.391 | 0.669 |
| Sex
(male/female) | 1.115 | 0.713 |
| Tumor size
(≥5/<5 cm) | 2.733 | 0.014a |
| Histological grade
(II–III/I) | 1.562 | 0.063 |
| Histology type
(adenocarcinoma/squamous) | 1.096 | 0.758 |
| Lymph node
metastasis (yes/no) | 4.256 | 0.005a |
| Distant metastasis
(yes/no) | 3.511 | 0.011a |
| Clinical stage
(III–IV/I–II) | 3.221 | 0.009a |
| Annexin A2
expression (high/low) | 2.653 | 0.018a |
 | Table III.Multivariate analysis of independent
prognostic factors of laryngeal cancer. |
Table III.
Multivariate analysis of independent
prognostic factors of laryngeal cancer.
| Variables | Hazard ratio | P-values |
|---|
| Tumor size | 2.153 | 0.037a |
| Lymph node
metastasis | 2.958 | 0.013a |
| Distant
metastasis | 3.316 | 0.002a |
| Clinical stage | 2.718 | 0.018a |
| Annexin A2
expression | 3.643 | 0.001a |
Discussion
Laryngeal squamous cell carcinoma accounts for
85–90% of all cases of laryngeal cancer and the mortality rate of
patients with larynx adenocarcinomas is high (14). In 2012, ~157,000 novel cases of
laryngeal carcinoma were diagnosed and ~1% of all cancer-associated
mortalities are estimated to be from laryngeal carcinoma (15). Due to the lack of a definitive method
of diagnosis and a frequent rate of recurrence, the prognosis of
patients with advanced laryngeal cancer remains poor (2). In addition, many patients develop
resistance to chemotherapy or radiotherapy, contributing to the
high mortality rate (3). Therefore,
it is necessary to improve understanding of the underlying
molecular mechanisms of laryngeal carcinoma and to identify more
effective diagnostic techniques and therapeutic targets.
Annexin A2, a calcium- and phospholipid-dependent
protein, is widely distributed in the nucleus, cytoplasm and
extracellular surface, and is primarily expressed in endothelial
cells, macrophages and tumor cells (11). Annexin A2 orchestrates multiple
biological processes and clinical associations, particularly during
cancer progression (16). It has
been demonstrated that Annexin A2 dysregulation is associated with
the onset, invasion, metastasis and drug resistance of cancer
(17). Furthermore, it has been
suggested that Annexin A2 may be used as a potential biomarker for
the diagnosis, treatment and prognosis of tumors (18). Zhang et al (18) reported that the soluble Annexin A2
concentration detected by ELISA in serum samples from 42 patients
with lung cancer was significantly higher than in 43 healthy
individuals. Furthermore, it has been demonstrated that Annexin A2
is upregulated in hepatocellular carcinoma tissues and serum
samples compared with benign liver disease samples and its
expression is associated with differentiated degree, intrahepatic
metastasis and tumor node metastasis staging (19). These results indicate that Annexin 2
may be an independent prognostic factor for hepatocellular
carcinoma (20). Previous studies
have demonstrated that the expression of Annexin A2 is
significantly associated with metastasis and poor survival in
nasopharyngeal and endometrial carcinoma, bladder cancer and serous
ovarian cancer (21–24). Using a proteomic assay, the present
study identified that Annexin A2 was highly expressed in laryngeal
carcinoma tissues, consistent with the results of the
aforementioned studies. This high expression of Annexin 2 was
confirmed by IHC staining. Furthermore, the expression of Annexin
A2 was significantly associated with tumor size, lymph node and
distant metastasis and the clinical stage. Therefore, the results
of the current study indicated that high Annexin A2 expression is
associated with poor prognosis in patients with laryngeal cancer,
suggesting that Annexin A2 may act as an independent prognostic
biomarker for evaluating the malignant progression of laryngeal
cancer. Measuring the expression of Annexin A2 as well as the
expression of other Annexins, including Annexins A4 and A3, may
accurately predict the prognosis of different types of cancer,
including urothelial carcinoma and cervical cancer (11,25,26). The
predictive efficiency of Annexin A2 combined with other Annexins in
laryngeal carcinoma requires further investigation.
Mechanically, silencing of Annexin A2 expression
suppresses cell proliferation, adhesion, migration, invasion and
vascular formation in nasopharyngeal carcinoma cells by
downregulating epithelial-mesenchymal transition-associated
signaling proteins (22). Decreased
expression of Annexin A2 causes defects in tumor growth in
vivo and cell proliferation in vitro without causing
cytotoxicity. This occurs by the induction of cell cycle arrest at
the G2 phase in non-small cell lung cancer cells and esophageal
squamous cell carcinoma, which occur in a p53-dependent or
-independent manner (27,28). Furthermore, it has been demonstrated
that decreased Annexin A2 expression alters cell polarity, disrupts
the formation of actin filaments and reduces C-X-C chemokine
receptor type 4 expression via the Rho/Rock pathway in renal cell
carcinoma (29). However, further
studies are required to determine the mechanism by which Annexin A2
promotes the development of laryngeal cancer.
In conclusion, to the best of our knowledge, the
current study is the first to demonstrate that Annexin A2 is
upregulated in laryngeal cancer and high Annexin A2 expression is
significantly associated with malignant progression and poor
prognosis in patients with laryngeal cancer. Therefore, the
expression of Annexin A2 may be a potential predictor for the
prognosis of patients with laryngeal cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81302355), the
National Natural Science Foundation of China (grant no. 81201740)
and Natural Science Foundation of Hunan Province (grant no.
2013FJ4225) and Xiangya Famous Doctors Foundation.
References
|
1
|
Li P, Liu H, Wang Z, He F, Wang H, Shi Z,
Yang A and Ye J: MicroRNAs in laryngeal cancer: Implications for
diagnosis, prognosis and therapy. Am J Transl Res. 8:1935–1944.
2016.PubMed/NCBI
|
|
2
|
Gama RR, Carvalho AL, Filho Longatto A,
Scorsato AP, López RV, Rautava J, Syrjänen S and Syrjänen K:
Detection of human papillomavirus in laryngeal squamous cell
carcinoma: Systematic review and meta-analysis. Laryngoscope.
126:885–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greco A, Rizzo MI, De Virgilio A, Gallo A,
Fusconi M, Pagliuca G, Martellucci S, Turchetta R and De Vincentiis
M: Cancer stem cells in laryngeal cancer: What we know. Eur Arch
Otorhinolaryngol. 273:3487–3495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CY and Lin CF: Annexin A2: Its
molecular regulation and cellular expression in cancer development.
Dis Markers. 2014:3089762014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu N, Liu S, Guo C, Hou Z and Sun MZ: The
role of Annexin A3 playing in cancers. Clin Transl Oncol.
15:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alves VA, Nonogaki S, Cury PM,
Wünsch-Filho V, de Carvalho MB, Michaluart-Júnior P, Moyses RA,
Curioni OA, Figueiredo DL, Scapulatempo-Neto C, et al: Annexin A1
subcellular expression in laryngeal squamous cell carcinoma.
Histopathology. 53:715–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR and Albarracin CT: Expression of annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: Association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Guo B, Zhang Y, Cao J and Chen T:
Silencing of the annexin II gene down-regulates the levels of
S100A10, c-Myc, and plasmin and inhibits breast cancer cell
proliferation and invasion. Saudi Med J. 31:374–381.
2010.PubMed/NCBI
|
|
10
|
Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen
F, Zhao YZ and Fu SB: Involvement of Annexin A2 in p53 induced
apoptosis in lung cancer. Mol Cell Biochem. 309:117–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng S, Wang J, Hou L, Li J, Chen G, Jing
B, Zhang X and Yang Z: Annexin A1, A2, A4 and A5 play important
roles in breast cancer, pancreatic cancer and laryngeal carcinoma,
alone and/or synergistically. Oncol Lett. 5:107–112.
2013.PubMed/NCBI
|
|
12
|
Yang J, Zhou M, Zhao R, Peng S, Luo Z, Li
X, Cao L, Tang K, Ma J, Xiong W, et al: Identification of candidate
biomarkers for the early detection of nasopharyngeal carcinoma by
quantitative proteomic analysis. J Proteomics. 109:162–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo Y, Wang X, Wang H, Xu Y, Wen Q, Fan S,
Zhao R, Jiang S, Yang J, Liu Y, et al: High Bak Expression Is
Associated with a Favorable Prognosis in Breast Cancer and
Sensitizes Breast Cancer Cells to Paclitaxel. PLoS One.
10:e1389552015. View Article : Google Scholar
|
|
14
|
Thompson LD: Laryngeal dysplasia, squamous
cell carcinoma, and variants. Surg Pathol Clin. 10:15–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mannelli G, Cecconi L and Gallo O:
Laryngeal preneoplastic lesions and cancer: Challenging diagnosis.
Qualitative literature review and meta-analysis. Crit Rev Oncol
Hematol. 106:64–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu XH, Pan W, Kang LH, Feng H and Song YQ:
Association of annexin A2 with cancer development (Review). Oncol
Rep. 33:2121–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauritzen SP, Boye TL and Nylandsted J:
Annexins are instrumental for efficient plasma membrane repair in
cancer cells. Semin Cell Dev Biol. 45:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Liu S, Guo C, Zong J and Sun MZ:
The association of annexin A2 and cancers. Clin Transl Oncol.
14:634–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Abd N, Fawzy A, Elbaz T and Hamdy S:
Evaluation of annexin A2 and as potential biomarkers for
hepatocellular carcinoma. Tumour Biol. 37:211–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Yao M, Wu W, Qiu L, Sai W, Yang
J, Zheng W, Huang J and Yao D: Up-regulation of annexin A2
expression predicates advanced clinicopathological features and
poor prognosis in hepatocellular carcinoma. Tumour Biol.
36:9373–9383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lokman NA, Pyragius CE, Ruszkiewicz A,
Oehler MK and Ricciardelli C: Annexin A2 and S100A10 are
independent predictors of serous ovarian cancer outcome. Transl
Res. 171:83–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CY, Lin YS, Chen CL, Chao PZ, Chiou
JF, Kuo CC, Lee FP, Lin YF, Sung YH, Lin YT, et al: Targeting
annexin A2 reduces tumorigenesis and therapeutic resistance of
nasopharyngeal carcinoma. Oncotarget. 6:26946–26959. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng L, Gao Y, Li X, Cai M, Wang H, Zhuang
H, Tan M, Liu S, Hao Y and Lin B: Expression and clinical
significance of annexin A2 and human epididymis protein 4 in
endometrial carcinoma. J Exp Clin Cancer Res. 34:962015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu H, Zhao J and Zhang M: Expression of
annexin A2 and its correlation with drug resistance and recurrence
of bladder cancer. Technol Cancer Res Treat. 15:NP61–NP68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi CH, Chung JY, Chung EJ, Sears JD, Lee
JW, Bae DS and Hewitt SM: Prognostic significance of annexin A2 and
annexin A4 expression in patients with cervical cancer. BMC Cancer.
16:4482016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu CM, Lin JJ, Huang HH, Ko YC, Hsu JL,
Chen JC, Din ZH and Wu YJ: A panel of tumor markers, calreticulin,
annexin A2, and annexin A3 in upper tract urothelial carcinoma
identified by proteomic and immunological analysis. BMC Cancer.
14:3632014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang CY, Chen CL, Tseng YL, Fang YT, Lin
YS, Su WC, Chen CC, Chang KC, Wang YC and Lin CF: Annexin A2
silencing induces G2 arrest of non-small cell lung cancer cells
through p53-dependent and -independent mechanisms. J Biol Chem.
287:32512–32524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Zheng S, Liu Q, Liu T, Liang M, Gao
X, Lu M, Sheyhidin I and Lu X: Under-expression of annexin A2 is
associated with Kazakh's esophageal squamous cell carcinoma. Mol
Carcinog. 54:779–788. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SF, Hsu HL, Chao TK, Hsiao CJ, Lin YF
and Cheng CW: Annexin A2 in renal cell carcinoma: Expression,
function, and prognostic significance. Urol Oncol. 33:222015.
View Article : Google Scholar : PubMed/NCBI
|