Introduction
Epilepsy is a neurological disorder characterized by
the chronic tendency of recurring, unprovoked seizures and ~30% of
patients have refractory epilepsy (1). For the majority of patients, refractory
epilepsy is caused by malformations of cortical development (MCD),
which is a heterogenous group of disorders that involves focal
cortical dysplasia, tuberous sclerosis and heteropia (2). Furthermore, epileptogenetic focus
resection is not suitable for all patients, and previous studies
have demonstrated that inflammatory pathways may be targeted as an
effective therapeutic strategy in refractory epilepsy (3,4).
Previous studies have supported the promotion of
inflammatory and immune processes in epileptogenesis (5), and the majority of them have been
described in patients with MCD (6,7).
Previous evidence has indicated that the high mobility group box 1
(HMGB1)-Toll-like receptor 4 (TLR4) axis was a proconvulsant
pathway in animal models of temporal lobe epilepsy (TLE) (8). Another study showed that, in epileptic
human tissues, TLR4 was expressed in astrocytes and dysplastic
neurons and HMGB1 was expressed in the glial cytoplasm, which
suggested that they may have an indispensable role in human
epileptogenesis associated with MCD (6). In addition, HMGB1 has been shown to
generate focal seizures by increasing the mean frequency of
spontaneous discharges and lowering the ictal event threshold in
animal models of TLE (9).
Ifenprodil, which is a selective antagonist of
N-methyl D-aspartate receptor subtype 2B (NR2B), which
contains N-methyl-D-aspartate (NMDA) receptors (10), is able to abrogate the
epileptogenetic effect of HMGB1 (8).
Despite these findings, the exact effect of ifenprodil on neuronal
levels and epileptic network activity remains to be fully
elucidated, particularly regarding human neocortical tissues. In
the present study, it was investigated whether ifenprodil affected
electrophysiological properties and spontaneous spikes in
neocortical pyramidal cells (PCs), in addition to the epileptic
network activity.
Materials and methods
Patients
Informed written consent for surgical resection of
human tissues for research was obtained from patients or their
relatives prior to surgery. The handling and use of human brain
tissue was approved by the Ethics Committee of Sanbo Brain
Hospital, Capital Medical University (Beijing, China). The study
included a total of 6 patients enrolled between October 2014 and
December 2015 (males, n=5; females, n=1; age, 11.6±9.6). These
cases consisted of 5 patients with refractory epilepsy with the
following inclusion criteria: i) Confirmed diagnosis of refractory
epilepsy; and ii) pathological diagnosis of malformations of
cortical development. Patients who had undergone radiotherapy or
chemotherapy were excluded from the study. There was 1 case of
glioma without epilepsy also included. Each patient received
cortical focal resection. For each case, at least 2 PCs were
recorded. The diagnosis of each patient was made by a
multidisciplinary team according to the International League
Against Epilepsy classification system (11).
Clinical information
The standardized information obtained from patients
included clinical history evaluation, routine neurological
examinations, such as motor, sensor, vision, language and auditory
sense examinations and scalp electroencephalogram (EEG)
video-recordings, magnetic resonance imaging (MRI) and
postoperative computed tomography. Clinical history was obtained
from medical records and included the following: Age at seizure
onset, age at surgery, clinical manifestation, type of surgery
(multi-lobar or lobar/focal), side of operation, sex, seizure
frequency, history of antiepileptic drugs (AEDs) and pathology
examination described previously (12), as indicated in Table I. In particular, the region of brains
sampled for in vitro electrophysiological studies were
recorded.
 | Table I.Clinical information of patients with
drug-resistant epilepsy caused by malformations of cortical
development (P1-5) or without epilepsy (P6). |
Table I.
Clinical information of patients with
drug-resistant epilepsy caused by malformations of cortical
development (P1-5) or without epilepsy (P6).
| Patient | Sex | Age at seizure
onset (years) | Age at surgery
(years) | Clinical
manifestation | Type of
surgery | Side of
surgery | Seizure
frequency | Situation of
AEDs | Pathology | Neurological
examinations | EEG
video-recordings | MRI | HFOs | Samples taken |
|---|
| P1 | Male | 4 | 8 | Contralateral
elementary motor signs; lower limbs clonic signs; generalized tonic
clonic seizure | Focal | Left | 6/month | None for 1
year | Tuberous
sclerosis | Negative | Ictal type in right
cerebral hemisphere | Bilateral multiple
abnormal signal, considering tuberous sclerosis | Around tubers | Left frontal lobe,
surrounding tissue of tubers |
| P2 | Male | 7 | 17 | Impairment of
consciousness; contralateral versive signs; facial expression;
integrated gestural motor behavior | Focal | Right | 7-9/month | Oxcarbazepine 600
mg bid; Valproate 500 mg bid | FCD IIId | Negative | Ictal type | Abnormal signals in
bilateral occipital lobe, considering ischemic change | None | Right superior
parietal lobe |
| P3 | Male | 8 | 13 | Impairment of
consciousness; both eyes contralateral turning with ipsilateral
head leading; contralateral upper limb tonic posture; integrated
gestural motor behavior with urinary incontinence | Focal | Left | 1/day | Oxcarbazepine 450
mg bid; Levetiracetam 750 mg bid | FCD IIId | Negative | Ictal type in
bilateral parietoocciptal lobe, especially left side | Right occipital
encephalomalacia | None | Left occipital
lobe |
| P4 | Male | 20 | 25 | Behavioral arrest;
contralateral proximal stereotypes; autonomic signs; fixed facial
expression | Focal | Right | 2/week | Oxcarbazepine 300
mg once daily | FCD Ib | Negative | Diffuse ictal
type | Abnormal signals in
right mesial temporal lobe | Right inferior
temporal gyrus+Right fusiform gyrus | Right inferior
temporal gyrus |
| P5 | Male | 4 | 14 | Autonomic aura;
integrated gestural motor behavior | Focal | Left | >10/day | Oxcarbazepine 900
mg 750 mg; Phenobarbital 30 mg bid | FCD IIb | Negative | Diffuse ictal type,
especially left side | Abnormal signals in
left superior frontal gyrus, considering focal cortical
dysplasia | None | Left superior
frontal gyrus |
| P6 | Female | No seizures | 35 | Headache for 1
year; exacerbated in 1 month | Focal | Right | N/A | N/A | Low grade glioma,
WHO II | Negative | N/A | Focal mass in left
frontotemporoinsula lobe, considering low grade glioma | N/A | Right frontal lobe,
surrounding tissue of tumor |
Electrophysiological methods
Human brain tissues, chosen as epileptic onset zone
according to preoperative evaluation (2/6 with high frequency
oscillations in stereotactic electroencephalogram [SEEG]), were
excised to investigate the electrophysiological character of
cortical neurons.
Tissues were removed microsurgically and directly
placed in ice-cold slicing solution containing 26 mM
NaHCO3, 2.5 mM KCl, 5 mM MgCl2, 1.25 mM
NaH2PO4, 10 mM dextrose, 213 mM sucrose, 1.0
mM CaCl2 and 1.0 mM MgSO4 (pH 7.2–7.4).
Within 1–2 h, slices 350 µm thick were cut using a Leica slicer and
incubated in regular artificial cerebrospinal fluid (ACSF)
containing 126 mM NaCl, 2.5 mM KCl, 1.25 mM
NaH2PO4, 26 mM NaHCO3, 25 mM
Dextrose, 2 mM MgSO4 and 2 mM CaCl2 (pH
7.2–7.4) for 45 min at 36°C and then at room temperature until
use.
During recordings, slices, without the cover slip,
from the original and ifenprodil groups were perfused at 35–36°C at
3 ml/min for 2 h in Mg2+-free modified ACSF composed of
126 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 26
mM NaHCO3, 25 mM dextrose and 2 mM CaCl2 (pH
7.2–7.4) to mimic epileptic activity (13,14).
Whole-cell recordings were achieved using glass pipettes (3–5 MΩ)
filled with 140 mM K-Gluconate, 3 mM KCl, 2 mM MgCl2, 10
mM HEPES, 0.2 mM EGTA, 2 mM Na2ATP and 0.2% biocytin (pH
7.25–7.30; osmolality, 280–290 mOsm) for voltage and current clamp
recordings. Spontaneous spikes lasting for at least 2 min, action
potential induced by depolarizing currents (40 pA current
increment; 500 msec) and membrane potential (Vm) responses to
hyperpolarized current (−100 pA; 500 msec) or a series of current
steps (500 msec in duration) were recorded, respectively, to
compare differences of intrinsic electrophysiological properties
prior to and after the application of ifenprodil (5 µM; I2892;
Sigma-Aldrich; Merk KGaA, Darmstadt, Germany).
The five patients with epilepsy underwent
epileptogenic zone resections and the patient without epilepsy
underwent tumor resection. Subsequently, patients with epilepsy
were split into the following groups: i) According to video EEG
recordings, patients were divided into a high frequency
oscillations (HFOs) group (n=3 patients) and a non-HFOs (n=2
patients) group; and according to pathological results, ii)
patients with focal cortical dysplasia type II and tuberous
sclerosis (TS) were classed as the TS complex (TSC)-related group
(n=3 patients) and iii) patients with focal cortical dysplasia type
I and type IIId were classed as the idiopathic group (n=2
patients). The patient without epilepsy was classed as the
non-epilepsy group.
For all brain tissue obtained from the 6 patients
enrolled in the current study, whole-cell recordings on
pyramidal-shaped neurons were performed and interneuron-shaped
neurons under the DIC (differential interference contrast)
microscope (×400) were avoided due the focus of the study being on
excitatory modulation. According to our previous study, PCs and
inhibitory interneurons were identified by their firing patterns
(15). Depolarizing currents were
applied (40 pA current increment; 500 msec) to induce action
potentials, and all cells were recorded at resting membrane
potential. Once the recordings were established, the type of neuron
was re-confirmed using staining techniques, after having identified
them by firing patterns.
Hemtoxylin and eosin staining
Tissue samples from all 6 patients were fixed in 10%
formalin at room temperature for 24 h and embedded in paraffin
prior to being cut into sections 4 µm thick. Sections were then
mounted on pre-coated glass slides (Star Frost, Waldemar Knittel
GmbH, Braunschweig, Germany). Sections of all specimens were
stained with hematoxylin and eosin at room temperature for 4 h, and
images were visualized using a Leica microscope, captured using an
Optronics DEI-750 three-chip camera equipped with a BQ 8000 sVGA
frame grabber (×400; Optronics, Goleta, CA, USA) and analyzed using
Bioquant software 2011 (Bioquant Image Analysis Corporation,
Nashville, TN, USA).
Drug treatment
For all slices obtained from patients enrolled in
the current study, including the epilepsy and non-epilepsy groups,
Vm responses to the hyperpolarized current, spontaneous spiking and
Vm responses to serials of hyperpolarized current steps were
recorded. Following original recordings for 20 min, brain tissue
sections from all 6 patients were immersed in 5 µM ifenprodil for 5
min in 36°C and the above-mentioned parameters were subsequently
examined again. Slices were then fixed in 4% paraformaldehyde for
12 h at room temperature for subsequent avidin staining.
Histological procedures
Alexa Fluor 488 (200 µM) and 0.2% biocytin were
added to internal solution (K-Gluconate 140 mM, KCl 3 mM,
MgCl2·6H2O 2 mM, HEPES 10 mM, EGTA 10 mM, Na2ATP 2 mM, 285 mOsm, pH
7.3) as described previously (15)
in order to study and record labeled cells. Following the
completion of recordings by patch clamp, 3 human brain slices from
the TSC, idiopathic and non-epilepsy groups were fixed in 8% PFA at
room temperature and 8% sucrose (w/v) in 0.1 Mmol PBS (pH 7.4) for
1 h. Slices were rinsed in 0.01 Mmol PBS 3 times, and subsequently
incubated in 0.5% Triton X-100 for 0.5 h at room temperature and
10% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used as a blocking solution at room temperature for 1
h. Once slices were washed in PBS 3 times for 10 min, they were
incubated in strept-avidin (1:1,000; cat no. SA-5000, Vector
Laboratories, Inc., Burlingame, CA, USA) for 2 h at room
temperature. Confocal images were taken with a laser scanning
confocal microscope (Nikon A1; Nikon Corporation; Tokyo, Japan)
with a ×40 objective. Z-stack images of labeled cells were attained
with an interval of 0.75 µm.
Statistical analysis
All of the electrophysiological values were
presented as mean ± standard error and all experiments were
repeated in triplicate. For two independent observations, a
two-sample Student's t-test was used for normally-distributed data,
whereas non-normal data were compared using Wilcoxon rank-sum test.
For multiple observations a one-way ANOVA followed by a
Student-Newman-Keuls test was used to examine the significance
between groups with a normal distribution, whereas when data did
not have a normal distribution a Kruskal-Wallis H test was used.
P<0.05 was considered to indicate a statistically significant
difference. Passive parameters were derived from Vm responses to
current steps.
Input resistance was calculated using the following
formula: Steady state Vm changes/current amplitudes. Action
potential (AP) amplitude was considered the voltage value between
the threshold and peak. AP threshold was the voltage value at the
time when the AP rising slope reached 20 V/sec. AP half-width was
determined as the AP duration at half amplitude. AP rising rate was
the highest value of voltage-time slope in the AP rising phase. To
detect the AP falling rate, the lowest value of the voltage-time
slope in the AP falling phase was used. Frequency-current intensity
curve (F-I curve) indicated the association between serials of
hyperpolarized currents and the number of spikes they evoked.
Every random spontaneous excitatory input was
identified, including subthreshold depolarizations and spontaneous
spikes, and then considered all-or-none random spontaneous bursts
into an event. In the statistical analysis, excitatory inputs with
an amplitude >15 mV were included. For each cell, the induced
spontaneous spikes and events within the selected 120 sec were
measured.
All analysis was performed using Matlab (R2014a, The
Math Works; Natick, MA, USA), SPSS 21 for Mac (IBM Corp., Armonk,
NY, USA) and Spike2 (7.11a; Cambridge electronic design; Cambridge,
UK).
Results
Patient clinical information
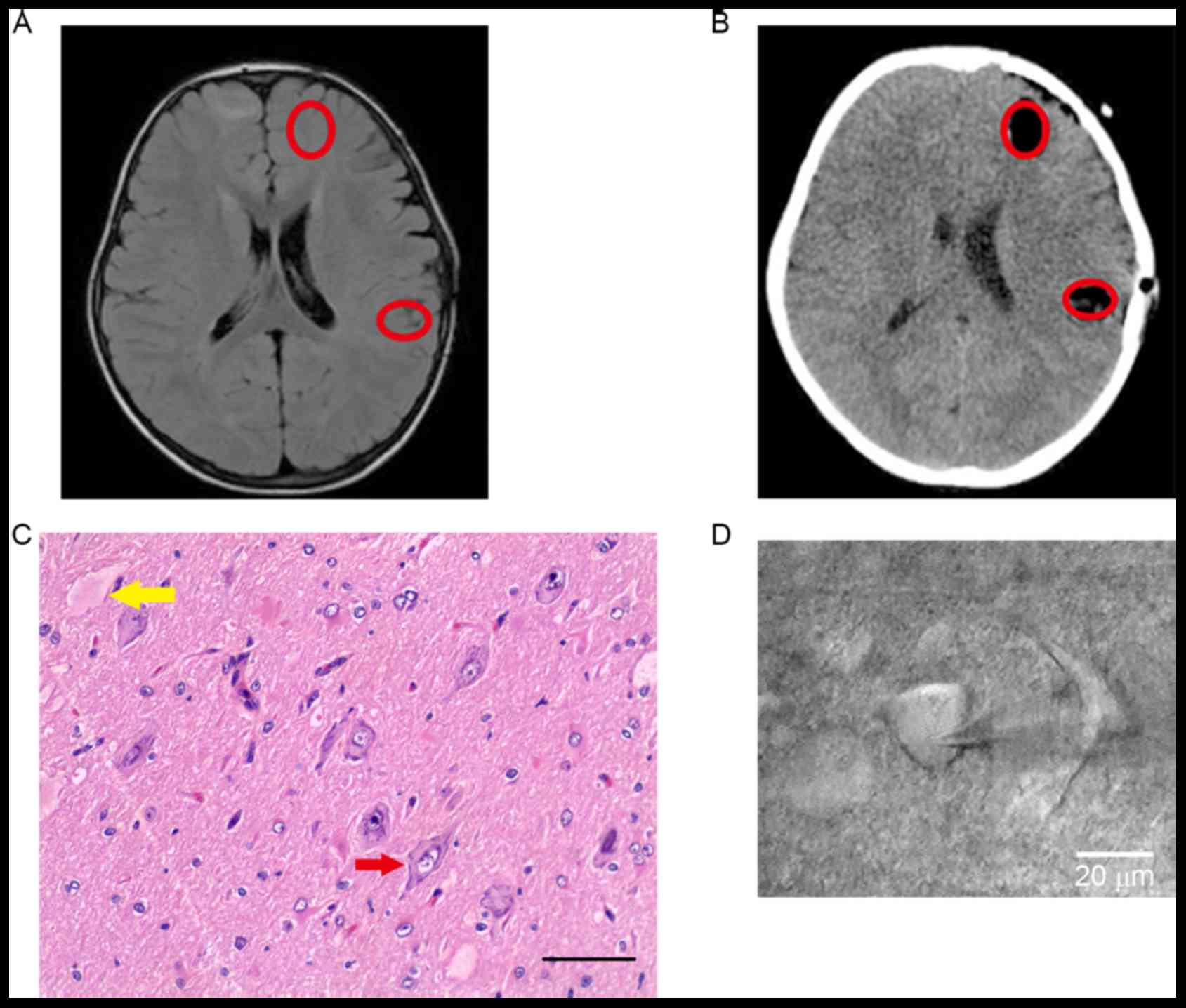
Clinical information is presented in Table I. Focal lesions were localized by
clinical manifestation, preoperative MRI (Fig. 1A) and SEEG (data not shown).
Following the combination of postoperative CT and pathological
results, the exact locations of samples were studied and dysmorphic
neurons and balloon cells were noted in Fig. 1B and C). The morphology of a
pyramidal neuron is presented in Fig.
1D.
Investigation of electrophysiological properties of
neocortical PCs in patients with MCD. To identify the correlation
between clinical manifestations and PC electrophysiological
properties, patients with focal cortical dysplasia type II and
tuberous sclerosis (TS) were categorized as the TS complex
(TSC)-related group, and patients with focal cortical dysplasia
type I and type IIId were classed as the idiopathic group.
According to video EEG recordings, patients were divided into the
HFOs group (n=3) and non-HFOs (n=2) group. In addition, one patient
without epilepsy was selected as the non-epilepsy group.
Electrophysiological properties of neocortical PCs
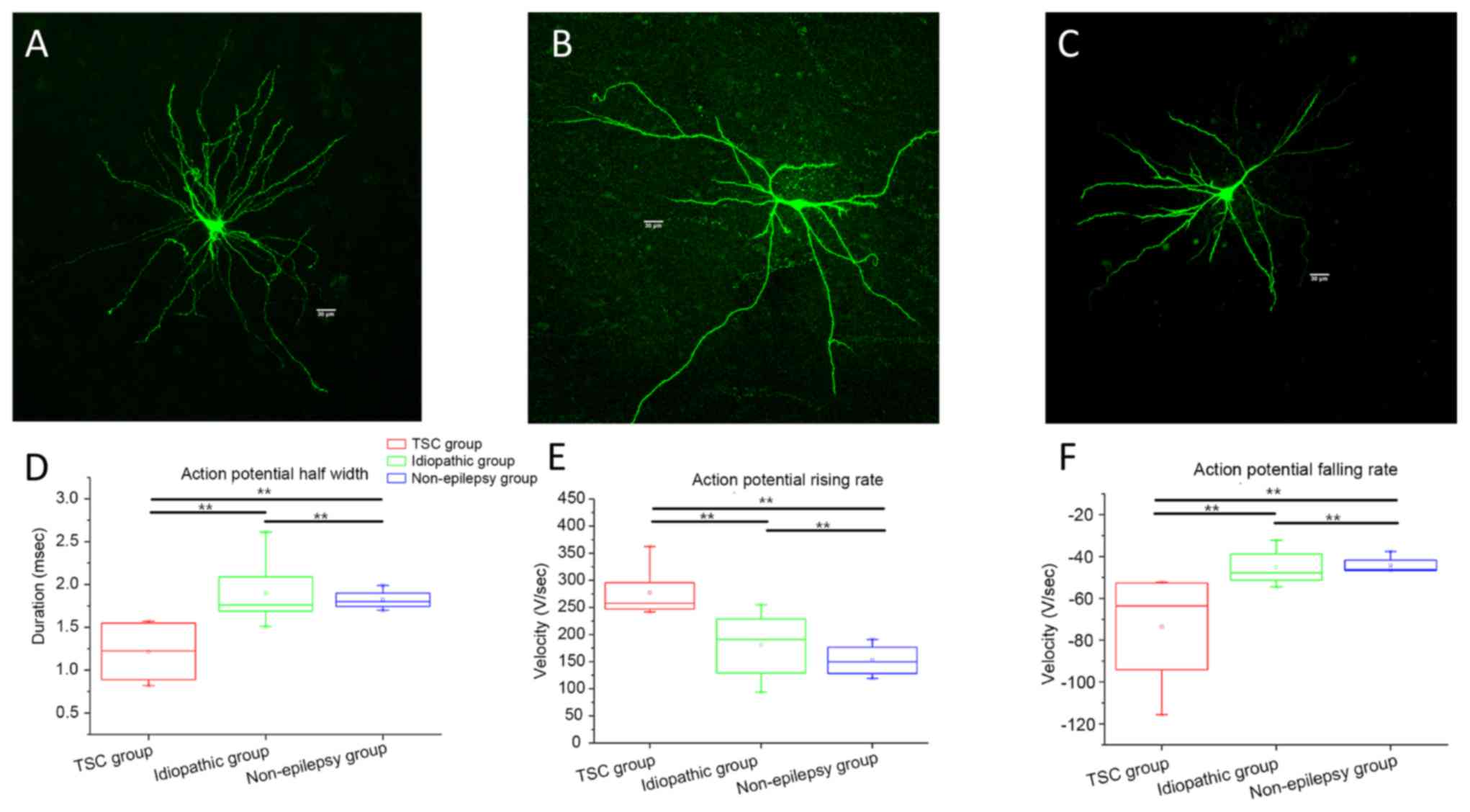
in patients with MCD were determined (Fig. 2). The morphology of recorded
pyramidal neurons, which were similar to those under physiological
condition, were indicated in Fig.
2A. The rising and falling rate of AP in the TSC-related group
was significantly faster than those in the idiopathic and
non-epilepsy groups (Fig. 2C and D,
respectively; P<0.01). The half-width of APs in the TSC-related
group was significantly decreased compared with those in idiopathic
and non-epilepsy groups (Fig. 2B;
P<0.01), whereas there was no significant difference in the
membrane input resistance, membrane capacitance, time constant,
amplitude of AP, and threshold potential among the 3 groups (data
not shown).
The characteristics determined by EEG indicated
there was no significant difference in the membrane input
resistance, membrane capacitance, time constant, AP rising rate,
threshold potential, amplitude of AP, half-width of AP and AP
falling rate between the HFOs group and non-HFOs group (data not
shown).
Modulation of electrophysiological
properties in PCs by ifenprodil
As HMGB1 modulates the excitability of PCs by
activating NR2B-containing NMDA receptors (16), the present study examined the exact
electrophysiological changes in PCs. Changes in whole-cell membrane
electrophysiological properties were obtained by injecting
hyperpolarizing and depolarizing currents steps, respectively.
Hyperpolarizing currents (−100 pA; 500 msec) were applied to the
recording cell to examine passive membrane properties.
Subsequently, depolarizing currents (40 pA current increment; 500
msec) were applied to induce action potentials. Cells were recorded
at resting membrane potential.
Electrophysiological membrane properties for each
cell were compared, including input resistance, time constant,
capacitance, AP amplitude, AP half-width, AP threshold, AP rising
rate and falling rate (Table II).
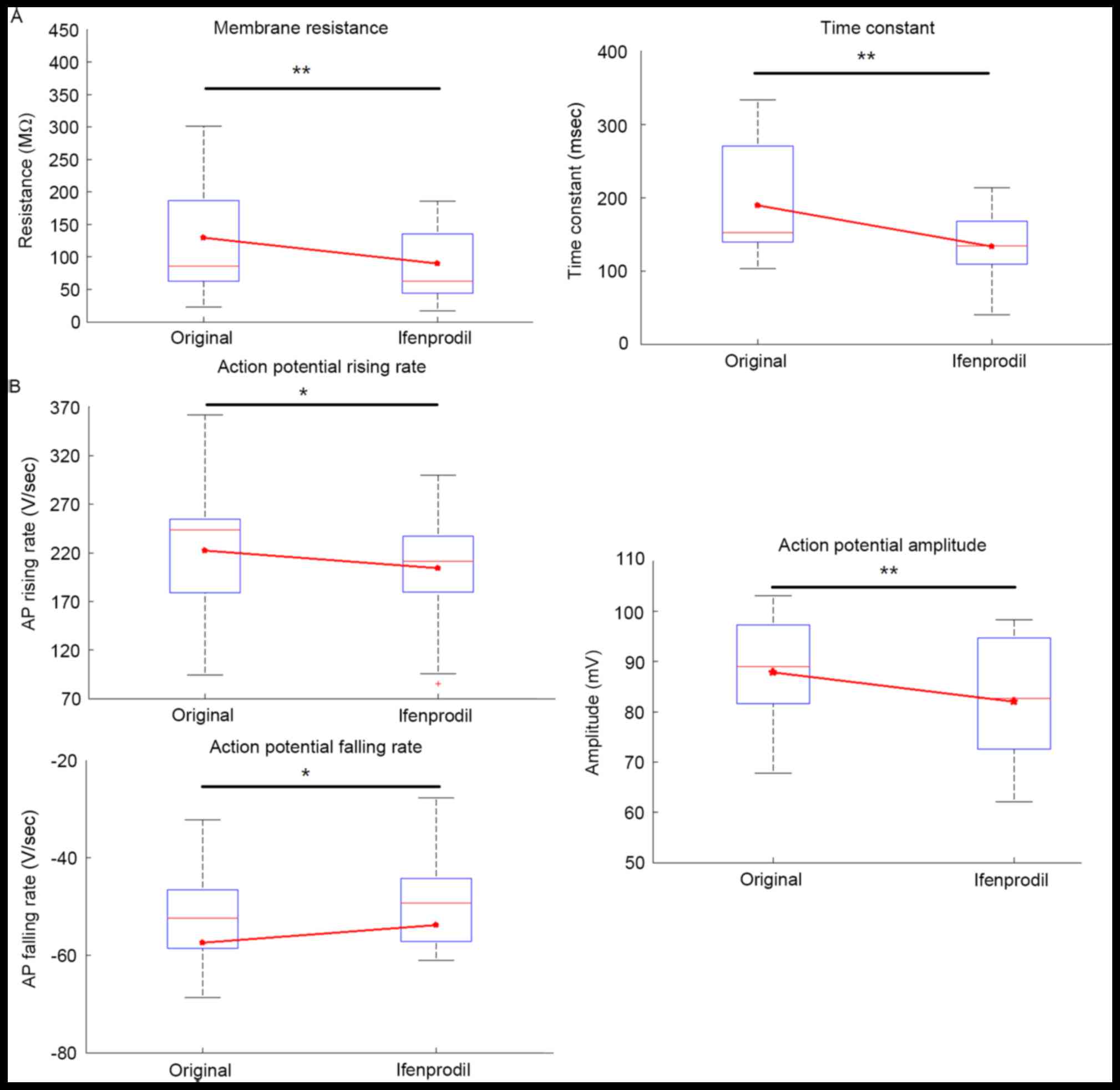
Among patients with epilepsy, ifenprodil significantly decreased
the input resistance and time constant compared with the original
group (Fig. 3A; P<0.01), whereas
no significant difference was indicated in capacitance (Table II; P=0.94). Furthermore, ifenprodil
treatment significantly decreased AP amplitude compared with the
original group (P<0.01; Table
II; Fig. 3B), but no significant
alteration in AP half-width was observed (Table II; Fig.
3B), which likely indicated decreased Ca2+ entry
following application of ifenprodil (17,18).
Additionally, AP rising and falling rates were significantly
decreased following ifenprodil treatment compared with the original
group, respectively (Table II;
Fig. 3B; P<0.05). However, the AP
threshold showed no significant difference prior to and after
application of ifenprodil. In patients without epilepsy, no
significant differences were noted in the membrane properties prior
to and after application of ifenprodil (Table II). In addition, no significant
difference in the membrane properties between patients with and
without epilepsy were exhibited, with one exception; significantly
increased membrane capacitance was detected in epilepsy patients
(Table II; P<0.05).
 | Table II.Modulation of electrophysiological
properties of pyramidal neurons. |
Table II.
Modulation of electrophysiological
properties of pyramidal neurons.
|
| Epilepsy | Non-epilepsy |
|---|
|
|
|
|
|---|
|
| Original | Ifenprodil |
| Original | Ifenprodil |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Property | Mean ± SE | n | Mean ± SE | n | P-value | Mean ± SE | n | Mean ± SE | n | P-value |
|---|
| Passive membrane
property |
|
|
|
|
|
|
|
|
|
|
|
Resistance (MΩ) |
140.665±29.768 | 14 |
102.683±21.365 | 14 | <0.01 |
191.297±22.947 | 4 |
218.875±15.168 | 4 | 0.144 |
| Tau
(msec) |
18.923±2.091 | 14 |
13.295±1.208 | 14 | <0.01 |
19.553±SE
3.943 | 4 |
21.752±3.236 | 4 | 0.465 |
|
Capacitance (pf) |
189.267±36.891 | 14 |
188.007±31.819 | 14 | 0.778 |
101.774±12.895 | 4 |
98.112±10.180 | 4 | 0.715 |
| AP
amplitude (mV) |
87.999±3.332 | 14 |
83.043±3.777 | 14 | <0.01 |
78.699±3.881 | 4 |
76.352±4.354 | 4 | 0.715 |
| AP half
width (msec) |
1.605±0.134 | 14 |
1.615±0.152 | 14 | 0.221 |
1.8223±0.0600 | 4 |
1.871±0.124 | 4 | 0.715 |
| Active membrane
property |
|
|
|
|
|
|
|
|
|
|
| AP
threshold (mV) |
−43.549±3.100 | 14 |
−44.327±2.594 | 14 | 0.778 |
−36.885±1.1450 | 4 |
−36.030±2.829 | 4 | 0.465 |
| AP
rising rate (V/sec) |
222.037±19.260 | 14 |
203.949±16.438 | 14 | <0.05 |
152.413±15.599 | 4 |
144.990±13.998 | 4 | 0.715 |
| AP
falling rate (V/sec) |
−57.454±6.013 | 14 |
−53.819±5.414 | 14 | <0.05 |
−44.219±2.205 | 4 |
−43.360±3.507 | 4 | 0.715 |
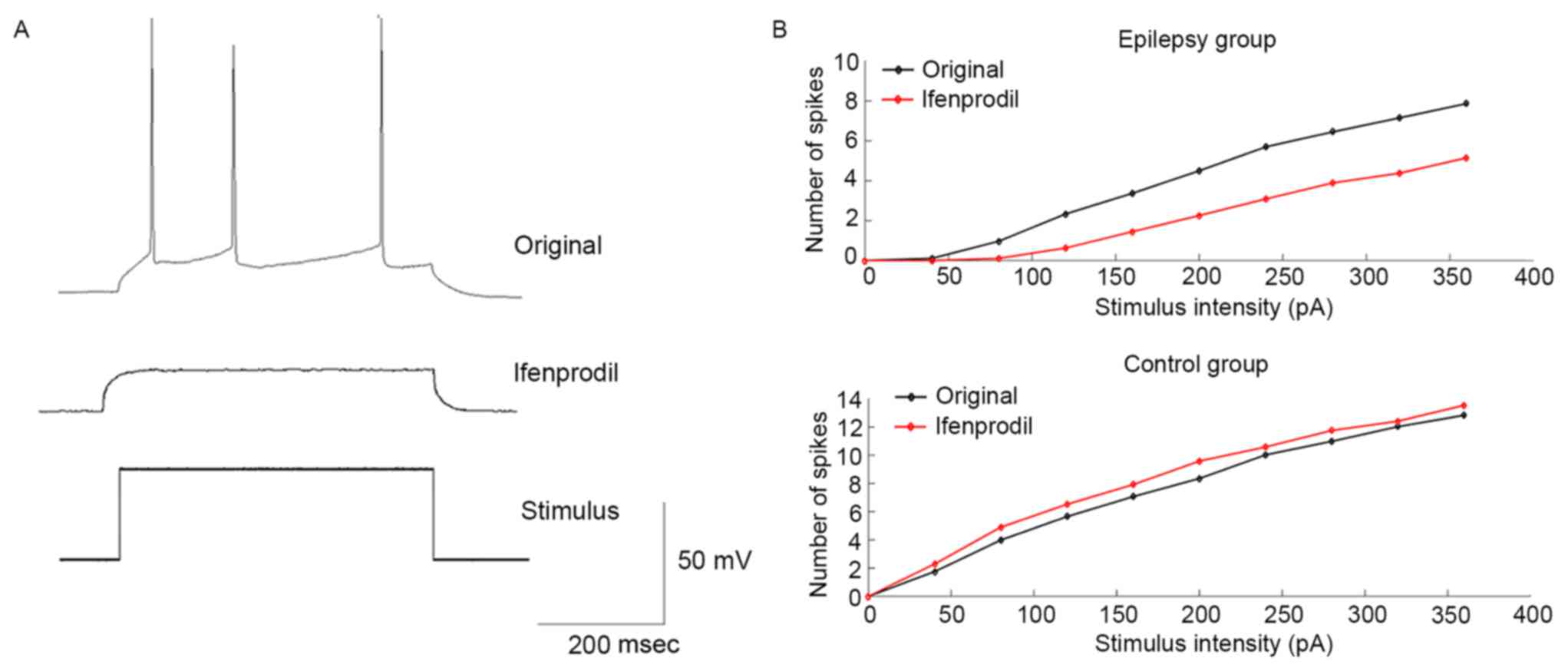
To investigate the modulation of neuronal
excitability of PCs by ifenprodil, a series of depolarizing current
steps were applied to generate an F-I curve. Fig. 4A indicates an example of the voltage
response of recording PCs under the current stimulus with the same
intensity prior to and after application of ifenprodil. Results
suggested that in the epilepsy group, ifenprodil decreased the mean
number of spikes compared with the original group; however, this
was not a statistically significant difference (Fig. 4B). Conversely, the mean number of
spikes was increased following the application of ifenprodil in
non-epilepsy patients, although this was not a statistically
significant difference (Fig. 4B).
These results indicate that ifenprodil may decrease neuronal
excitability of PCs in patients with epilepsy.
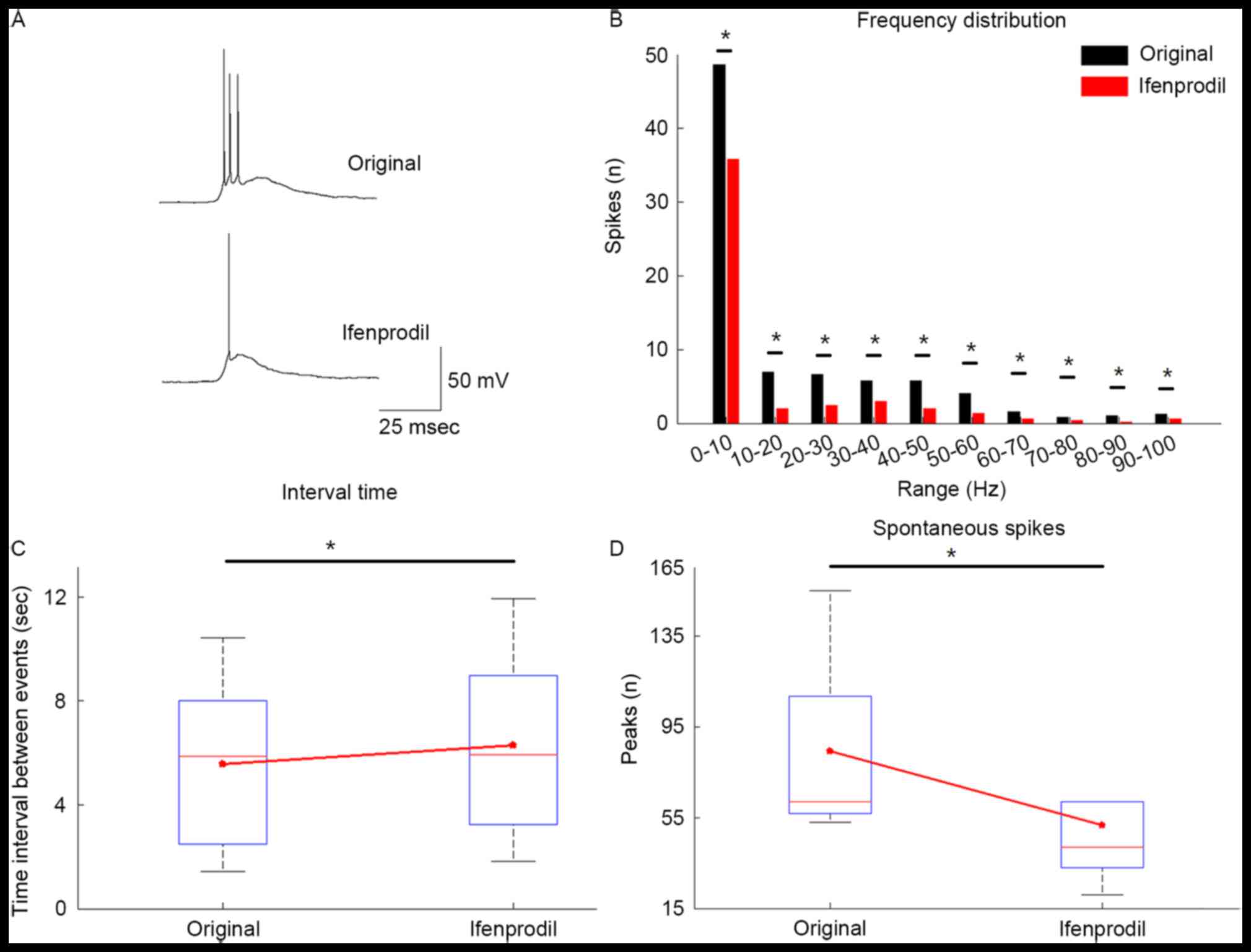
Modulation of epileptic network
activity by ifenprodil
To mimic epileptic activity, the brain tissues were
perfused in Mg2+-free ACSF instead of normal ACSF. A
total of 85.7% of slices (12/14) generated epileptiform activity in
cortical tissue obtained from epilepsy patients. Once a stable
epileptic network activity was achieved in 5 slices for ≥25 min,
ifenprodil was applied and the generation of epileptiform activity
was decreased after applying ifenprodil (Fig. 5). The number of spikes for each
depolarized event was decreased in the Ifenprodil group compared
with the original group (Fig. 5A).
As defined above, depolarizing potentials with amplitude >15 mV
and duration >300 msec were considered as an event. Bath
application of 5 µM ifenprodil significantly decreased the number
of depolarizing events compared with the original group (Fig. 5D; P<0.05) and significantly
increased the inter-burst-interval (Fig.
5C; P<0.05). Furthermore, the frequency distribution plot
indicated that ifenprodil significantly reduced the quantity of
depolarizing events in every 10-Hz step within the selected 120 sec
by (Fig. 5B; P<0.05).
Discussion
Immune mediators are functional in the immune
response to infection; however, recent studies have demonstrated
that they also have alternative roles (19,20).
Increasing experimental evidence has demonstrated that immune
mediators are associated with neuromodulation (21). In the last two decades, findings have
suggested that immune mediators, including pathogen associated
molecular patterns, danger associated molecular patterns (DAMPs)
and extracellular matrix components, modulate voltage-dependent ion
channels, receptor-dependent ion channel and synaptic strength
(22). HMGB1, which is a type of
DAMP, was expressed in the nucleus in both neurons and glial cells
under normal physiological conditions, and is produced from cell
necrosis or active release following stimulation (23). In an animal model, the HMGB1/TLR4
axis increased Ca2+ influx through phosphorylation of
the NMDA-NR2B receptor (16). The
present study revealed that AP amplitude in PC was significantly
decreased following the application of ifenprodil in the epilepsy
group, which indicated a reduction in Ca2+ influx
(17). However, Ca2+
channels are categorized in three families and mediate different
types of currents (24); therefore,
further investigation is required to identify which family was
associated with this effect. As Ca2+ influx is necessary
to activate the release of HMGB1 (25), it was postulated that ifenprodil
likely decreased the release of HMGB1 in PCs. HMGB1 also provokes
astrocytes to release immune mediators (26), and application of ifenprodil
potentially decreases the release of a variety of immune
mediators.
As HMGB1 and NMDA receptors are upregulated in the
nidus of the neuron among patients with MCD (27), it was expected that application
ifenprodil would exhibit pathological-specific modulation of
neuronal excitability in PCs. In the present study, the application
of ifenprodil reduced neuronal excitability of PCs in patients with
epilepsy, but marginally enhanced PC neuronal excitability in
patients without epilepsy. Coincidentally, application of
ifenprodil significantly decreased membrane input resistance in
patients with epilepsy. Previous studies showed that NR2B was
predominantly expressed in the extrasynaptic region (28) and extrasynaptic neurotransmitter
receptor activation appeared to modulated membrane potential
(29). These findings suggest that
NR2B blockage by application of ifenprodil may hyperpolarize
membrane potential, which results in an increase in membrane
conductance by HCN channel activation (30).
Neuronal excitability was determined by AP
characteristics and spiking pattern, thereby neuronal excitability
should be described by both AP shape and F-I curve alteration. The
present results indicated that application of ifenprodil altered
the AP characteristics in the epilepsy group by decreasing either
AP amplitude, AP rising rate or AP falling rate. These findings
suggested that the application of ifenprodil likely decreased
Ca2+ influx during the generation of APs, and decreased
the conductance of Ca2+-dependent K+ channel
in AP falling phase (31). Using
calcium imaging in an animal model, a previous study demonstrated
that HMGB1 increased spontaneous ictal-like discharges, which were
blocked by tetrodotoxin, which is a voltage-gated sodium channel
blocker (9). The present study
showed that application of ifenprodil decreased the total number of
spontaneous spikes and increased inter-burst-interval time among
PCs in patients with epilepsy.
With the exception of HMGB1, experimental evidence
has revealed interactions between interleukin-1β and NR2B subunits
in hippocampal neurons (32). The
interaction between HMGB1 and IL1β also exhibited amplification of
the inflammatory response in osteoarthritis (33). We subsequently speculated that the
application of ifenprodil likely had a potent anti-epileptic effect
via the direct blockage of HMGB1/TLR4 axis (8).
In clinical practice, antiepileptic agents have
non-specific effects on preventing epileptic activity by decreasing
excitation or increasing the inhibition in every neuron within the
cortex (34,35). In patients with epilepsy and MCD, the
expression of HMGB1 was upregulated in dysmorphic neurons and
translocated from the nucleus to the cytoplasm in glial cells in a
previous study (27). No similar
findings in patients without epilepsy were identified in the
present study. In addition, animal experiments have revealed that
the activation of HMGB1/TLR4 axis also has long term effects on
epileptogenesis by reducing the epileptogenetic threshold (36).
Ifenprodil has been proven to be safe for humans in
the treatment of ischemic brain injury (37,38) as a
non-competitive and highly selective antagonist of NMDA receptors
containing the NR2B subunit (10),
and an effective neuroprotectant for modulating NR2B receptor
activity by changing the binding rate of the receptor (39). In the present study, ifenprodil
showed specific antiepileptic effects by reducing PC neural
excitability in patients with epilepsy and marginally increasing
neural excitability in the patient without epilepsy. Furthermore,
ifenprodil inhibited the neural network activity in brain tissue
slices transformed from patients with MCD.
The limitations of the study are as follows: A small
cohort of patients was used, which makes it challenging to avoid
the bias brought by variables and in terms of the effect of
ifenprodil, interneurons have not been involved in the current
study. Therefore, further investigation for the expression,
regulation, function of ifenprodil on interneurons is required.
In conclusion, although further studies are
necessary to investigate whether ifenprodil exerts an effect on
inhibitory interneurons and glial cells, the present findings
suggest that ifenprodil may be utilized as a treatment method for
epilepsy caused by MCD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81571275).
References
|
1
|
Perucca E, French J and Bialer M:
Development of new antiepileptic drugs: Challenges, incentives, and
recent advances. Lancet Neurol. 6:793–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aronica E, Becker AJ and Spreafico R:
Malformations of cortical development. Brain Pathol. 22:380–401.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vezzani A, Conti M, De Luigi A, Ravizza T,
Moneta D, Marchesi F and De Simoni MG: Interleukin-1beta
immunoreactivity and microglia are enhanced in the rat hippocampus
by focal kainate application: Functional evidence for enhancement
of electrographic seizures. J Neurosci. 19:5054–5065.
1999.PubMed/NCBI
|
|
4
|
van Scheppingen J, Broekaart DW, Scholl T,
Zuidberg MR, Anink JJ, Spliet WG, van Rijen PC, Czech T,
Hainfellner JA, Feucht M, et al: Dysregulation of the
(immuno)proteasome pathway in malformations of cortical
development. J Neuroinflammation. 13:2022016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aronica E and Crino PB: Inflammation in
epilepsy: Clinical observations. Epilepsia. 3 52 Suppl:S26–S32.
2011. View Article : Google Scholar
|
|
6
|
Zurolo E, Iyer A, Maroso M, Carbonell C,
Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A
and Aronica E: Activation of Toll-like receptor, RAGE and HMGB1
signalling in malformations of cortical development. Brain.
134:1015–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boer K, Jansen F, Nellist M, Redeker S,
van den Ouweland AM, Spliet WG, van Nieuwenhuizen O, Troost D,
Crino PB and Aronica E: Inflammatory processes in cortical tubers
and subependymal giant cell tumors of tuberous sclerosis complex.
Epilepsy Res. 78:7–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maroso M, Balosso S, Ravizza T, Liu J,
Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi
AA, et al: Toll-like receptor 4 and high-mobility group box-1 are
involved in ictogenesis and can be targeted to reduce seizures. Nat
Med. 16:413–419. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiavegato A, Zurolo E, Losi G, Aronica E
and Carmignoto G: The inflammatory molecules IL-1β and HMGB1 can
rapidly enhance focal seizure generation in a brain slice model of
temporal lobe epilepsy. Front Cell Neurosci. 8:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chenard BL and Menniti FS: Antagonists
selective for NMDA receptors containing the NR2B subunit. Curr
Pharm Des. 5:381–404. 1999.PubMed/NCBI
|
|
11
|
Fisher RS, Cross JH, D'Souza C, French JA,
Haut SR, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, et
al: Instruction manual for the ILAE 2017 operational classification
of seizure types. Epilepsia. 58:531–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hemb M, Velasco TR, Parnes MS, Wu JY,
Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon
N, et al: Improved outcomes in pediatric epilepsy surgery: The UCLA
experience, 1986–2008. Neurology. 74:1768–1775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoffman WH and Haberly LB:
Bursting-induced epileptiform EPSPs in slices of piriform cortex
are generated by deep cells. J Neurosci. 11:2021–2031.
1991.PubMed/NCBI
|
|
14
|
Hoffman WH and Haberly LB: Bursting
induces persistent all-or-none EPSPs by an NMDA-dependent process
in piriform cortex. J Neurosci. 9:206–215. 1989.PubMed/NCBI
|
|
15
|
Wang B, Yin L, Zou X, Ye M, Liu Y, He T,
Deng S, Jiang Y, Zheng R, Wang Y, et al: A Subtype of inhibitory
interneuron with intrinsic persistent activity in human and monkey
neocortex. Cell Rep. Mar 3–2015.(Epub ahead of print).
|
|
16
|
Viviani B, Bartesaghi S, Gardoni F,
Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M,
Galli CL and Marinovich M: Interleukin-1beta enhances NMDA
receptor-mediated intracellular calcium increase through activation
of the Src family of kinases. J Neurosci. 23:8692–8700.
2003.PubMed/NCBI
|
|
17
|
Geiger JR and Jonas P: Dynamic control of
presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in
hippocampal mossy fiber boutons. Neuron. 28:927–939. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Connors BW, Gutnick MJ and Prince DA:
Electrophysiological properties of neocortical neurons in vitro. J
Neurophysiol. 48:1302–1320. 1982.PubMed/NCBI
|
|
19
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Zhang X, Liu L, Cui L, Yang R, Li
M and Du W: Tanshinone II A down-regulates HMGB1, RAGE, TLR4,
NF-kappaB expression, ameliorates BBB permeability and endothelial
cell function, and protects rat brains against focal ischemia.
Brain Res. 1321:143–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang R, Lao L, Ren K and Berman BM:
Mechanisms of acupuncture-electroacupuncture on persistent pain.
Anesthesiology. 120:482–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bianchi ME and Manfredi AA: Immunology.
Dangers in and out. Science. 323:1683–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bianchi ME: DAMPs, PAMPs and alarmins: All
we need to know about danger. J Leukoc Biol. 81:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catterall WA: Structure and regulation of
voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 16:521–555.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huttunen HJ and Rauvala H: Amphoterin as
an extracellular regulator of cell motility: From discovery to
disease. J Intern Med. 255:351–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pedrazzi M, Patrone M, Passalacqua M,
Ranzato E, Colamassaro D, Sparatore B, Pontremoli S and Melloni E:
Selective proinflammatory activation of astrocytes by high-mobility
group box 1 protein signaling. J Immunol. 179:8525–8532. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Najm IM, Ying Z, Babb T, Mohamed A, Hadam
J, LaPresto E, Wyllie E, Kotagal P, Bingaman W and Foldvary N:
Epileptogenicity correlated with increased N-methyl-D-aspartate
receptor subunit NR2A/B in human focal cortical dysplasia.
Epilepsia. 41:971–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas CG, Miller AJ and Westbrook GL:
Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured
hippocampal neurons. J Neurophysiol. 95:1727–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Botta P, Demmou L, Kasugai Y, Markovic M,
Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, et al: Regulating
anxiety with extrasynaptic inhibition. Nat Neurosci. 18:1493–1500.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waters J and Helmchen F: Background
synaptic activity is sparse in neocortex. J Neurosci. 26:8267–8277.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bean BP: The action potential in mammalian
central neurons. Nat Rev Neurosci. 8:451–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gardoni F, Boraso M, Zianni E, Corsini E,
Galli CL, Cattabeni F, Marinovich M, Di Luca M and Viviani B:
Distribution of interleukin-1 receptor complex at the synaptic
membrane driven by interleukin-1β and NMDA stimulation. J
Neuroinflammation. 8:142011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García-Arnandis I, Guillén MI, Gomar F,
Pelletier JP, Martel-Pelletier J and Alcaraz MJ: High mobility
group box 1 potentiates the pro-inflammatory effects of
interleukin-1β in osteoarthritic synoviocytes. Arthritis Res Ther.
12:R1652010. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Howard P, Twycross R, Shuster J, Mihalyo
M, Rémi J and Wilcock A: Anti-epileptic drugs. J Pain Symptom
Manage. 42:788–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neels HM, Sierens AC, Naelaerts K, Scharpé
SL, Hatfield GM and Lambert WE: Therapeutic drug monitoring of old
and newer anti-epileptic drugs. Clin Chem Lab Med. 42:1228–1255.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Neill LA and Bowie AG: The family of
five: TIR-domain-containing adaptors in toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CX and Shuaib A: NMDA/NR2B selective
antagonists in the treatment of ischemic brain injury. Curr Drug
Targets CNS Neurol Disord. 4:143–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tu W, Xu X, Peng L, Zhong X, Zhang W,
Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, et al: DAPK1
interaction with NMDA receptor NR2B subunits mediates brain damage
in stroke. Cell. 140:222–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perin-Dureau F, Rachline J, Neyton J and
Paoletti P: Mapping the binding site of the neuroprotectant
ifenprodil on NMDA receptors. J Neurosci. 22:5955–5965.
2002.PubMed/NCBI
|