Introduction
Hypertension is a leading risk factor for morbidity
and mortality (1,2). The elevation of blood pressure is
accompanied by increased reactive oxygen species production,
decreased nitric oxide bioavailability and decreased antioxidant
capacity (2,3). These factors may contribute to the
elevation of arterial blood pressure as well as end organ damage in
hypertension (2,3). Furthermore, matrix metalloproteinases
(MMPs) may be involved in end organ damage in hypertension
(2). MMPs are a family of proteases
that degrade extracellular matrix (ECM) proteins (4,5). They
are produced as pro-MMPs, which are then cleaved into active MMPs
(4,5). Classes of MMP include collagenases,
gelatinases, stromelysins, matrilysins and membrane-types (4). MMP-2 (gelatinase A) and MMP-9
(gelatinase B) are expressed in the uterus of humans and bovines,
as well as other mammals, and they serve a role in animal and human
endometrial tissue remodeling during the estrous and menstrual
cycles, and in pregnancy (4).
Curcumin (diferuloylmethane) is a component of
turmeric (2–5%, w/w) a spice that gives the yellow color to curry
powder. In traditional Ayurveda medicine, turmeric has been
described as a potent anti-inflammatory agent (6). The suppression of cellular
transformation, proliferation, invasion, angiogenesis and
metastasis of tumors by curcumin has been reported (6). On the other hand, a low concentration
of curcumin (500 nM) results in a neurological protective effect,
through increasing the proliferation of neural stem cells in the
hippocampus (7). It has been shown
that curcumin protects the vascular endothelium, through the
production of endothelial heme-oxigenase, while in Alzheimer's
disease it counteracts β-amyloid-induced oxidative stress (7). Some of these observations can be
explained by the activating and inhibitory actions of curcumin on a
number of key signaling pathways, including those of MAP kinase,
protein kinase C and brain-derived neurotrophic factor. Given its
activity on histone deacetylases (HDACs) (7), curcumin may also be considered an
epigenetic drug. Furthermore, curcumin can partially protect
against fructose-induced impairment in vascular contractility via
an antioxidant effect and reduction of elevated intracellular
calcium (8).
HDACs regulate the expression of cancer-associated
genes, which makes them promising targets for the development of
novel cancer drugs (9). Several HDAC
inhibitors, including suberanilohydroxamic acid, valproic acid and
trichostatin A, have been used within a clinical setting for the
treatment of cancer, or are undergoing clinical trials (9). The HDAC family contains 18 proteins,
which are grouped into four classes (I–IV) based on their structure
and homology. Classes I, II and IV contain 11 family members and
they are known as the classical HDACs, while the seven class III
family members are referred to as sirtuins (9).
The aim of the current study is to examine the
therapeutic effects and mechanism of curcumin on vascular injury
induced by hypertension in spontaneous hypertensive rats (SHRs).
The protective effects of curcumin against hypertension-caused
injury by regulating gene expression levels of MMP-2, HDAC1, tissue
inhibitor of metalloproteinase 1 (TIMP1) and transforming growth
factor β (TGFβ) are investigated.
Materials and methods
Animals
A total of 42 male SHRs (weight, ~200 g), aged 8–10
weeks, were obtained from the Shanghai Research Center for Model
Organisms (Shanghai, China). This study was approved (permit no.
SRCMR20130016) by the Animal Ethics Committee of Shanghai Research
Center for Model Organisms, and the experimental protocols were in
compliance with the Experimental Animal Regulations of the Ministry
of Science and Technology (Beijing, China). All rats were
maintained in colonies of 2 per cage for 14 days, and housed in a
temperature-controlled room under a standard light-dark cycle, with
free access to food and water. The animals were divided into 7
groups as follows: Blank control group (n=6) without any treatment,
negative control group (n=6) that received saline only (by
intraperitoneal injection) and 5 experimental groups (n=6 in each)
that were administered curcumin by a single intraperitoneal
injection (200 µl) every 2 days, for a total of 56 days, in
concentrations of 25, 50, 100, 200 and 400 mg/kg body weight.
Curcumin was obtained from Shanghai Sinopharm Chemical Reagent
Company, Ltd. (Shanghai, China). A total of 8 weeks following the
start of treatment, the rats (weight ~200 g) were anesthetized with
1.5% Nembutal (30 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) administered by intraperitoneal injection. Following
anesthesia the rats were sacrificed by cervical dislocation and
additional experiments were conducted.
Histopathological analysis
For histopathological analysis, coronary artery
tissues were stained with hematoxylin and eosin (H&E) and
Massion stains. Briefly, all fresh tissues were washed three times
with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) for 30 min, dehydrated through a graded series of ethanol,
vitrified in xylene and embedded in paraffin. Next, serial 6-µm
sections were cut and stained with H&E. The Massion stain was
performed according to the manufacturer's protocol (Sigma-Aldrich;
Merck KGaA).
Immunohistochemistry (IHC)
analysis
Briefly, all steps were carried out as previously
described (10). The animals were
sacrificed by cervical dislocation and the aorta was subsequently
isolated from the root to the abdominal aortic bifurcation for
disconnection. The coronary artery was harvested, cut
longitudinally and washed with saline. All fresh coronary artery
tissues were washed three times with PBS and fixed with 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 30 min, dehydrated
through a graded series of ethanol (cat. no. 10009218), vitrified
in xylene (cat. no. 10023418) and embedded in paraffin (cat. no.
69018961) (all Shanghai Sinopharm Chemical Reagent Company, Ltd.).
Next, serial 6-µm-thick sections were cut and rinsed with 3% PBS
(Sigma-Aldrich; Merck KGaA). Antigen retrieval was performed at
100°C using an antigen repairing solution Improved Citrate Antigen
Retrieval solution (cat. no. P0083; Beyotime Institute of
Biotechnology, Haimen, China). Rabbit anti-human HDAC1 (cat. no.
34589), MMP-2 (cat. no. 87809) and TIMP1 (cat. no. 8946), rabbit
anti-histone H3 acetyl (cat. no. 9927) and anti-TGFb (cat. no.
3711) primary antibodies (all 1:200; Cell Signaling Technology,
Inc., Danvers, MA, USA) were added and the sections were incubated
for 60 min at room temperature, then horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2768; 1:200;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was added and the
sections were incubated for 60 min at room temperature. Finally, a
VECTASTAIN Elite ABC kit (cat. no. PK-6100; Vector Laboratories,
Inc., Burlingame, CA, USA) was used for the color reaction.
Meanwhile, PBS (pH 7.4) was used as a negative control in the place
of the first antibody. Five random fields of each tissue section
were observed (magnification, ×200) and analyzed using Image-Pro
Plus version 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA). ImageJ 1.42q software (National Institutes of Health,
Bethesda, MD, USA) was used to analyze the area of the positively
stained cells.
Chromatin immunoprecipitation (ChIP)
assays
ChIP experiments were performed on coronary artery
tissue sections, which were prepared as described above, using
rabbit anti-human HDAC1 (cat. no. 34589) and rabbit anti-histone H3
acetyl (cat. no. 9927) primary antibodies (both 1:100; Cell
Signaling Technology, Inc.), and normal rabbit immunoglobulin G
(cat. no. 12-370; Upstate Biotechnology; Merck Millipore,
Billerica, MA, USA) as a negative control. All steps were carried
out as previously described (11).
In brief, the cells were fixed with 1% formaldehyde (Sigma-Aldrich;
Merck KGaA) for 30 min at 37°C, then quenched with 125 mM glycine
for 10 min at room temperature to form DNA-protein cross-links.
Samples were sonicated on ice until chromatin fragments were
200–1,000 bp in size, then incubated with antibodies at 4°C
overnight. PCR amplification was performed under the following
conditions: Thirty-three cycles in total, consisting of
denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 30 sec.
Statistical analysis
Each experiment was performed at least three times.
Data are shown as the mean ± standard error and were analyzed using
the Student's t-test by GraphPad Prism software, version 5.00
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of curcumin on coronary artery
structure and blood pressure in SHRs
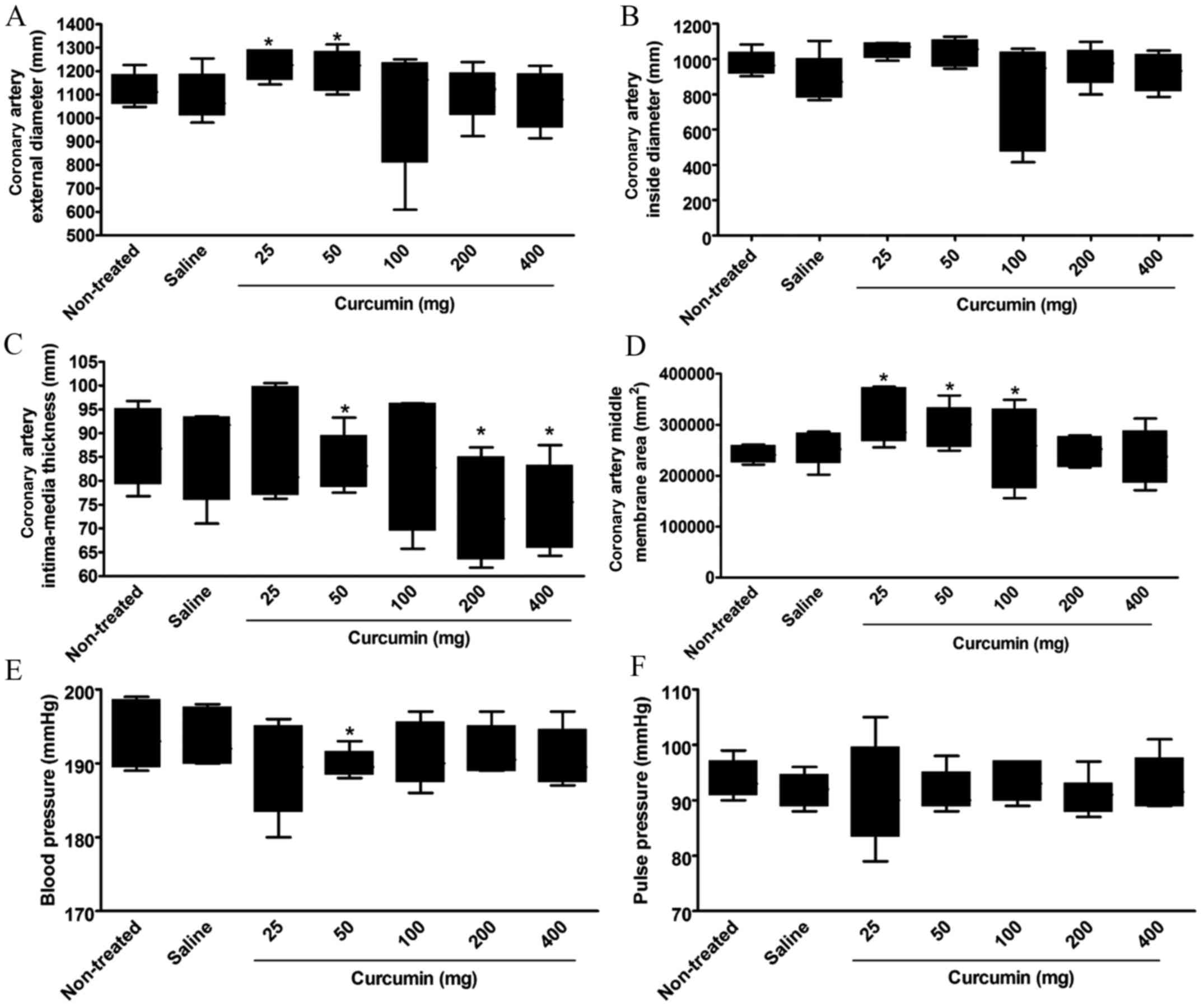
At 8 weeks after treatment, the coronary artery
structure, blood pressure and pulse pressure in each SHR group were
determined. There was no significant difference in the coronary
artery external diameter between the non-treatment group and the
saline group or the curcumin treatment groups with dosages of 100,
200 or 400 mg. However, the external diameter of the coronary
artery in the curcumin treatment groups with dosages of 25 or 50 mg
was significantly increased as compared with the non-treatment
group (P<0.05; Fig. 1A, Table I).
 | Table I.Coronary artery structure, blood
pressure and pulse pressure of spontaneous hypertension rats at 8
weeks after curcumin treatment. |
Table I.
Coronary artery structure, blood
pressure and pulse pressure of spontaneous hypertension rats at 8
weeks after curcumin treatment.
|
|
|
| Curcumin treatment
(mg) |
|---|
|
|
|
|
|
|---|
| Variable | Non-treated | Saline | 25 | 50 | 100 | 200 | 400 |
|---|
| Coronary artery
external diameter (mm) |
1,12±24.99 |
1,09±37.84 |
1,23±23.15a |
1,21±31.66a |
1,07±97.87 |
1,11±41.99 |
1,08±46.10 |
| Coronary artery
inside diameter (mm) |
971.40±25.17 |
884.80±48.06 |
1,06±16.06 |
1,04±27.65 |
820.40±111.90 |
962.40±39.54 |
925.00±40.57 |
| Coronary artery
intima-media thickness (mm) |
87.04±3.17 |
87.04±3.72 |
85.83±4.43 |
83.75±2.22a |
82.83±5.09 |
73.50±3.99a |
74.88±3.39a |
| Coronary artery
middle membrane area (mm2) |
241,854±6,336 |
253,373±12,102 |
308,564±20,539a |
296,495±15,360a |
254,771±29,075a |
248,710±10,843 |
236,958±19,914 |
| Blood pressure
(mmHg) |
193.71±1.75 |
193.22±1.42 |
189.32±2.33 |
189.83±0.70a |
191.01±1.59 |
191.52±1.26 |
190.51±1.48 |
| Pulse pressure
(mmHg) |
93.67±1.256 |
91.83±1.108 |
91.00±3.502 |
92.00±1.397 |
93.00±1.215 |
91.14±1.317 |
92.67±1.838 |
No significant difference was found in the coronary
artery inside diameter between the groups at 8 weeks after
treatment (Fig. 1B, Table I).
The coronary artery intima-media thickness
significantly decreased in the curcumin treatment groups with
dosages of 50, 200 and 400 mg at 8 weeks after treatment as
compared with the non-treatment group (P<0.05). However, no
significant decrease was found in the curcumin treatment groups
with dosages of 25 or 100 mg (Fig.
1C, Table I).
The coronary artery middle membrane area
significantly increased in the curcumin treatment groups with
dosages of 25, 50 and 100 mg, as compared with the non-treated
group (P<0.05). However, no significant difference was found
between the curcumin treatment groups with dosages of 200 or 400 mg
and the non-treated group (Fig. 1D,
Table I).
A significant increase in blood pressure was only
found in the curcumin treatment group with a dosage of 50 mg as
compared with the non-treated group (P<0.05). All other dosages
showed no significant difference in blood pressure compared with
the non-treated group (Fig. 1E,
Table I).
No significant difference was found in the pulse
pressure between the groups at 8 weeks after treatment (Fig. 1F, Table
I).
Effect of curcumin on coronary artery
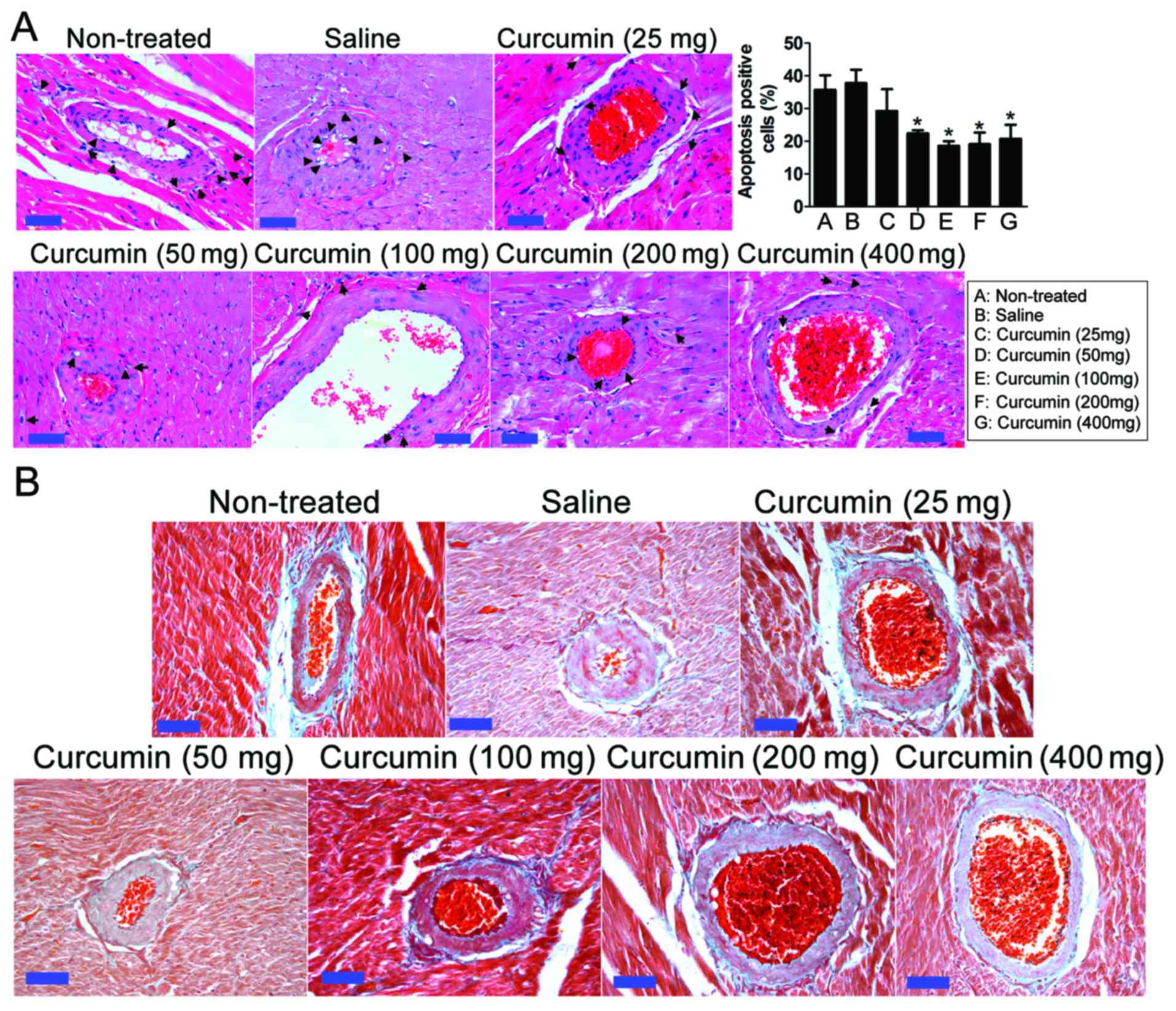
pathology in SHRs
Hypertension can cause coronary artery stenosis and
blood flow resistance, and promote inflammatory cell hyperplasia
and atherosclerosis plaque formation. Consistent with this, the
coronary arteries of SHRs demonstrated vascular adventitial
fibrosis, smooth muscle cell hyperplasia, coronary artery
endometrial glass-like lesions and vascular endothelial cell injury
and hyperplasia (Fig. 2). However,
at 8 weeks after treatment, in the curcumin treatment groups with
dosages of 25, 50 and 100 mg, H&E (Fig. 2A) and Massion (Fig. 2B) stains revealed that coronary
artery inflammation in vascular endothelial cells had decreased and
the apoptosis of endothelial cells had decreased. Following H&E
staining, apoptotic cells exhibit a clear nuclear contraction and
cytoplasmic expansion phenotype. The proportion of apoptotic cells
to the total cell number was calculated and the statistical results
indicated that curcumin treatment (50–400 mg) significantly
decreased the apoptotic rate of vascular endothelial cells and
smooth muscle cells in rats (P<0.05). In each assay, the inner
wall of the blood vessel had thickened and atherosclerotic plaques
were reduced. In addition, the accumulation of perivascular
collagen and fibrosis was reduced.
Effect of curcumin on expression
levels of HDAC1, MMP-2, TIMP1 and TGFβ in the coronary arteries of
SHRs
ImageJ 1.42q software was used to analyze the area
of positive cells on IHC staining (Table II and Fig. 3). IHC analysis indicated that at 8
weeks following treatment with 25 mg curcumin, the expression of
HDAC1 and TGFβ were significantly lower compared with the
non-treated group (P<0.05). However, the expression of TIMP1 was
significantly higher in the curcumin treatment groups (25–100 mg)
compared with the non-treated group (P<0.01). At 8 weeks
following treatment with 50 mg curcumin, the expression of HDAC1
(P<0.05), MMP2 (P<0.01) and TGFβ (P<0.05) were all
significantly lower compared with the non-treated group, however
the expression of TIMP1 was significantly higher (P<0.01). At 8
weeks following treatment with 100 mg curcumin, only the expression
of MMP2 was significantly decreased compared with the non-treated
group (P<0.05), however, the expression of TIMP1 was
significantly higher (P<0.01). At 8 weeks following treatment
with 200 mg curcumin, the expression of MMP2 (P<0.05), TGFβ
(P<0.05) and HDAC1 (P<0.01) were significantly lower compared
with the non-treated group, however, the expression of TIMP1 was
not significantly higher. At 8 weeks following treatment with 400
mg curcumin, only the expression of HDAC1 was significantly lower
in the curcumin treatment groups than in the non-treated group
(P<0.05).
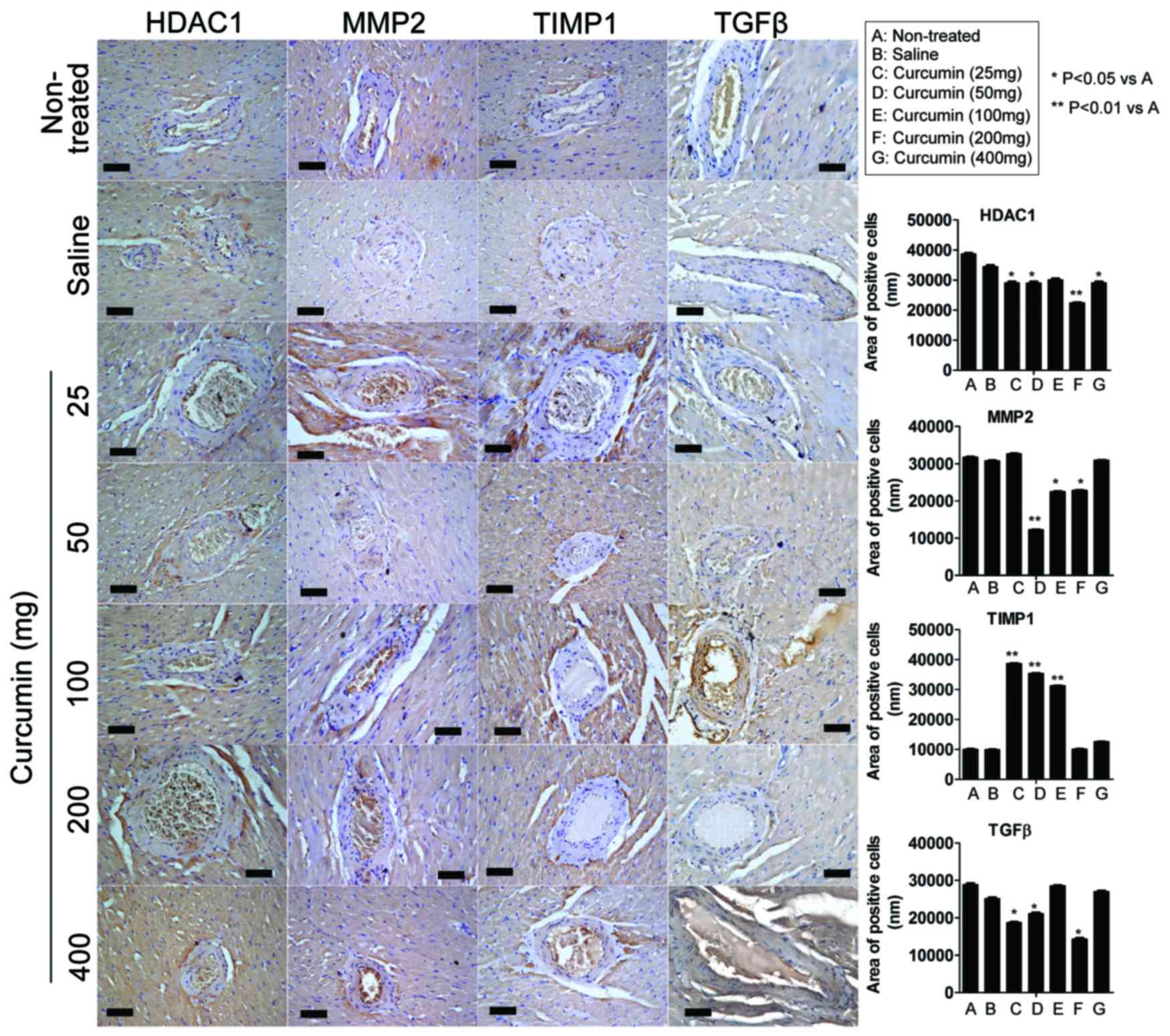 | Figure 3.Effect of curcumin on protein
expression in the coronary arteries of spontaneous hypertensive
rats. The expression levels of HDAC1, MMP-2, TIMP1 and TGFβ
proteins were determined by immunohistochemical staining. At 8
weeks following treatment, immunohistochemistry analysis indicated
that the expression of HDAC1, MMP-2 and TGFβ was significantly
lower in certain curcumin treatment groups when compared with the
non-treated group. Certain curcumin treatment groups demonstrated
significantly higher levels of TIMP1 expression compared with the
non-treated group. Scale bar, 30 µm; magnification, ×200. HDAC1,
histone deacetylase 1; MMP-2, matrix metalloproteinase-2; TIMP1,
tissue inhibitor of metalloproteinase 1; TGFβ, transforming growth
factor β. |
 | Table II.Area of positively stained cells
following immunohistochemistry staining. |
Table II.
Area of positively stained cells
following immunohistochemistry staining.
|
|
|
| Curcumin treatment
(mg) |
|---|
|
|
|
|
|
|---|
| Protein | Non-treated | Saline | 25 | 50 | 100 | 200 | 400 |
|---|
| HDAC1 |
38,694±350.19 |
34,444±525.68 |
29,076±446.81a |
28,976±560.42a |
30,118±423.49 |
22,416±172.58b |
29,067±511.25a |
| MMP2 |
31,700±150.61 |
30,726±161.64 |
32,579±144.78 |
12,201±121.31b |
22,436±145.66a |
22,791±138.96a |
30,871±109.97 |
| TIMP1 |
10,031±166.22 |
9,889±152.34 |
38,671±143.77b |
35,356±187.15b |
31,209±140.04b |
10,042±126.58 |
12,562±111.32 |
| TGFβ |
28,876±349.06 |
25,081±329.62 |
18,690±255.52a |
21,048±353.79a |
28,545±156.22 |
14,282±345.32a |
26,981±242.04 |
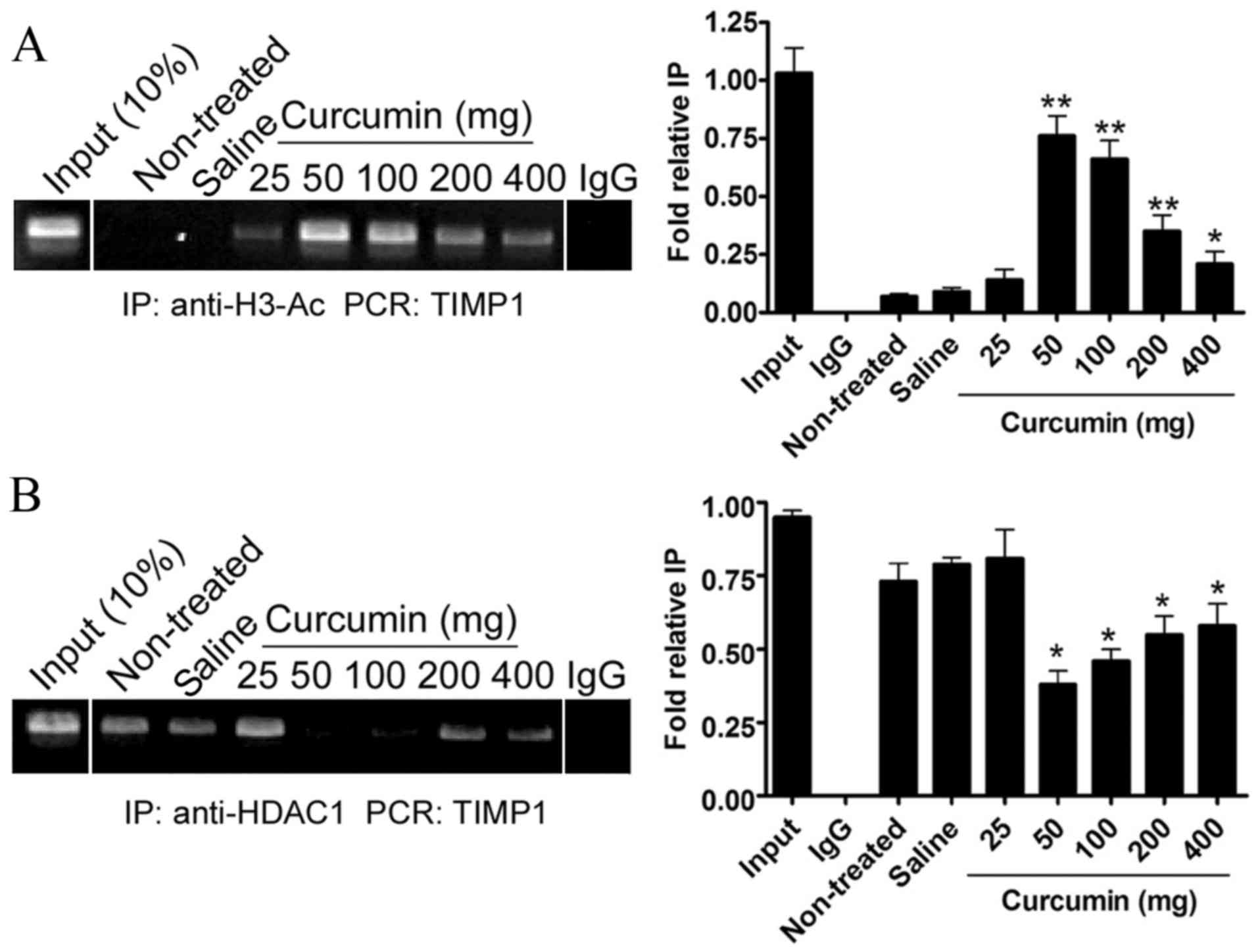
Effect of curcumin on the
transcriptional activity of TIMP1 genes
A ChIP assay was performed to evaluate histone H3
acetylation levels of TIMP1 promoters in each SHR group. At 8 weeks
after treatment, the curcumin treatment groups with dosages of
50–400 mg demonstrated significantly higher levels of acetylation
of histone H3 in the TIMP1 promoter regions as compared with the
non-treated group (P<0.01, P<0.05; Fig. 4A). However, the acetylation levels of
histone H3 in the TIMP1 promoter regions did not significantly
increase in the saline group or in the curcumin group with a dosage
of 25 mg as compared with the non-treated group.
In addition, a ChIP assay was performed to evaluate
HDAC1 levels at the TIMP1 promoter regions in SHRs. At 8 weeks
after treatment, the levels of HDAC1 at the TIMP1 promoter regions
had not significantly decreased in the saline group or the curcumin
group with a dosage of 25 mg, as compared with the non-treated
group. However, in the curcumin treatment groups with dosages of
50–400 mg), the levels of HDAC1 at the TIMP1 promoter regions had
significantly decreased as compared with the non-treated group
(P<0.05; Fig. 4B). These findings
suggested that curcumin was capable of increasing the activation of
the TIMP1 promoter through suppressing HDAC1 expression and
increasing histone H3 acetylation.
Discussion
Hypertension is a major risk factor for
cardiovascular events (12). In
addition to the alterations triggered by chronic hypertension on
cardiomyocytes, important modifications occur in the cardiac ECM
integrity during the progression of left-ventricular hypertrophy
(12). A previous study demonstrated
that perturbed ECM synthesis and degradation were the primary
contributors to cardiac remodeling observed in hypertensive heart
disease and were caused by an imbalance in the ratio of MMPs and
their inhibitors, TIMPs (13).
MMP-2, MMP-9 and TIMP1 expression levels were previously
demonstrated to be enhanced in the myocardium of Dahl
salt-sensitive rats during the transition to congestive heart
failure (13,14). Moreover, MMP inhibition results in
the remodeling of vascular, glomerular and tubulointerstitial
spaces by altering the turnover of ECM proteins (14). A previous study also indicated that
MMP-2 played a key role in hypertensive cardiac remodeling and in
other cardiovascular disorders (12).
In the current study, it was revealed that curcumin
effectively improved vascular structure and reduced pathological
damage to the coronary arteries. Although a range of concentrations
of curcumin were useful for the treatment of coronary artery injury
in SHR rats, the best results were observed with moderate doses
(approximately 50 mg/kg body weight). Therefore, we speculated that
this dosage of curcumin had a potential therapeutic effect.
In previous studies, curcumin could inhibit the
activity of HDACs (7). In the
current study, the expression levels of HDAC1, MMP-2, TIMP1 and
TGFβ proteins were determined after treatment with curcumin in
dosages from 25 to 400 mg. The results indicated that the
expression levels of HDAC1, MMP-2 and TGFβ decreased in the
curcumin treatment groups, as compared with the non-treated group
or the negative control group. However, the expression of TIMP1 did
not decrease in the curcumin treatment groups.
The main function of HDACs is to remove acetyl
groups from the N-acetyl lysines on a histone, modify the chromatin
structure and modulate gene transcription (9). The current study investigated the
regulation mechanism of curcumin on covalent histone modifications
and the expression levels of HDAC1. It was found that curcumin
could suppress HDAC1 expression and increase histone H3 acetylation
at the TIMP1 promoter region in SHR rats, which may promote TIMP1
transcription activation.
In conclusion, curcumin could relieve extracellular
matrix degradation and interstitial fibrosis induced by
hypertension, elevate blood pressure and improve vascular structure
through inhibiting the expression of HDAC1, promote TIMP1
transcription activation and inhibit the expression of MMP-2 and
TGFβ. However, the results of the present study were conducted in
an animal model, so are not necessarily wholly representative of
the effect curcumin may have in humans. In the present study, the
epigenetic mechanisms underlying the effect of curcumin on the
regulation of vascular wall collagen was preliminarily
demonstrated. However, further research is required to clarify how
curcumin induces histone acetylation and the deacetylation of other
proteins, as this was not investigated in depth within the present
study. Further research may also focus on the effects of curcumin
on the regulation of lipid metabolism and its protein interaction
networks.
Acknowledgements
The present study was supported by a grant from the
Shanghai Municipal Health Bureau Fund (grant no. 20114312), awarded
to Professor Jun Hu (Xuhui Central Hospital, Shanghai, China), and
by a grant from the Shanghai Natural Science Fund (grant no.
17ZR1426800), awarded to Professor Fu Zhu (Xuhui Central
Hospital).
References
|
1
|
Kakar P and Lip GY: Towards understanding
the aetiology and pathophysiology of human hypertension: Where are
we now? J Hum Hypertens. 20:833–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duansak N and Schmid-Schönbein GW: The
oxygen free radicals control MMP-9 and transcription factors
expression in the spontaneously hypertensive rat. Microvasc Res.
90:154–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sedeek M, Hébert RL, Kennedy CR, Burns KD
and Touyz RM: Molecular mechanisms of hypertension: Role of Nox
family NADPH oxidases. Curr Opin Nephrol Hypertens. 18:122–127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Mata KM, Mazzuca MQ and Khalil RA:
Altered matrix metalloproteinase-2 and −9 expression/activity links
placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and
vascular remodeling and collagen deposition in hypertensive
pregnancy. Biochem Pharmacol. 89:370–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luizon MR, Palei AC, Sandrim VC, Amaral
LM, Machado JS, Lacchini R, Cavalli RC, Duarte G and Tanus-Santos
JE: Tissue inhibitor of matrix metalloproteinase-1 polymorphism,
plasma TIMP-1 levels, and antihypertensive therapy responsiveness
in hypertensive disorders of pregnancy. Pharmacogenomics J.
14:535–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggarwal BB: Prostate cancer and curcumin:
Add spice to your life. Cancer Biol Ther. 7:1436–1440. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panighini A, Duranti E, Santini F, Maffei
M, Pizzorusso T, Funel N, Taddei S, Bernardini N, Ippolito C,
Virdis A and Costa M: Vascular dysfunction in a mouse model of rett
syndrome and effects of curcumin treatment. PLoS One. 8:e648632013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahmoud MF and El Bassossy HM: Curcumin
attenuates fructose-induced vascular dysfunction of isolated rat
thoracic aorta rings. Pharm Biol. 52:972–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song C, Zhu S, Wu C and Kang J: Histone
deacetylase (HDAC) 10 suppresses cervical cancer metastasis through
inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J
Biol Chem. 288:28021–28033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen DZ, Xin SL, Chen C and Liu T: Effect
of atorvastatin on expression of TLR4 and NF-κB p65 in
atherosclerotic rabbits. Asian Pac J Trop Med. 6:493–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Huang Y, Huang Q, Jiang L, Guo L
and Liu Z: Use of human amniotic epithelial cells as a feeder layer
to support undifferentiated growth of mouse spermatogonial stem
cells via epigenetic regulation of the Nanog and Oct-4 promoters.
Acta Biol Hung. 63:167–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizzi E, Guimaraes DA, Ceron CS, Prado CM,
Pinheiro LC, Martins-Oliveira A, Gerlach RF and Tanus-Santos JE:
β1-Adrenergic blockers exert antioxidant effects, reduce matrix
metalloproteinase activity and improve renovascular
hypertension-induced cardiac hypertrophy. Free Radic Biol Med.
73:308–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinoshita T, Ishikawa Y, Arita M,
Akishima-Fukasawa Y, Fujita K, Inomata N, Suzuki T, Namiki A,
Mikami T and Ikeda T: Antifibrotic response of cardiac fibroblasts
in hypertensive hearts through enhanced TIMP-1 expression by basic
fibroblast growth factor. Cardiovasc Pathol. 23:92–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pushpakumar SB, Kundu S, Metreveli N,
Tyagi SC and Sen U: Matrix metalloproteinase inhibition mitigates
renovascular remodeling in salt-sensitive hypertension. Physiol
Rep. 1:e000632013. View
Article : Google Scholar : PubMed/NCBI
|