Introduction
Dimethyl carbonate (DMC) is an environmentally
friendly organic solvent, which may have a wide range of potential
applications, such as being used as a new solvent or fuel additive
(1). Due to high market demand and
environmental regulations, DMC may be a potential substitute for
the organic solvents and chemical midbodies that are currently
used, for example DMC could be used as a substitute of methyl
chloroform for organic solvents or carbonyl chloride for the
production of polycarbonate and carbamic acid ester. However,
compared with other commercially used chemicals, the toxicity of
DMC remains unknown. It has been demonstrated that 10 day
inhalation exposure to 3,000 ppm DMC in pregnant female mice during
days 6–15 of pregnancy for 6 h/day reduces the weight of mothers
and fetuses, and increases the number of malformed and hypogenetic
fetuses (2). In recent years, the
adverse health effects of various chemicals, including the toxicity
of chemicals to the female reproductive system, have become more
topical. It has been demonstrated that various industrially used
chemicals may disrupt hormonal regulation and follicular
development in the mammalian ovary (3). Environmental contaminants have been
detected in the ovarian follicular fluid, further indicating the
effect of such chemicals on ovarian function (4). However, the toxicity of DMC on the
reproductive system remains largely unknown.
Autophagy is a primary cytoprotective mechanism and
is characterized by the formation of isolation membranes. These
membranes sequester unnecessary cellular components or damaged
organelles, including mitochondria and endoplasmic reticulum, and
form autophagosomes to recycle building blocks for cell survival
when the cell is under stress (5,6).
Previous studies have demonstrated that autophagy serves a role in
regulating testicular homeostasis following exposure to toxins
(7,8); however few relevant studies have
investigated the effects of toxins on the ovaries. In the testes,
autophagy is generally induced by the upregulation of reactive
oxygen species (ROS) in tissues following exposure to toxins
(9). Previous studies have also
revealed that exposure to toxins is associated with ROS
accumulation in the ovaries (10,11). In
addition, it has been demonstrated that the hypoxia inducible
factor 1 α subunit (HIF-1α) signaling pathway serves a role in
regulating cellular ROS levels by stimulating mitochondrial
selective autophagy to reduce ROS production (12,13).
Other studies have demonstrated that HIF-1α activation is a
self-protective mechanism that occurs under adverse conditions, as
it upregulates genes encoding glucose transporters and glycolytic
enzymes, downregulates mitochondrial genes (14) and activates autophagic signaling
pathways (15). In mammalian
ovaries, HIF-1α is ubiquitously expressed during various stages of
folliclular and corpus luteum development, and serves a pivotal
role in the maintenance of ovarian homeostasis (16,17).
Due to the important role served by HIF-1α signaling
in ovarian homeostasis, it was hypothesized that the HIF-1α
signaling pathway serves a role in the regulation of ovarian
function following exposure to DMC. Therefore, the present study
investigated the histological changes and expression pattern of
apoptosis-related proteins in the ovary to determine whether DMC
exposure induces injury in the ovary and assessed the role served
by the HIF-1α signaling pathway in doing this.
Materials and methods
Animals and treatment
A total of 40 healthy adult female Kunming mice
weighing 18–22 g (age, 6–8 weeks) were purchased from Wushi
Experimental Animal Supply Co. Ltd. (Fuzhou, China). Mice were kept
in a room with a constant temperature of 21–25°C, a relative
humidity of 40–60% and a 12 h light/dark cycle, and fed a standard
diet and tap water ad libitum. Mice were housed in cages and
each cage contained 3–4 mice. Prior to grouping, the toxicity of
DMC was pre-tested by extra experiments (results not shown).
According to the results of the pre-test, all mice were randomly
distributed into the following 4 groups (all n=10): A control group
(Ctrl) treated with corn oil, a low dose treatment group (group A)
treated with 0.06 g/kg DMC; a medium dose treatment group (group B)
treated with 0.6 g/kg DMC; and a high dose treatment group (group
C) treated with 6.0 g/kg DMC. All mice in the treatment groups were
administered DMC (Sinopharm Chemical Reagent Co., Ltd, Shanghai,
China) via gavage. DMC is unable to dissolve in water, therefore
corn oil (Shandong Luhua Group Co., Ltd., Laiyang, China) was used
as a solvent and different dilutions were prepared prior to
administration. The dilutions were 0.3, 3 and 30% (v/v) and the
volume of treatment dosages was 0.4 ml. All mice were administrated
an equal volume of solution by gavage every 2 days over a treatment
period of 30 days. The mice in the control group were treated with
corn oil alone via gavage. The current study was approved by the
Animal Ethical and Welfare Committee of Funjian Normal University
(Fujian, China).
Tissue preparation and
histopathological examination
At the end of treatment, mice were sacrificed by
cervical dislocation. The bilateral ovaries were immediately
removed and weighed. The left ovary of each animal was used for
histopathological examination and the right ovary was used to
examine the expression of different functional proteins. Ovarian
tissues for histological study were fixed in 4% paraformaldehyde at
37°C for 24 h, then dehydrated and embedded in paraffin; sections
5-µm thick were subsequently prepared. Ovary sections were dewaxed
and rehydrated prior to staining with hematoxylin. The sections
were stained with hematoxylin at room temperature for 20–30 sec and
then observed under a light microscope.
Immunohistochemistry of light chain
(LC)-3 and HIF-1α
Immunohistochemical staining of LC-3 and HIF-1α were
performed following the manufacturer's protocols and previously
reported studies (18,19). Briefly, paraffin-embedded tissue
sections (prepared as described above) were dewaxed and rehydrated.
Sections were then subjected to antigen microwave antigen retrieval
using 0.01 M citric acid buffer at 100°C for 10 min. Endogenous
peroxide was inhibited by incubation of the sections in 3%
H2O2 for 30 min. Sections were then incubated
in 5% bovine serum albumin at room temperature for ~20 min to block
non-specific reactions. Primary antibodies (50 µl) were incubated
overnight at 4°C. The primary antibodies used were anti-LC-3B
antibody (1:500, cat. no. ab48394, Abcam, Cambridge, MA, USA) and
anti-HIF-1α antibody (1:400, cat. no. sc-53546, Santa Cruz
Biotechnology Inc., Dallas, TX, USA). Following washing with PBS,
slides were incubated with the secondary antibodies (PV-9001 and
PV-9002, OriGene Technologies, Beijing, China) at room temperature
for 20 min, respectively. For visualization, all sections were
stained with diaminobenzidine tetrahydrochloride chromogen at room
temperature for 3–6 min. All sections were then counterstained with
hematoxylin at room temperature for 5 min, dehydrated and mounted.
The sections were observed under a light microscope.
Western blot analysis of cleaved
caspase-3, Bcl-2, Bax, LC-3II, beclin-1, HIF-1α and BNIP3
expression
Ovarian tissues from each group were homogenized in
ice-cold radioimmunoprecipitation assay lysate buffer (cat. no.
P0013, Beyotime Institute of Biotechnology, Haimen, China) and
centrifuged at 15,000 × g for 15 min at 4°C. The supernatant was
then collected. Nuclear proteins were extracted using a kit from
Beyotime Institute of Biotechnology (Nuclear and Cytoplasmic
Protein Extraction kit, cat. no. P0027) and protein concentrations
were determined using a BCA Protein assay kit (cat. no. P0011,
Beyotime Institute of Biotechnology). Protein samples were diluted
into equal concentrations and 20 µg protein samples were then
subjected to 12% SDS-PAGE gel electrophoresis and
electrophoretically transferred onto polyvinylidene difluoride
membranes (Pall Life Sciences, Port Washington, NY, USA). Membranes
were washed with TBS with 0.2% Tween-20 (TBST; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and blocked with 5% nonfat milk in Tris
buffered saline-Tween-20 (TBST, pH 7.4) for 1 h at room
temperature. Membranes were then incubated with anti-cleaved
caspase-3 antibody (1:1,000; cat. no. 5A1E; Cell Signaling
Technology, Danvers, MA, USA), anti-Bcl-2 antibody (1:1,000; cat.
no. 12789; Proteintech Group, Wuhan, China), anti-Bax antibody
(1:2,000; cat. no. 60,267; Proteintech Group), anti-LC-3II antibody
(1:1,000; cat. no. ab48394; Abcam), anti-Beclin-1 antibody
(1:1,500; cat. no. 11306; Proteintech Group), anti-p62 antibody
(1:1,000, cat. no. ab56416; Abcam), anti-HIF-1α antibody (1:500,
cat. no. sc-53,546; Santa Cruz Biotechnology), anti-BNIP3 antibody
(1:1,000, cat. no. ab10433; Abcam) and anti-β-actin antibody
(1:4,000; cat. no. 66,009; Proteintech Group) overnight at 4°C.
Following three washes with TBST, the membrane was incubated in
horseradish peroxidase-conjugated goat anti-rabbit (cat. no. A0208)
or mouse (cat. no. A0216) immunoglobulin (1:4,000, Beyotime
Institute of Biotechnology) for 1 h at room temperature. Bands were
then visualized using an enhanced chemiluminscence kit (Beyotime
Institute of Biotechnology). Blots were quantified using ImageJ
1.49 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The significance of differences of mean values within and
between multiple groups was evaluated using one-way analysis of
variance, followed by Tukey's range test. P<0.05 was determined
to indicate a statistically significant difference. #
P<0.05 vs. the control, & P<0.05 vs. Group A,
*P<0.05 vs. Group B.
Results
Effect of DMC exposure on ovarian
histopathology
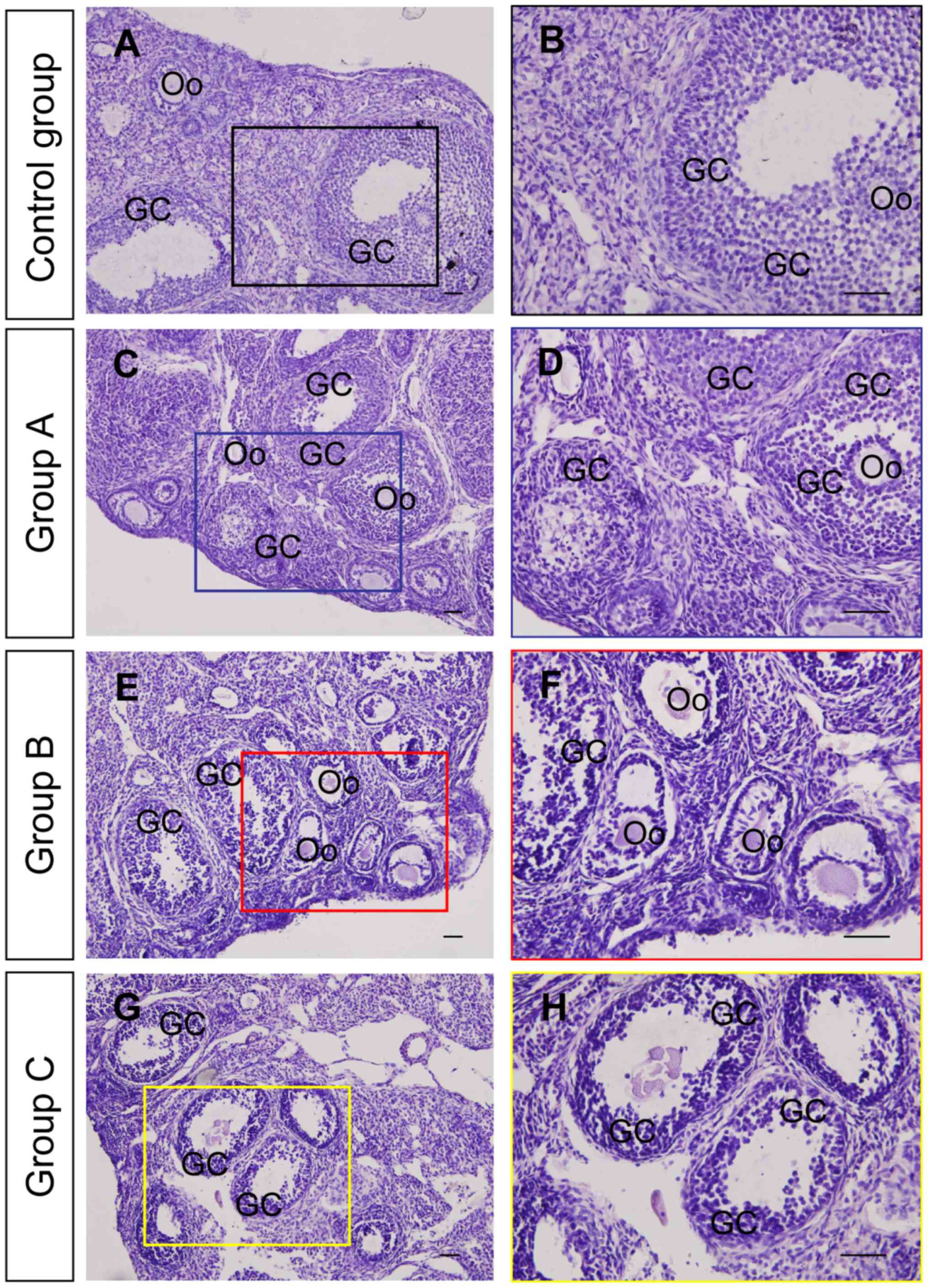
To clarify whether the exposure of DMC led to
impairments in the ovary, the morphology of ovaries from the
different groups of mice was examined (Fig. 1). Staining indicated that the
granulosa cells of developing follicles from mice in the control
group were well organized and tightly arranged around the inner of
the follicular theca and that the connection between granulosa
cells and oocytes was well maintained (Fig. 1A and B). The morphological structure
of the ovaries was largely maintained in group A (Fig. 1C and D); however, it was notably
altered in groups B (Fig. 1E and F)
and C (Fig. 1G and H) compared with
the control group. The primary histopathological changes following
exposure to DMC included follicular atresia and dispersal of
granulosa cells in the developing follicles.
DMC exposure increases apoptosis in
ovarian tissue
To determine whether DMC exposure activates the
apoptotic pathway, the expression of apoptosis-related proteins was
measured using western blotting. Bcl-2 primarily functions as a
factor to maintain the survival of cells, whereas Bax promotes the
activation of capase-3 and induces the apoptosis of cells (20).
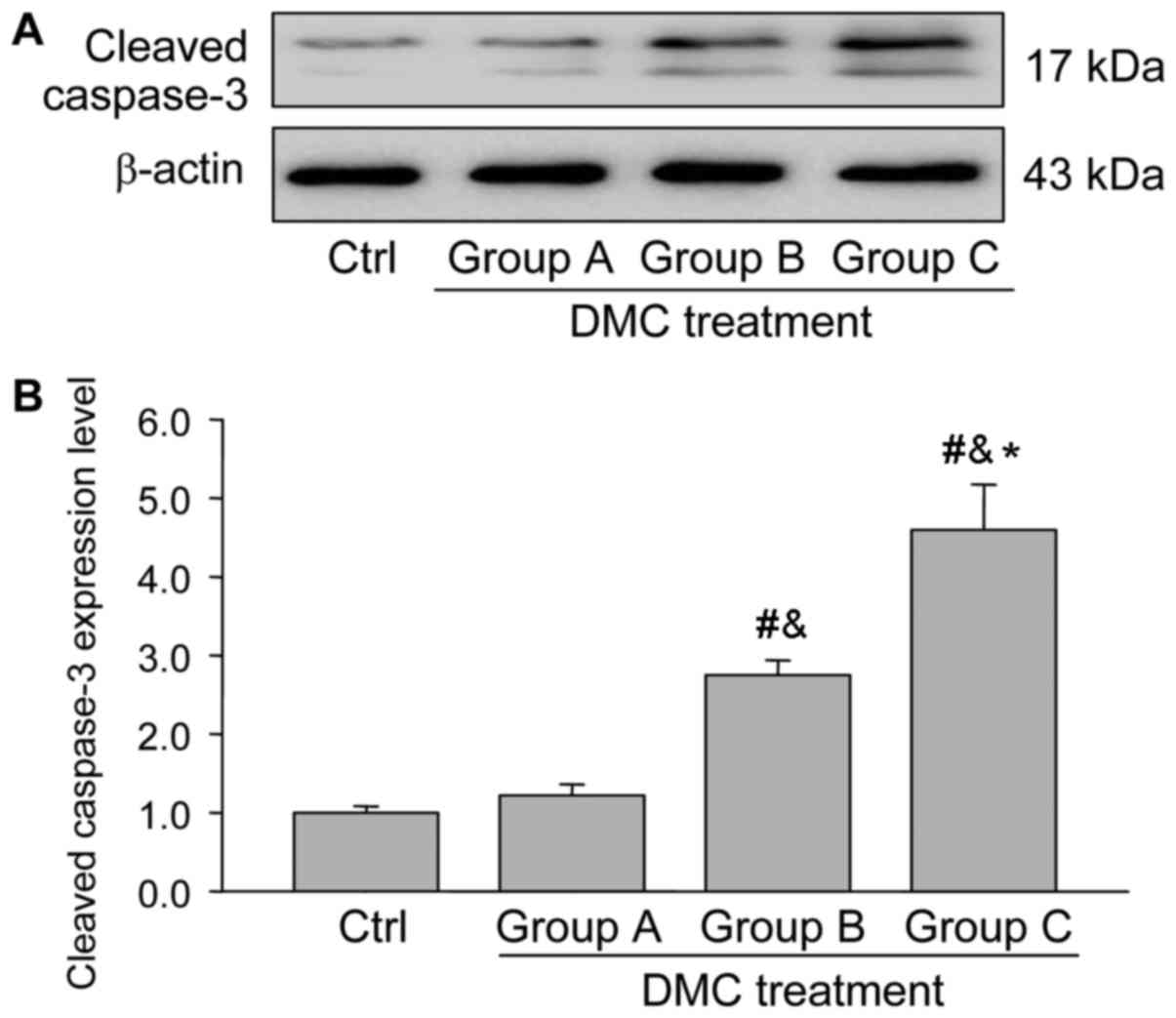
The results demonstrated that the expression of
cleaved caspase-3, a marker of cellular apoptosis, increased
significantly following DMC treatment, in a dose-dependent manner
(Fig. 2). Further analysis indicated
that the Bcl-2 expression was significantly increased in group A
and significantly decreased in groups B and C (Fig. 3A and B), whereas the expression of
Bax was similar to the control group in group A and significantly
increased in groups B and C (Fig.
3C). The ratio of Bax to Bcl-2 indicates whether cells are
pro-survival or pro-death. It was observed that the ratio of
Bax/Bcl-2 exhibited a similar pattern to that of cleaved caspase-3
(Figs. 2B and 3D). These results suggest that low doses of
DMC do not affect cellular apoptosis, whereas higher doses of DMC
increase the rate of apoptosis in the mammalian ovaries.
DMC exposure activates autophagy in
the ovaries
Previous studies have identified the involvement of
autophagy in the regulation of reproductive system following
exposure to toxins (1,2). To determine the possible role of
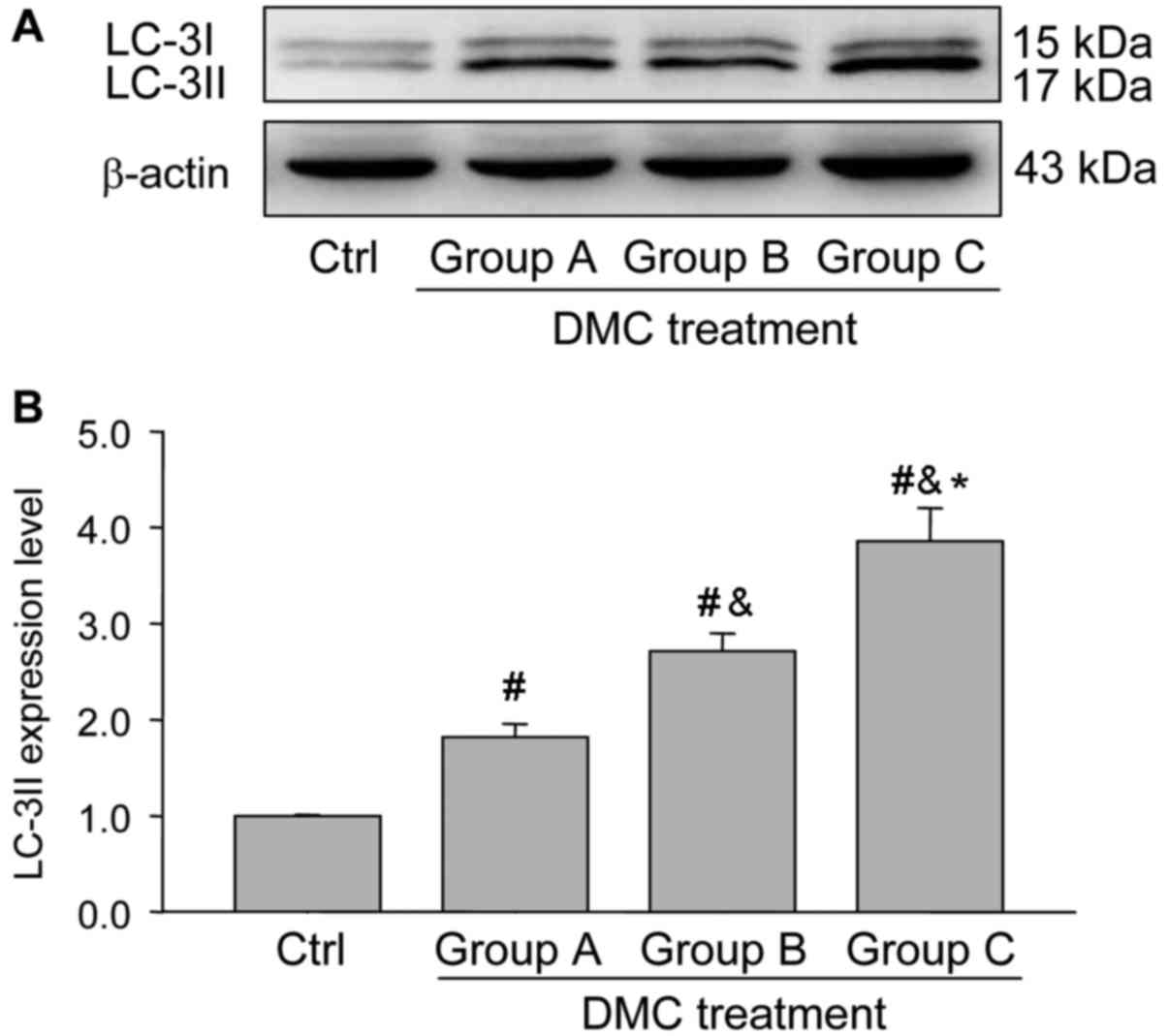
autophagy in the ovaries following DMC exposure, the expression of
LC3-II, a marker of autophagy, was determined using immunostaining
(Fig. 4) and western blotting
(Fig. 5). Immunohistochemistry
detected positive staining of LC-3II in groups A, B and C following
DMC exposure (Fig. 4) and identified
that LC3-II expression was located specifically in the ovarian
granulosa cells (Fig. 4). Western
blot analysis demonstrated that DMC exposure significantly
activated autophagy in the ovaries following DMC treatment in a
dose-dependent manner (Fig. 5).
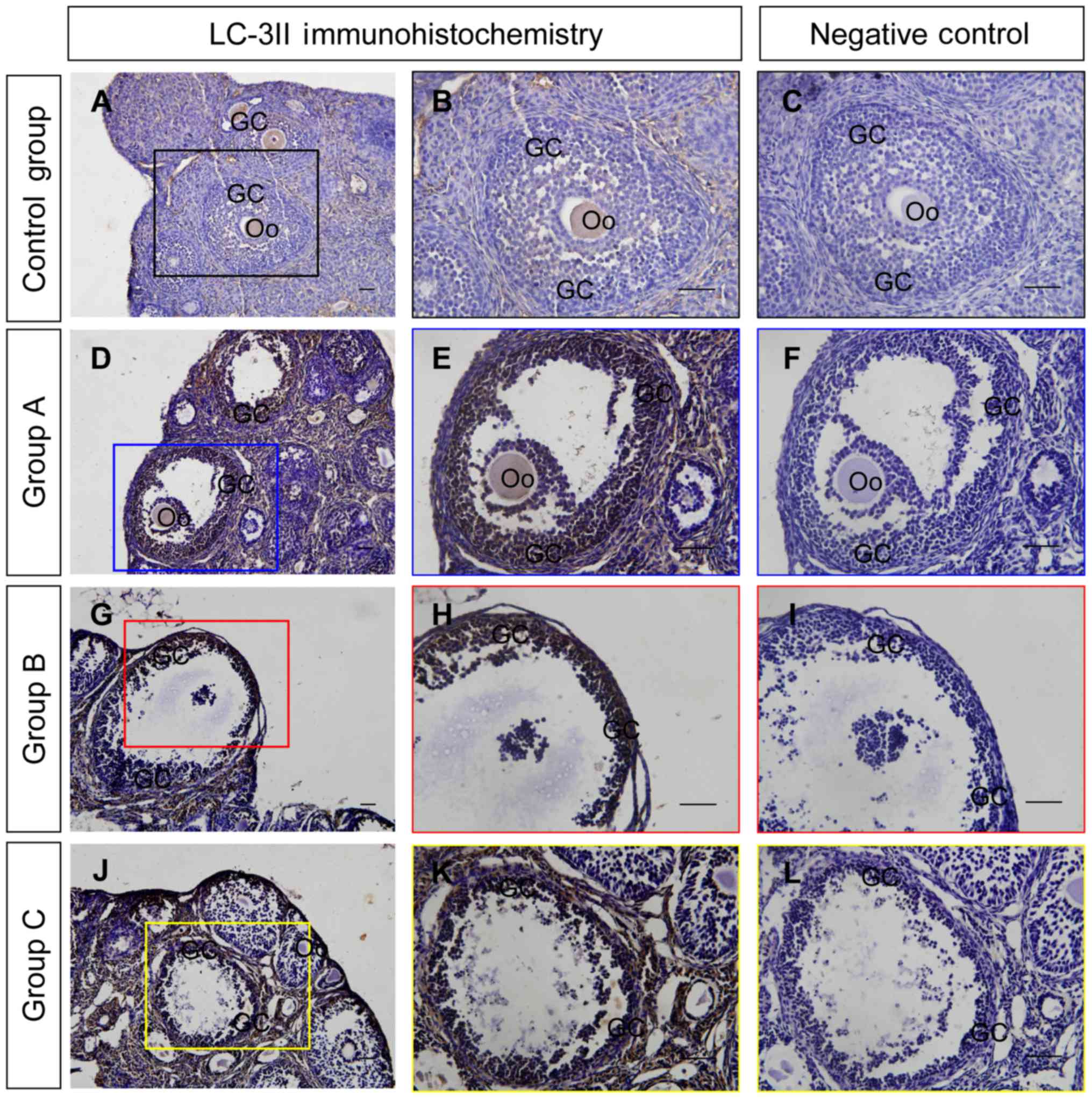 | Figure 4.Immunohistochemical examination of
LC-3II in the ovaries from each group. LC-3II immunohistochemical
signals are brown and the counterstaining background are blue.
Negative controls remained unstained, as they lacked primary
antibody. (A and B) LC-3II immunohistochemical staining in ovaries
from the control group. (C) Negative control of the control group.
(D and E) LC-3II immunohistochemical staining in ovaries from group
A. (F) Negative control of group A. (G and H) LC-3II
immunohistochemical staining in ovaries from group B. (I) Negative
control of group B. (J and K) LC-3II immunohistochemistry in
ovaries from group C. (L) Negative control of group C. Scale
bar=100 µm. Coloured boxes on the left side panels indicate the
area exhibited on the middle and right side panels. GC, granulosa
cell, Oo, oocyte; LC-3II, light chain 3 II; control group, mice
treated with corn oil; group A, mice treated with a low dose of
DMC; group B, mice treated with a medium dose of DMC; group C, mice
treated with a high dose of DMC; DMC, dimethyl carbonate. |
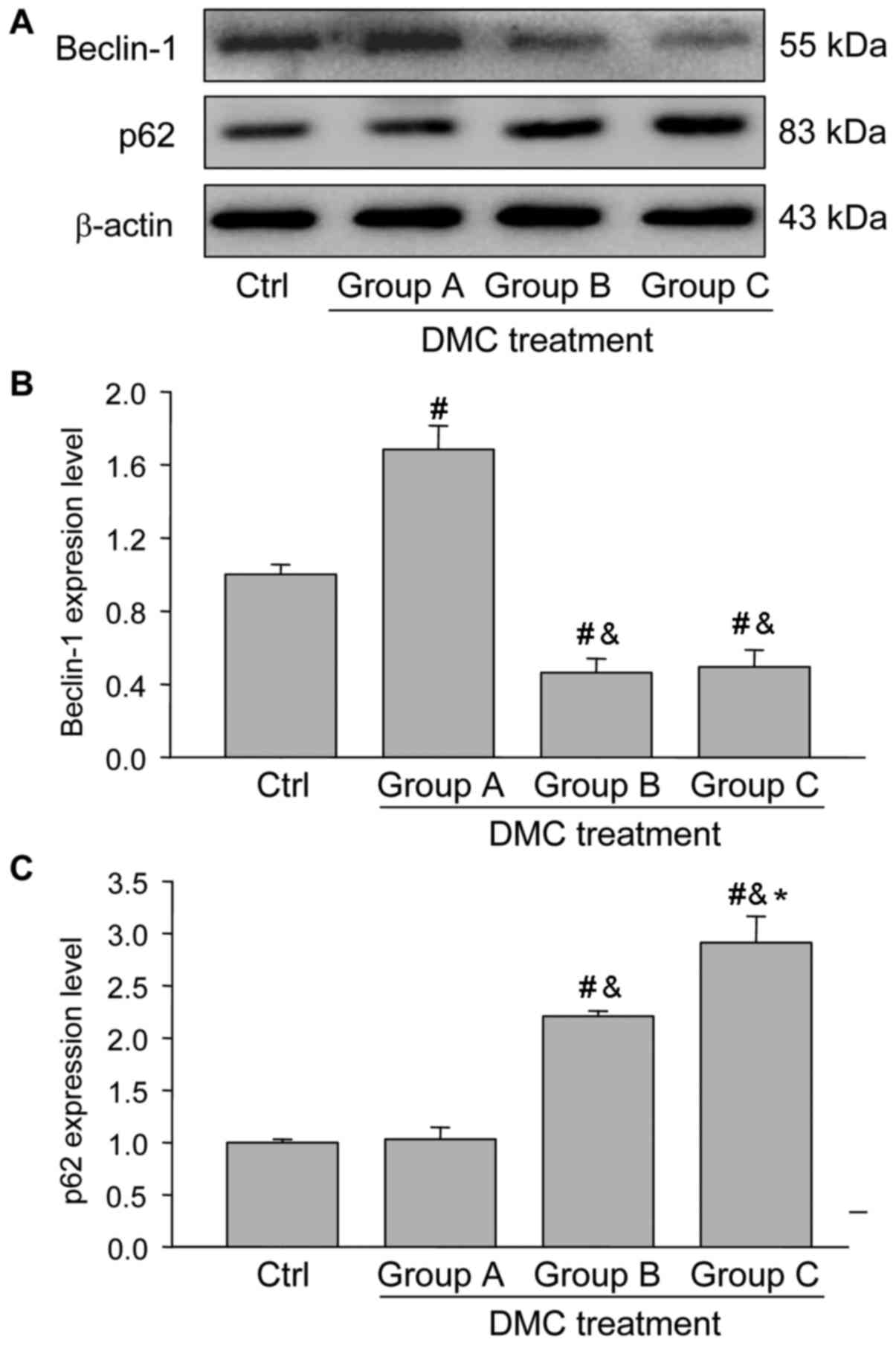
To further determine the involvement of autophagy in
ovarian injury following exposure to DMC, the expression of
Beclin-1 and p62 were also measured. Beclin-1 expression was
significantly increased in group A but was significantly decreased
in groups B and C, compared with the control group (Fig. 6A and B), indicating that Beclin-1, as
an autophagy inducing protein, is involved in the induction of
autophagy. p62 expression was significantly increased following DMC
treatment in a dose-dependent manner (Fig. 6A and C). This suggested that p62 is a
marker protein involved in the degradation of autophagosomes, the
upregulation of which indicates an accumulation of autophagosomes.
In these results, it was observed that p62 was involved in
autophagic vesicle accumulation.
HIF-1α/BNIP3 signaling is involved in
DMC-induced autophagy
Previous studies have demonstrated that the HIF-1α
signaling pathway is activated under adverse conditions to maintain
cell survival and does so either by activating survival related
genes or initiating autophagy (3,4). To
determine whether HIF-1α is involved in the regulating autophagy in
the ovary, the expression and localization of HIF-1α were detected
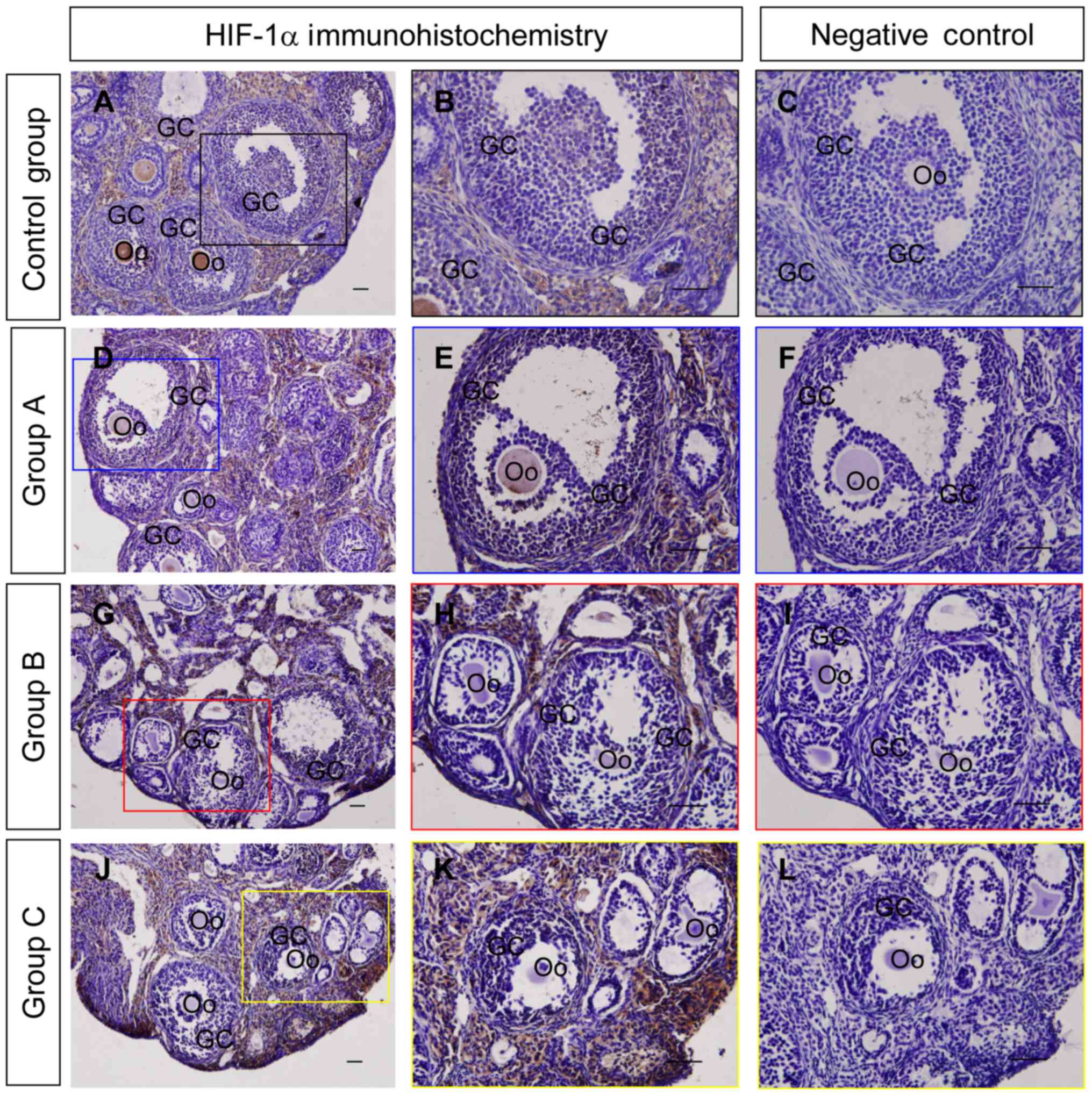
using immunohistochemistry and western blotting. The results
indicated that the HIF-1α staining intensity in granulosa cells
from group A was increased compared with those from groups B and C
(Fig. 7), which was also consistent
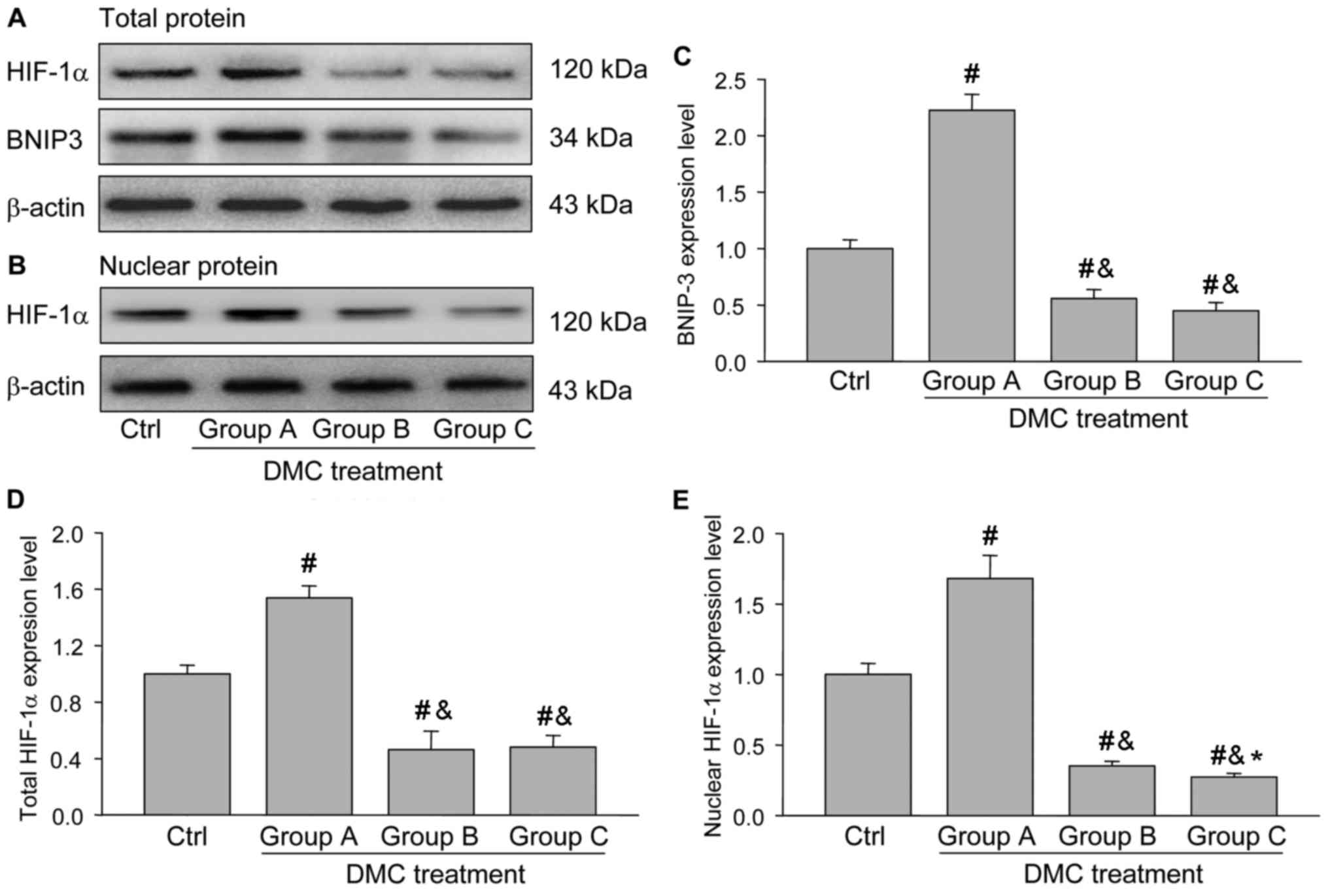
with the results of the western blot analysis (Fig. 8A and B).
In addition, the expression of BNIP3, a protein
downstream from HIF-1α, was also determined using western blotting
and the results demonstrated that its expression was significantly
increased in group A compared with the control group and groups B
and C (Fig. 8C), which was similar
to the expression pattern of total or nuclear HIF-1α (Fig. 8D and E). These results suggest that
HIF-1α/BNIP3 signaling was activated in the ovaries of group A and
may be involved in DMC-induced autophagy.
Discussion
To the best of our knowledge, the present study was
the first to investigate the potential toxic effects of DMC
exposure on follicular development in the ovary. The results of
histological examination indicated that high doses of DMC
administered by gavage significantly impaired the development of
follicles. This was indicated primarily by the arrangement of
granulosa cells and the irregular shape of oocytes within the
ovarian follicles following DMC administration. In addition, the
expression of apoptotic related proteins, including cleaved
capase-3 and Bax were significantly increased, whereas Bcl-2 was
concomitantly decreased. Compelling evidences have been
demonstrated that Bax is the activator of caspase-3 and Bcl-2 is
the inhibitor of caspase-3 (20).
Thus, these results further demonstrated that high doses of DMC
induced ovarian follicular atresia by activating the caspase-3
dependent apoptotic-signaling pathway. At present, the
organ-specific toxicity of DMC has not been evaluated, although
acute toxicity studies have been conducted (5,6). Indeed,
the material safety data sheet for DMC states that its
toxicological properties remain untested (6). It has been suggested that chemicals
exhibiting low general toxicity may impair the reproductive system
of mammals (7,8), which is consistent with the results of
the current study.
In female mammals, reproductive capacity is largely
dependent on the normal functions of the ovary, including the
successful development of follicles (21). Autophagy is a major catabolic pathway
that serves a role in the delivery of misfolded proteins and
damaged organelles to lysosomes and is frequently induced in
response to nutrient starvation or under stressful conditions,
including hypoxic stress, heat stress and chemical insults
(10–12). The induction of autophagy may
adversely affect cellular physiology; its induction may lead to
cell survival, however, excessive autophagy or accumulation of
autophagosomes may also activate caspase-3 dependent apoptosis
(22). During the induction of
autophagy, autophagy-related proteins, including Atg5, Atg6, Atg7
and Beclin-1 regulate the process of autophagosome formation,
transportation and fusion (23). It
has been suggested that the conversion of LC-3I into LC-3II may be
a marker of autophagy and that LC-3II expression is associated with
the extent of autophagosome formation (13). Previous studies have identified the
regulatory role of autophagy in the impairment of the reproductive
system of mammals induced by exposure to chemicals (14–17),
however, the majority of studies have focused on the effects of
such chemicals in the testes. In the present study, to investigate
whether autophagy serves a role in regulating ovarian functions
following exposure to DMC, LC-3II expression in the ovaries was
measured. The results demonstrated that LC-3II expression was
significantly upregulated in all treatment groups compared with the
control group. This indicates that autophagy is significantly
increased following DMC treatment even in groups exposed to low
doses of DMC. To determine the primary site of autophagy, LC-3II
distribution was determined in the ovaries of all different groups.
The results indicated that the staining intensities of ovarian
follicles from the DMC treatment groups were stronger than the
control group, suggesting that the ovarian follicles may be the
primary site of DMC induced-autophagy. The expression of LC-3II was
significantly increased in group A and was accompanied by the
maintenance of apoptosis, implying that autophagy may serve a
cytoprotective role following exposure to low doses of DMC.
However, LC-3II expression was also significantly increased in a
dose-dependent manner in the medium and high dose treatment groups
and the expression of apoptosis-related proteins was significantly
increased, as indicated by the increase in the Bax/Bcl-2 expression
ratio and cleaved caspase-3 expression. Previous studies have
demonstrated that exposure to high doses of toxins may disrupt the
process of autophagy (2,7). To explore whether autophagy was induced
following exposure to DMC, the present study measured the
expression of Beclin-1, a protein involved in the initiation of
autophagy (24) and p62, a marker
protein that indicates whether autophagosomes were successfully
degraded (25). In group A, the
expression of Beclin-1 was increased but p62 expression was similar
to that of control group. By contrast, in groups B and C, Beclin-1
expression was decreased but p62 expression was increased compared
with the control. This suggests that the accumulation of
autophagosomes may increase the Bax/Bcl-2 ratio, thereby leading to
the activation of caspase-3 in the ovaries of mice in the high dose
groups. It has been demonstrated that high concentrations of toxins
may enable the maintenance of Beclin-1 expression despite the
upregulation of LC-3II (2,7), which is consistent with the results of
the current study.
HIF-1 is a member of the basic-Helix-Loop-Helix-PAS
family of transcription factors, which has been characterized as a
transcriptional activator of several oxygen-sensitive genes,
including transferrin, erythropoietin, hemeoxygenases and several
glycolytic enzymes (26–29). The protein products of these genes
serve vital roles in developmental and physiological processes,
including iron transport, angiogenesis, erythropoiesis, glycolysis
and cell proliferation/survival (30–34). It
has been demonstrated that the expression of HIF-1α, a subunit of
HIF-1, is induced by a decrease in O2 concentration, an
increase in ROS and the stimulation of follicle-stimulating hormone
in the mammalian ovary (9,31,35,36).
Under conditions of stress, the upregulation of HIF-1α allows cells
to successfully adapt to, or overcome their oxygen and
nutrient-deprived state and survive in otherwise hostile
environments (37). Studies have
indicated that HIF-1α may maintain cell survival by activating the
downstream protein BNIP3 and subsequently inducing autophagy
(38,39). Exposure to toxins may significantly
aggravate the cellular microenvironment by increasing ROS levels
(40,41), which may activate HIF-1α expression.
To determine whether the HIF-1α/BNIP3 signaling pathway was
involved in the induction of autophagy following exposure to DMC,
the expression of HIF-1α and BNIP3 were measured. The results
indicated that levels of HIF-1α and BNIP3 were increased in group A
compared with the control group, and subsequently downregulated in
groups B and C following exposure to increased doses of DMC, which
is consistent with the tendency of Beclin-1. The activation of
HIF-1α/BNIP3 signaling may contribute to the induction of a
self-protective response by autophagy, whereas high doses of DMC
may disrupt the cytoprotective mechanism of the ovaries by
upregulating the expression of p62, Bax and cleaved caspase-3,
which are signals of aberrant autophagosome accumulation (42,43).
Thus, the role served by autophagy in the ovaries is dependent on
the dose of DMC. Chen et al (44) demonstrated that autophagy induced by
hypoxia served dual roles and was regulated by different signaling
pathways depending on whether the duration of hypoxia was short or
prolonged. The present study did not investigate the regulatory
mechanisms underlying the downregulation of HIF-1α/BNIP3 signaling
and the upregulation of LC-3II in the groups exposed to high doses
of DMC; therefore further investigations are required.
In conclusion, to the best of our knowledge, the
present study was the first to evaluate the toxicity of DMC
exposure on ovarian function and the involvement of autophagy
activation in ovarian injuries. The results demonstrated that DMC
exposure induces the activation of autophagy in the ovaries, which
may protect follicular development following exposure to low doses
of DMC via activation of HIF-1α/BNIP3 signaling pathway. DMC
exposure also induced follicular atresia in the groups exposed to
medium and high doses of DMC by activating the apoptotic signaling
pathway. This may be an important mechanism regulating follicular
development and ovarian function in the mammalian ovaries.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31271255; Beijing,
China), Fujian Provincial Natural Science Foundation (grant nos.
2016J01145 and 2017J01626; Fujian, China) and Fujian Province
Science and Technology Project of The Education Department (grant
nos. JAT160118 and JZ160426, Fujian, China).
References
|
1
|
Zhang G, Liu K, Ling X, Wang Z, Zou P,
Wang X, Gao J, Yin L, Zhang X, Liu J, et al: DBP-induced
endoplasmic reticulum stress in male germ cells causes autophagy,
which has a cytoprotective role against apoptosis in vitro and in
vivo. Toxicol Lett. 245:86–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Niu Q, Gao H, Ma R, Lei R, Zhang
C, Xia T, Li P, Xu C, Wang C, et al: Excessive apoptosis and
defective autophagy contribute to developmental testicular toxicity
induced by fluoride. Environ Pollut. 212:97–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lall R, Ganapathy S, Yang M, Xiao S, Xu T,
Su H, Shadfan M, Asara JM, Ha CS, Ben-Sahra I, et al: Low-dose
radiation exposure induces a HIF-1-mediated adaptive and protective
metabolic response. Cell Death Differ. 21:836–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellot G, Garcia-Medina R, Gounon P,
Chiche J, Roux D, Pouysségur J and Mazure NM: Hypoxia-induced
autophagy is mediated through hypoxia-inducible factor induction of
BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol.
29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bevan C and Beyer B: Developmental
toxicity evaluation of dimethylcarbonate by inhalation in CD-1
mice. Int Toxicol. 7:721995.
|
|
6
|
Anderson SE, Franko J, Anderson KL, Munson
AE, Lukomska E and Meade BJ: Immunotoxicity and allergic potential
induced by topical application of dimethyl carbonate (DMC) in a
murine model. J Immunotoxicol. 10:59–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan C, Wang C, Duan P, Huang W, Chen W,
Tang S and Yang K: Bisphenol a induces autophagy and apoptosis
concurrently involving the Akt/mTOR pathway in testes of pubertal
SD rats. Environ Toxicol. 32:1977–1989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ML, Wang JL, Wei J, Xu LL, Yu M, Liu
XM, Ruan WL and Chen JX: Tri-ortho-cresyl phosphate induces
autophagy of rat spermatogonial stem cells. Reproduction.
149:163–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan
M, Shi Y and Yang K: 4-Nonylphenol induces apoptosis, autophagy and
necrosis in Sertoli cells: Involvement of ROS-mediated
AMPK/AKT-mTOR and JNK pathways. Toxicology. 341–343:28–40. 2016.
View Article : Google Scholar
|
|
10
|
Li Y, Wang Y, Kim E, Beemiller P, Wang CY,
Swanson J, You M and Guan KL: Bnip3 mediates the hypoxia-induced
inhibition on mammalian target of rapamycin by interacting with
Rheb. J Biol Chem. 282:35803–35813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang M, Jiang M, Bi Y, Zhu H, Zhou Z and
Sha J: Autophagy and apoptosis act as partners to induce germ cell
death after heat stress in mice. PLoS One. 7:e414122012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang SH, Shih YL, Ko WC, Wei YH and Shih
CM: Cadmium-induced autophagy and apoptosis are mediated by a
calcium signaling pathway. Cell Mol Life Sci. 65:3640–3652. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu LL, Liu ML, Wang JL, Yu M and Chen JX:
Saligenin cyclic-o-tolyl phosphate (SCOTP) induces autophagy of rat
spermatogonial stem cells. Reprod Toxicol. 60:62–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Liu K, Ling X, Wang Z, Zou P,
Wang X, Gao J, Yin L, Zhang X, Liu J, et al: DBP-induced
endoplasmic reticulum stress in male germ cells causes autophagy,
which has a cytoprotective role against apoptosis in vitro and in
vivo. Toxicol Lett. 245:86–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Zhou Y, Wang X, Qian W and Han X:
Microcystin-LR induces autophagy and apoptosis in rat Sertoli cells
in vitro. Toxicon. 76:84–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gannon AM, Stämpfli MR and Foster WG:
Cigarette smoke exposure elicits increased autophagy and
dysregulation of mitochondrial dynamics in murine granulosa cells.
Biol Reprod. 88:632013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Obert LA, Sobocinski GP, Bobrowski WF,
Metz AL, Rolsma MD, Altrogge DM and Dunstan RW: An
immunohistochemical approach to differentiate hepatic lipidosis
from hepatic phospholipidosis in rats. Toxicol Pathol. 35:728–734.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nahdi A, Hammami I, Kouidhi W, Chargui A,
Ben Ammar A, Hamdaoui MH, El May A and El May M: Protective effects
of crude garlic by reducing iron-mediated oxidative stress,
proliferation and autophagy in rats. J Mol Histol. 41:233–245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin XM, Oltvai ZN and Korsmeyer SJ: BH1
and BH2 domains of Bcl-2 are required for inhibition of apoptosis
and heterodimerization with Bax. Nature. 369:321–323. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canipari R: Oocyte-granulosa cell
interactions. Hum Reprod Update. 6:279–289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C and Klionsky DJ: The molecular
mechanism of autophagy. Mol Med. 9:65–76. 2003.PubMed/NCBI
|
|
24
|
Nazarko VY and Zhong Q: ULK1 targets
Beclin-1 in autophagy. Nat Cell Biol. 15:727–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:pp. 5510–5514. 1995, View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li
PL and Li N: Hypoxia-inducible factor-1α contributes to the
profibrotic action of angiotensin II in renal medullary
interstitial cells. Kidney Int. 79:300–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Zhu Q, Li PL, Dhaduk R, Zhang F,
Gehr TW and Li N: Silencing of hypoxia-inducible factor-1α gene
attenuates chronic ischemic renal injury in two-kidney, one-clip
rats. Am J Physiol Renal Physiol. 306:F1236–F1242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ and
Li N: Hypoxia-inducible factor prolyl-hydroxylase 2 senses
high-salt intake to increase hypoxia inducible factor 1alpha levels
in the renal medulla. Hypertension. 55:1129–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yaba A, Bianchi V, Borini A and Johnson J:
A putative mitotic checkpoint dependent on mTOR function controls
cell proliferation and survival in ovarian granulosa cells. Reprod
Sci. 15:128–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C, Georgescu MM, Simons JW and Semenza GL: Modulation of
hypoxia-inducible factor 1alpha expression by the epidermal growth
factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human
prostate cancer cells: Implications for tumor angiogenesis and
therapeutics. Cancer Res. 60:1541–1545. 2000.PubMed/NCBI
|
|
32
|
Wulff C, Dickson SE, Duncan WC and Fraser
HM: Angiogenesis in the human corpus luteum: Simulated early
pregnancy by HCG treatment is associated with both angiogenesis and
vessel stabilization. Hum Reprod. 16:2515–2524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishimura R and Okuda K: Hypoxia is
important for establishing vascularization during corpus luteum
formation in cattle. J Reprod Dev. 56:110–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyazawa M, Yasuda M, Fujita M,
Hirabayashi K, Hirasawa T, Kajiwara H, Muramatsu T, Miyazaki S,
Harasawa M, Matsui N, et al: Granulosa cell tumor with activated
mTOR-HIF-1alpha-VEGF pathway. J Obstet Gynaecol Res. 36:448–453.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jewell UR, Kvietikova I, Scheid A, Bauer
C, Wenger RH and Gassmann M: Induction of HIF-1alpha in response to
hypoxia is instantaneous. FASEB J. 15:1312–1314. 2001.PubMed/NCBI
|
|
36
|
Zhang Z, Wang Q, Ma J, Yi X, Zhu Y, Xi X,
Feng Y and Jin Z: Reactive oxygen species regulate FSH-induced
expression of vascular endothelial growth factor via Nrf2 and HIF1α
signaling in human epithelial ovarian cancer. Oncol Rep.
29:1429–1434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ietta F, Wu Y, Winter J, Xu J, Wang J,
Post M and Caniggia I: Dynamic HIF1A regulation during human
placental development. Biol Reprod. 75:112–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen J, Bai M, Ning C, Xie B, Zhang J,
Liao H, Xiong J, Tao X, Yan D, Xi X, et al: Gankyrin facilitates
follicle-stimulating hormone-driven ovarian cancer cell
proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway.
Oncogene. 35:2506–2517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bellot G, Garcia-Medina R, Gounon P,
Chiche J, Roux D, Pouysségur J and Mazure NM: Hypoxia-induced
autophagy is mediated through hypoxia-inducible factor induction of
BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol.
29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia
J, Sun ZJ, Wang YN and Zhao YF: Autophagy regulates hypoxia-induced
osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J
Cell Physiol. 227:639–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Xu L, Shen J, Wang J, Ruan W, Yu M
and Chen J: Involvement of oxidative stress in tri-ortho-cresyl
phosphate-induced autophagy of mouse Leydig TM3 cells in vitro.
Reprod Biol Endocrin. 14:302016. View Article : Google Scholar
|
|
42
|
Sun L, Li T, Wei Q, Zhang Y, Jia X, Wan Z
and Han L: Upregulation of BNIP3 mediated by ERK/HIF-1α pathway
induces autophagy and contributes to anoikis resistance of
hepatocellular carcinoma cells. Future Oncol. 10:1387–1398. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen G, Zhang W, Li YP, Ren JG, Xu N, Liu
H, Wang FQ, Sun ZJ, Jia J and Zhao YF: Hypoxia-induced autophagy in
endothelial cells: A double-edged sword in the progression of
infantile haemangioma? Cardiovasc Res. 98:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|