Introduction
Gonadotropin-releasing hormone (GnRH) is a
decapeptide originally discovered as a factor of hypothalamic
origin that controls secretions of the anterior pituitary gland.
GnRH has a direct effect on reproductive processes by regulating
the synthesis and release of pituitary gonadotropins. In addition,
it has been reported that GnRH has neurotrophic effects on
dendritic spine density and cultured cerebral neurons of rat
embryos by increasing growth and neurite number as well as
modifying neurofilament expression (1,2).
Several synthetic GnRH agonists, designed to have an
increased biological effect, have been used for different
therapeutic applications, including the treatment of cancer and
precocious puberty as well as in vitro fertilization
techniques (3).
Leuprolide acetate (LA) is a synthetic analog of
GnRH composed of nine amino acids with a high biological effect due
to its increased affinity to GnRH receptors and its prolonged
action compared with those of endogenous GnRH (3,4). This
agonist has an increased resistance to enzymatic degradation and
high protein binding due to its non-natural amino acids and
prolonged serum half-life (5,6). This
agonist has been previously used for the same purposes as those
mentioned above (7). Furthermore, it
has been used as a neurological recovery factor in experimental
autoimmune encephalomyelitis and in spinal cord injury (8–10).
Studies in humans and experimental models have
evidenced that therapeutic application of GnRH or administration of
agonists thereof has the secondary effect of increasing body weight
(8,9,11). The
mechanisms involved in this process remain elusive; however, in the
last two decades, different approaches have been proposed to
elucidate the association between energy balance and adipogenesis
with the aim of correlating endocrine and metabolic pathways. In
this sense, the development of adipogenesis under non-pathological
conditions corresponds with a high food consumption and loss of
physical activity. Under this concept, ghrelin an orexigenic
peptide have a fundamental role, acting on the brain to regulate
food intake, body weight, adiposity and glucose metabolism
(12). Furthermore, lipoprotein
lipase (LPL) is an enzyme with numerous physiological activities,
which mainly include the regulation of fatty acid supply to various
tissues for either storage or oxidation (13). Changes in ghrelin and LPL expression
depend on physiological stages and under stimuli by GnRH agonists,
this condition may be favored to increase or suppress food
consumption and changes in body weight. However, it is necessary to
know the main changes in body composition that occur in the
treatment with this agonist and the implications this may have in
neuroregeneration studies, where weight gain is possibly associated
with a better recovery or similarly, in other therapeutic
applications, such as oncological, fertility or precocious puberty
treatments, in which nutritional care is necessary in order to
control undesired effects. The present study hypothesized that
long-term use of LA may generate changes in body composition and
modify the mRNA expression of ghrelin and LPL, two important
molecules with high repercussion in the control of hunger and
adipogenesis. In order to confirm this hypothesis, the aim of the
present study was to evaluate a long-term effect of LA
administration on body composition and determine the mRNA
expression of ghrelin and LPL in non-ovariectomized and
ovariectomized rats.
Materials and methods
Animal groups and housing
conditions
A total of 96 female Wistar rats (weight, 120–150 g;
age, 6 weeks) were obtained from the bioterium of the Universidad
Autónoma de Aguascalientes (Aguascalientes, México) and treated
according to the Guide for the Care and Use of Experimental Animals
by the USA National Institutes of Health. They were maintained in
separated cages (4 rats/cage of 50×50×18 cm) under controlled
conditions (temperature 23±2°C; relative humidity 48%) with a 12 h
alternate light/dark cycle. They were provided a diet of standard
rodent pellets (Purina Nutricubes®; Nestle, Vevey,
Switzerland) and water ad libitum. The animals were divided
into four groups: Untreated (CTRL group), treated with LA (LA
group), ovariectomized (OVX group) and ovariectomized treated with
LA (OVX+LA group). All rats were habituated for 10 days prior to
the experiment. The present study received approval from the Ethics
Committee of the University of Aguascalientes (Aguascalientes,
México).
Ovariectomy and LA administration
On the 10th day of habituation, two groups of
animals were surgically ovariectomized. The animals were then kept
in a stable and clean environment and were allowed to recover from
the surgery for 10 days. For animals in the LA and OVX+LA groups,
the treatment with LA (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) began on day 20 with by intramuscular administration of 5
µg/kg in 0.1 ml saline solution (0.9% NaCl) every 3 days until day
120. The other two groups, CTRL and OVX, received only saline
solution with the same treatment schedule.
Murinometric evaluations and
nutritional measurements
Food consumption (g/24 h/100 g) was measured daily
at the same time (between 09:00 and 10:00 h) and body weight was
measured once a week to adjust the LA dose and to observe weight
changes in treated and non-treated groups over the course of the
experiment. Food was weighed in a digital balance (500 g capacity
×0.1 g; V31X501; Ohaus, Parsippany, NJ, USA). Body length
(nose-anus length) was determined in all groups at 0, 20, 40, 60,
80, 100 and 120 days of the experiment with a metric tape fixed to
a worktable. Body weight and body length were used to determine
body mass index (BMI; g/cm2) and ponderal index (PI;
g/3√cm). Animal weight and food consumption was used to
determine the specific rate of body mass gain in g/kg. All measures
were determined according to equations described by Novelli et
al (14), where the specific
rate of body mass gain is defined as follows: Body mass gain
(g/kg)=dM/Mdt, where dM represents the gain of body weight during
dt=t2-t1 and M is the rat body weight at the time-point t1.
Body composition analysis in vivo
Body composition changes were registered in
vivo throughout the different evaluation periods. To determine
body density, hydrostatic weighing was performed every 20 days
until day 120 as reported by Hohl et al (15). In brief, a device was designed with
two graduated cylinders linked by a flexible hose were the rat was
placed inside of one and changes in water volume in both cylinders
were recorded. Recommendations for calibration and animal
measurements were followed. Once the animal's density was
determined, a densitometry-based equation was used to calculate the
fat mass (FM) (16). The data
obtained were converted to grams in order to compare the values
with those from the dissection study. In the present study, the
fat-free mass (FFM) was considered as the difference between total
body weight and FM.
Carcass analysis and chemical lipid
extract
Every 20 days, four rats from each group were
sacrificed under anaesthesia. Blood samples were collected from the
central aorta and weighed to calculate the residual mass. Rats were
eviscerated and the skin was completely dissected from the neck
region to the front and rear trunnions. All components were weighed
using a calibrated balance (Ohaus V31X501) and were classified
under the following criteria: The carcass was considered a major
component of the FFM, the previously shaved skin as a FM component
and a third component, the residual mass, was composed of blood and
viscera. From the skin, the previously dissected fat mass was
extracted in accordance with the Bligh and Dyer method, which
comprises homogenization of a 1:2:1 mixture of methanol, chloroform
and tissue (17).
RNA isolation and reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR) assay
Total RNA was isolated by tissue lysis with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) from tissue samples of the stomach and central aorta to
measure ghrelin and LPL, respectively. The RT reactions were
performed with a High Capacity cDNA RT kit (cat. no. 4368813;
Applied Biosystems; Thermo Fisher Scientific, Inc.) using 3 µg RNA
with 0.8 µl of 25X deoxynucleotidetriphosphates (dNTPs; 100 mM), 2
µl 10X random primers, 2 µl 10X RT buffer and 1 U Multiscribe RT in
a final volume of 20 µl topped up with nuclease-free water using a
thermocycler (FGEN02TP; Techne Genius; Cole-Parmer, Stone, UK) with
the following incubation conditions: 25°C for 10 min, 37°C for 120
min and 85°C for 5 min. Each PCR was performed in a final volume of
25 µl containing 0.25 µl complementary (c)DNA, 2.5 µl 10X PCR
Buffer Minus Mg, 10 mM dNTPs mix, 50 mM MgCl2, 10 µM of
each ghrelin, LPL and GAPDH-specific primer and 0.125 U Taq
polymerase (all, Invitrogen; Thermo Fisher Scientific, Inc). A
347-DNA fragment coding for rat ghrelin was amplified with forward
primer 5′-TTGAGCCCAGAGCACCAGAAA-3′ and reverse primer
5′-AGCTTCTGCCTCCTCTGCAACT-3′, with the oligonucleotides designed
from the sequence of the gene (GenBank accession no. AB029433.1).
In the case of LPL, a 292-bp DNA fragment was amplified with
forward primer 5′-CCCCAGCAAGGCATACAGGT-3′ and reverse primer
5′-CGGCAGGGTGAAGGGAATGT-3′, with the oligonucleotide designed from
the sequence of the gene (GenBank accession no. NM_012598.2). As an
internal control for amplification, a 207-bp fragment of rat GAPDH
was amplified from the same cDNA, with the forward primer
5′-AGACAGCCGCATCTTCTTGT-3′ and the reverse primer
5′-CTTGCCGTGGGTAGAGTCAT-3′ designed from the sequence of the gene
(GenBank accession no. NM_017008.4). All primers used in the
experiment were custom-made (Invitrogen; Thermo Fisher Scientific,
Inc.). Optimal PCR conditions were as follows: an initial
incubation of 94°C for 3 min followed by 35 cycles at 94°C for 30
sec, 56°C for 30 sec and 72°C for 30 sec and a final extension step
at 72°C for 10 min. PCR products were analyzed in ethidium
bromide-stained agarose gels (Invitrogen; Thermo Fisher Scientific,
Inc.). They underwent horizontal gel electrophoresis for 80 min at
60 V and 15 min staining in ethidium bromide solution 5 µg/ml
(Sigma-Aldrich; Merck KGaA). The intensity of the amplified bands
was analyzed using QuantityOne® version 4.6.6 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Band intensities
were normalized to the GAPDH signal (ghrelin/GAPDH and LPL/GAPDH
rates).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Comparisons among groups were performed using two-way
analysis of variance (ANOVA) followed by Bonferroni's post-hoc test
using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA) in most experiments. PCR results were analyzed by one-way
ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a significant difference between groups.
Results
Body weight, length, food consumption
and specific weight gain
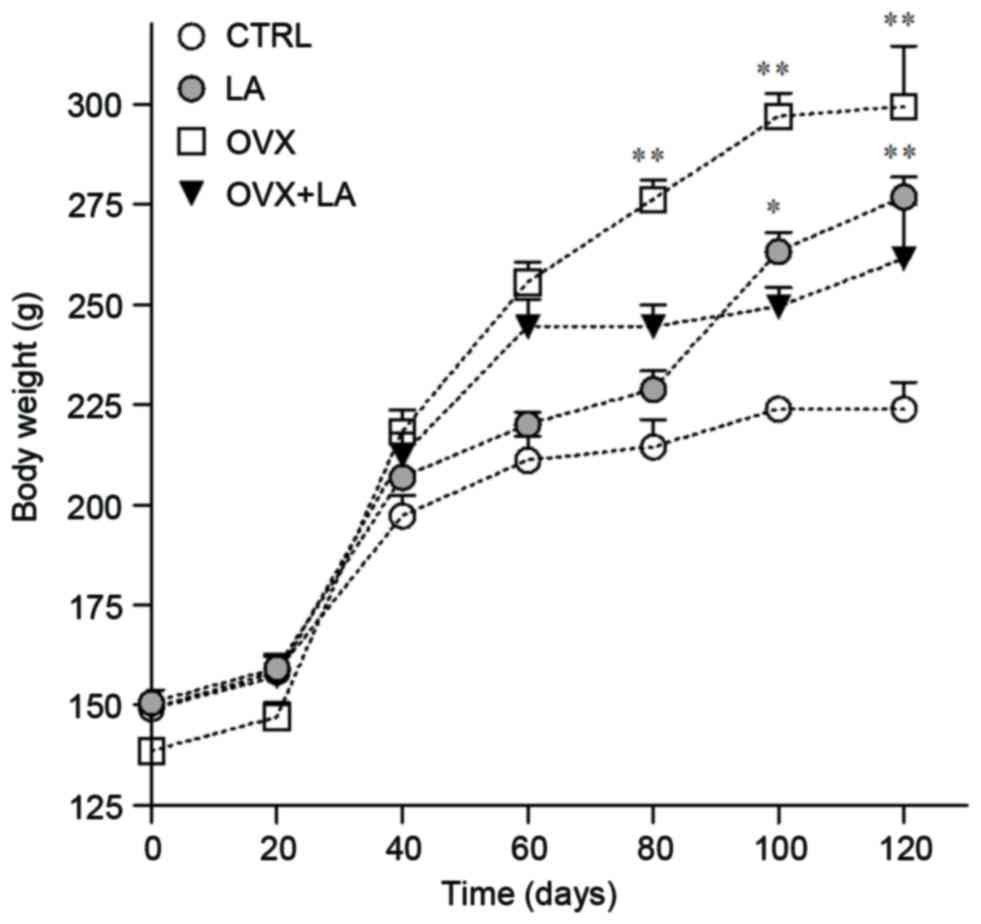
From day 40 of treatment, all groups exhibited
obvious differences in body weight until the end of the experiment.
A higher weight gain was identified in the OVX, OVX+LA and LA
groups (216.1, 175.4 and 183.7%, respectively) compared with that
in the CTRL group (150.1%). At the end of the experiment, the
groups treated with LA had a significantly higher weight compared
with that in their equivalent groups who received saline only
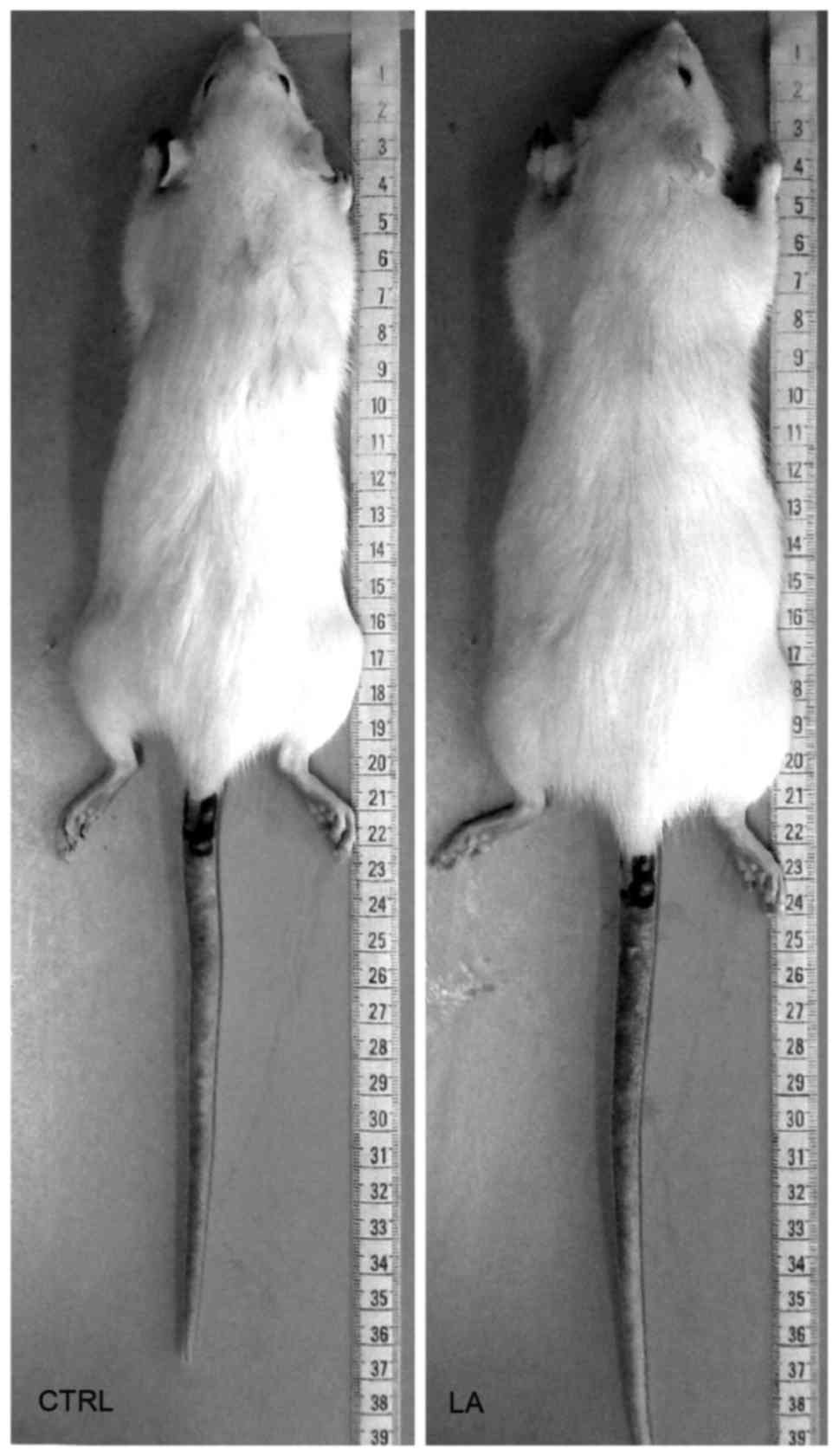
(Fig. 1). Regarding body length
(Fig. 2), animals that received LA
injections had a slight increment in their body length (~4.3%) in
comparison with LA-untreated animals at 120 days (data not
shown).
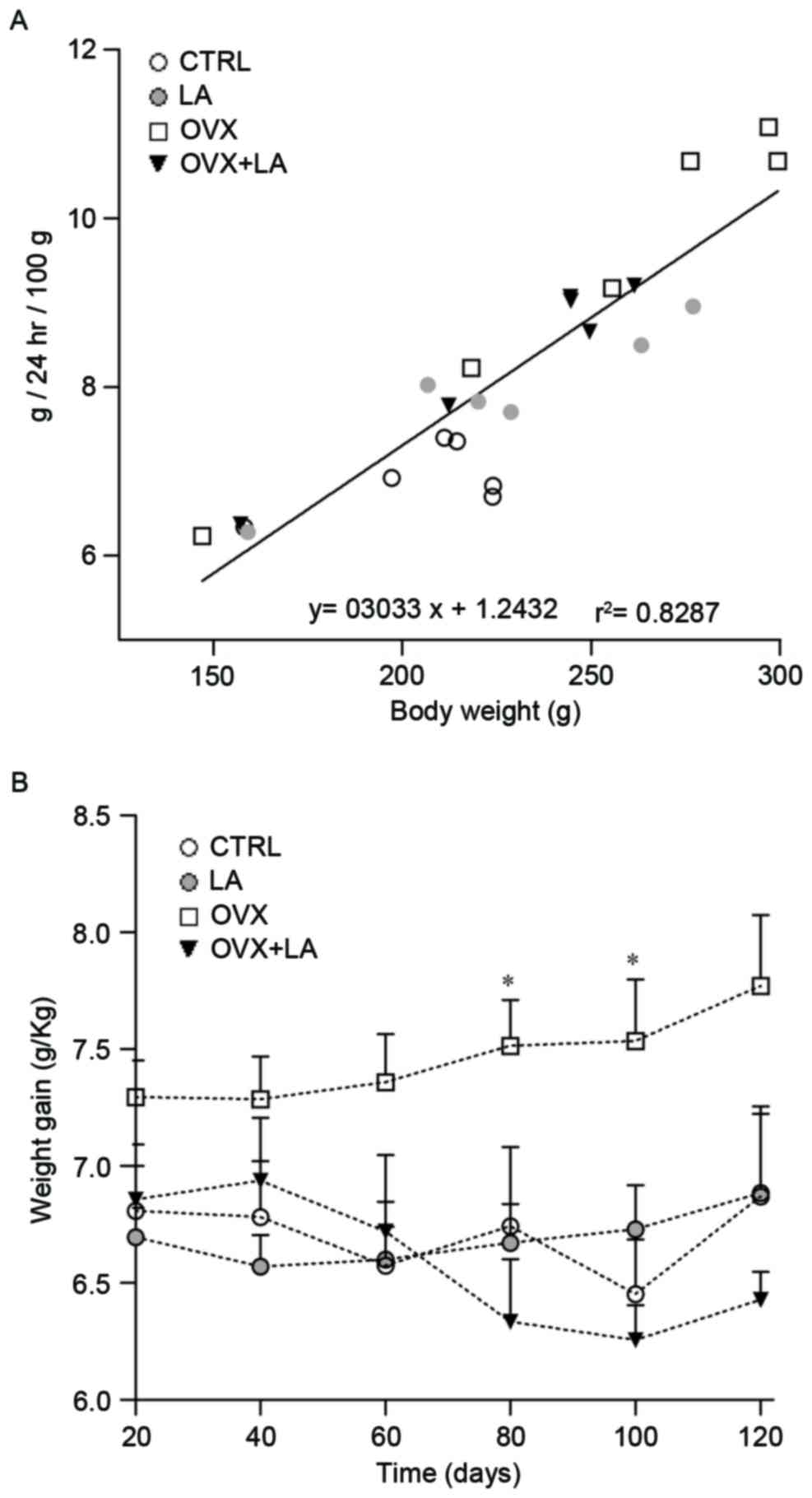
Food consumption (FC) was positively correlated with
body weight (BW), as presented in Fig.
3A, which correlates the cumulative results of FC and BW
(measured once a day and weekly, respectively) every 20 days for
the duration of the experiment. High correlation was observed
between food intake and weight gain in the LA and OVX+LA groups.
The OVX group consumed more food and gained more weight compared
with the CTRL group, which practically did not change their food
consumption and weight gain was minimal (r=0.874, P<0.0001, FC
vs. BW). Over the entire experiment, the specific rate of body mass
gain in the OVX group was higher compared with that in the other
groups. No significant differences were observed between the CTRL
and LA groups; however, the OVX+LA group exhibited a marked
reduction in weight gain compared with that in the OVX group with
significant differences on days 80 (6.33±0.26 vs. 7.51±0.19 g;
P<0.05) and 100 of treatment (6.25±0.15 vs. 7.53±0.26 g;
P<0.05; Fig. 3B). At 120 days
there were notable differences in weight gain between the two
groups but they were not statistically significant (6.4±0.12 vs.
7.7±0.30 g).
Body composition measured by
hydrodensitometry
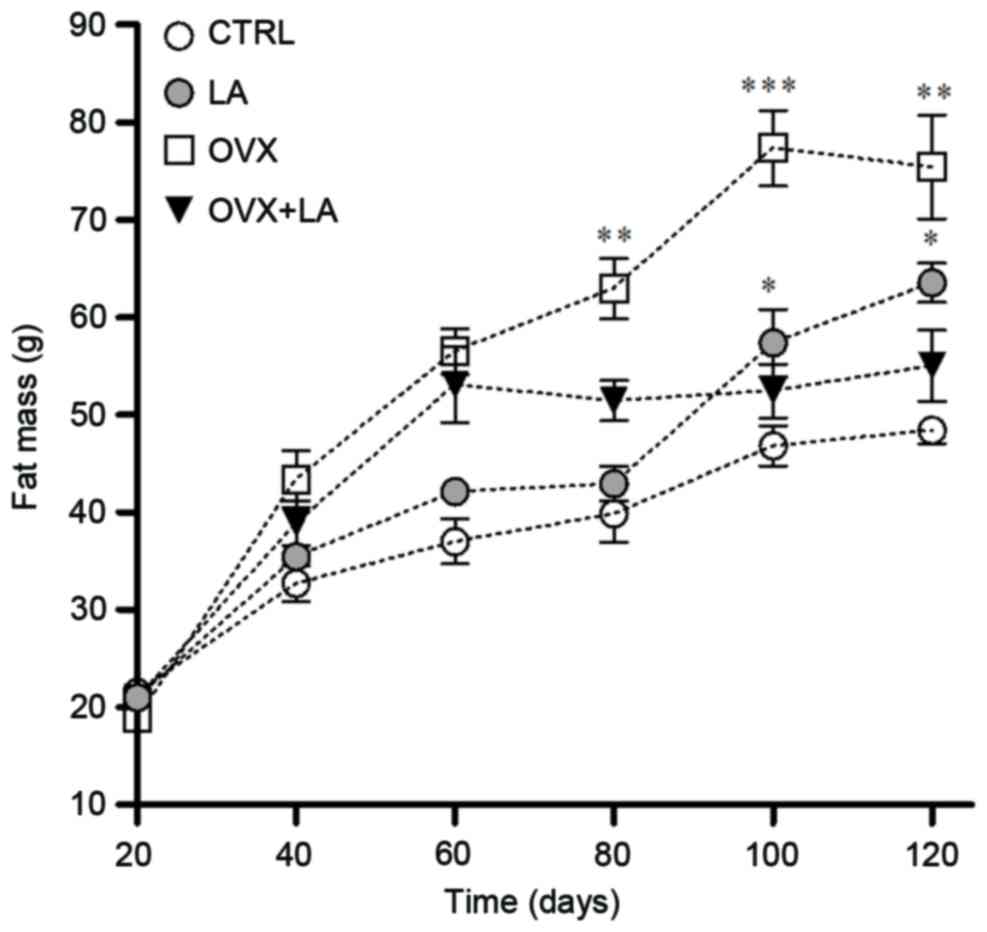
Significant differences in FFM (data not shown) and
FM were observed on days 100 and 120 between equivalent groups
(CTRL vs. LA and OVX vs. OVX+LA). The FM in the LA group was 18.3%
higher compared with that in the CTRL group at day 100 (P<0.05;
Fig. 4) and 23.9% at day 120
(P<0.05; Fig. 4). Furthermore,
the OVX+LA group had a significantly lower FM compared with that in
the OVX group on days 80, 100 and 120. At the end of the
experiment, the FM in the OVX group was increased to 27.01%
compared with that in the OVX+LA group (P<0.01; Fig. 4).
Carcass analysis and total lipids
extracted from skin
All groups exhibited substantial changes in residual
mass and skin weight compared with CTRL animals (data not shown).
All groups exhibited substantial changes skin weight compared with
the CTRL animals. Analyses from equivalent groups for each
component revealed significant differences for skin weight in the
case of LA vs. CTRL at day 100 (36.2±1.0 vs. 28.5±0.3 g; P<0.05)
and day 120 (38.6±2.3 vs. 28.4±0.9 g; P<0.01; data not shown).
No substantial differences were found between the OVX and OVX+LA
groups; however, at day 120, each of these two groups had higher
values than the CTRL and LA groups in skin weight (data not shown).
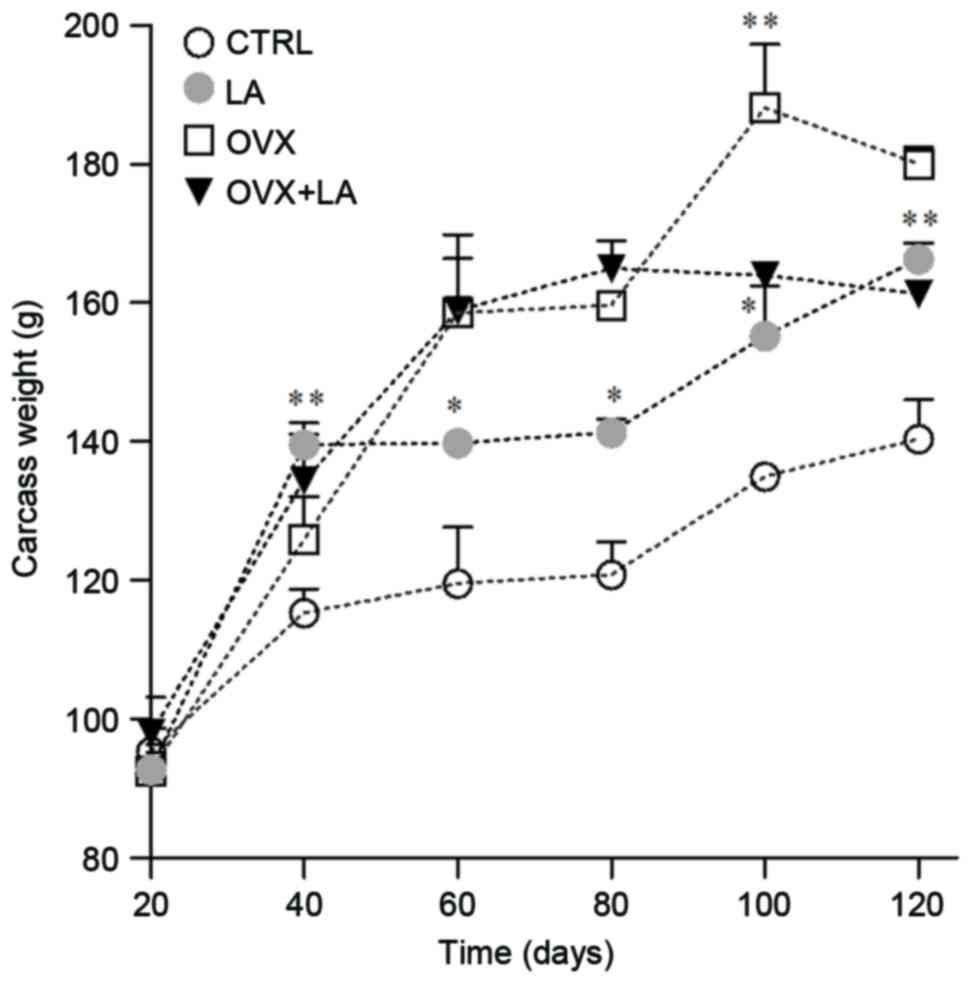
At the end of the experiment, the carcass weight in the LA group
had increased by ~179%, the CTRL group had increased by 147%, the
carcass weight in the OVX group exhibited a high increase of ~194%
and an increase of 164% was observed in the OVX+LA animals in
comparison from the basal values of each group (Fig. 5). The comparisons between equivalent
groups resulted in a significantly higher carcass weight for LA
group in comparison with CTRL group from day 40 to 120 (day 40,
139.5±3.1 vs. 115.3±3.4 g; P<0.01; day 60, 139.7±1.4 vs.
119.5±6.1 g; P<0.05; day 80, 141.3±1.9 vs. 120.8±4.6 g;
P<0.05; day 100, 155.1±6.3 vs. 134.9±1.4 g; P<0.05; day 120,
166.3±2.2 vs. 140.3±5.6 g; P<0.01; Fig. 5). The OVX and OVX+LA groups had
similar carcass weights from 20 to 80 days and a significant
difference at 100 days (188.1±9.1 vs. 163.9±1.1 g; P<0.05),
however this difference was reduced at 120 days (180.05±2.3 vs.
161.2±1.2 g; P>0.05; Fig. 5).
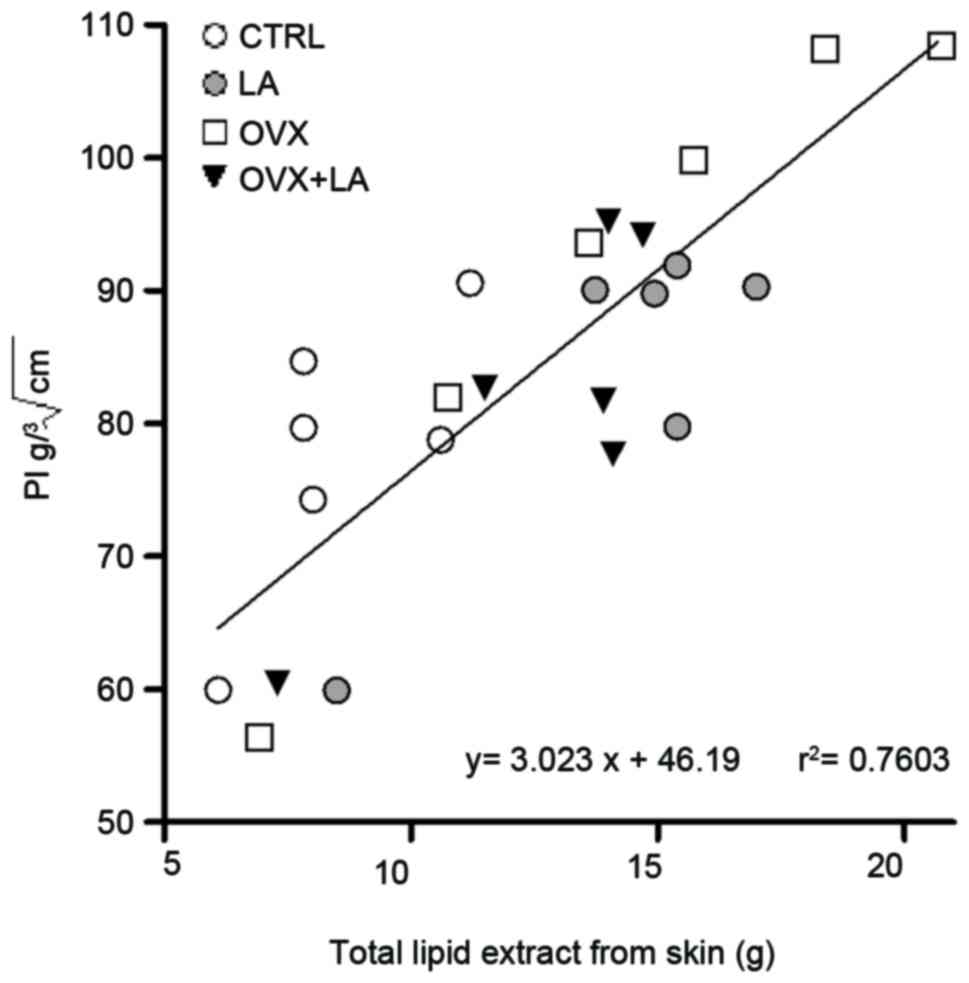
The PI was correlated with the lipid extracted from
the skin of all groups (r=0.7603, P<0.0001; Fig. 6). The highest values of PI and total
lipid extracted from skin were observed in OVX animals, while the
lowest values were in the CTRL group. The LA and OVX+LA had similar
results in this correlation analysis (Fig. 6).
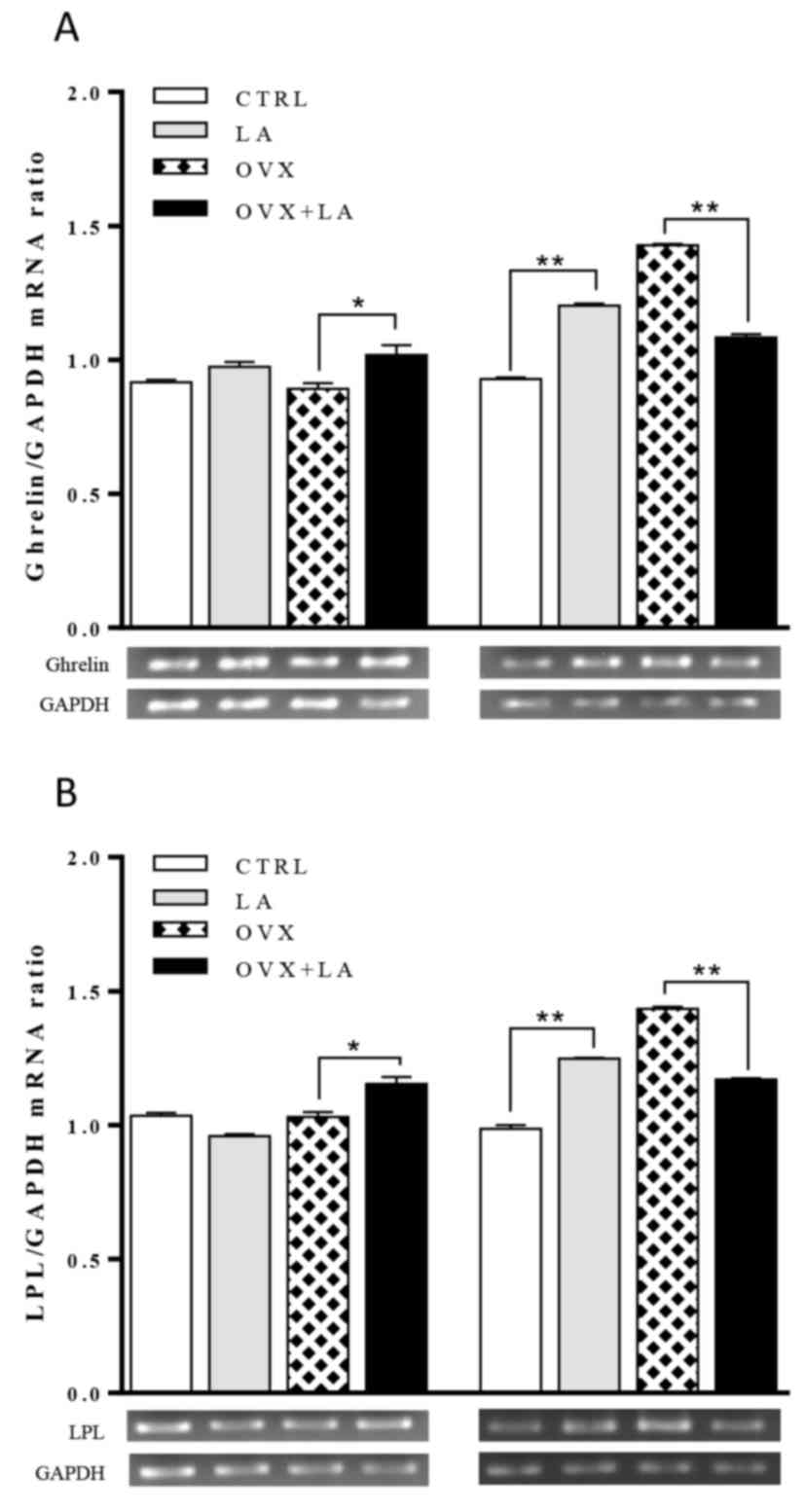
Ghrelin and LPL mRNA expression
As presented in Fig.
7A, ghrelin mRNA expression in the LA group increased by ~20%
compared with that in the CTRL group at the end of the experiment
(P<0.01). Furthermore, simultaneous treatment with LA resulted
in ~24% less ghrelin mRNA expression in comparison with that in the
OVX group (P<0.05). Indeed, the OVX group had the highest
ghrelin mRNA expression among all groups. Similar results were
obtained for LPL mRNA (Fig. 7B); the
LA group had a ~20% higher expression than the CTRL group
(P<0.05), and of note, the OVX+LA group had ~18% less LPL
expression in comparison with that in the OVX group
(P<0.01).
Discussion
It has been reported that LA is a safe drug for
treating certain types of cancer and hormonal disorders associated
with the reproductive system (7).
Recently, GnRH and its agonist LA have been used in experimental
studies on neuronal regeneration (8–10).
However, weight gain is one of the side effects reported more
frequently by these treatments, while only few studies have
addressed body composition and the possible pathways responsible
for this. The present study demonstrated that LA promoted changes
in body composition and variations in the mRNA expression of
ghrelin and LPL in non-ovariectomized and ovariectomized rats. It
was revealed that long-term LA administration causes an increase in
body weight in rats compared with that in the control group. This
subsequent effect has been reported in humans mainly in treatments
for precocious puberty using GnRH and LA (18,19), as
well as cancer (20,21) and fertility regulation (22,23).
Of note, the OVX group demonstrated the highest
weight gain among all other groups (CTRL, OVX+LA and LA). OVX by
itself causes this condition, which is in accordance with previous
studies (24). Nguyen et al
2004 (25), reported a 53% increase
in body weight in cats after OVX compared with only 27% in a group
that underwent sham surgical intervention. This increase in
corporal weight was highly associated with food consumption. In the
present study, an association was observed between high food
consumption and increased body weight, which was also supported by
the ovariectomy-specific weight gain due to the weight in the OVX
group being comparatively higher during the entire experiment. In
an osteoporosis model induced by ovariectomy, Jiang et al
2008 (26) identified that
hyperphagia is the main cause of gain weight in Sprague Dawley
rats. An outcome to highlight is the stabilization in weight gain
for animals in the OVX+LA group, which had minimum variations from
day 60 of treatment until the end of the experiment.
Regarding the analysis of body composition by
hydrodensitometry, an increase of FM was identified in the LA group
at the end of the experiment and no difference was observed in FFM
when compared with that in the CTRL group. In clinical trials with
androgen deprivation therapy using LA to treat prostate cancer, an
increase in weight and FM measured by dual-energy x-ray
absorptiometry and bioelectrical impedance devices has been
reported (21,27). It is well established that long-term
therapy with GnRH agonists causes a desensitization of the
pituitary gonadotrophs, which leads to a complete or acute lack of
response of follicle-stimulatory hormone or luteinizing hormone in
eugonadal subjects (28). This
condition restrains gonadal steroidal production avoiding a
negative feedback at the hyphophyseal level in GnRH secretion
(29). However, not only steroidal
production exerts this function; Shimizu et al (30) reported reduced levels of circulating
estradiol (E2) in response to total bilateral
ovariectomy and increases in the mRNA expression of neuropeptide Y
(NPY) at the hyphophyseal level. It is possible that NPY, an
orexigenic peptide, may induce hyperphagia with subsequent weight
gain. This may explain for the weight gain in the AL group, in
which long-term administration of LA partially decreased the
production of E2 and favored an increase in food
consumption. It has been reported that decreasing E2
causes not only an overproduction of NPY, but also an impaired
leptin sensitivity in ovariectomized rats (31). Estrogen replacement has been studied
as a method to prevent increases in body weight in experimental and
clinical trials (32,33). However, in the present study, the
OVX+LA group exhibited less FM and FFM compared with that in the
OVX group.
To confirm the results of previous in vivo
assays, the present study demonstrated that the LA group had a
higher FFM (carcass weight) and FM (skin weight) than the CTRL
group, while the OVX+LA and OVX groups had a similar skin weight
but the carcass weight in the OVX+LA group did not change from day
60. Several studies indicated that therapy with GnRH agonists
modifies the body composition with the FM being the main affected
component and slight reductions in the FFM (20,34–36).
However, these therapies were performed to treat specific
pathophysiological conditions with no dietary control. In addition,
the present study identified a positive correlation between PI and
the total lipid extract from the skin, which means that animal size
is not only associated with this indicator but also with a FM under
the skin.
At present, no information is available on the
effect of the use of GnRH agonists after ovariectomy or its direct
long-term effects on body composition under these conditions.
Furthermore, a recent study report that GnRH may have different
roles outside the hypothalamus-pituitary-reproductive axis
(34).
The present study reported an increased ghrelin mRNA
expression in animals in the LA group, which also had a high food
consumption and weight gain in comparison with CTRL animals. At
present, the manufacturers of commercial LA preparations warn about
rapid weight gain during treatments, for instance in central
precocious puberty (18,37), but these phenomena are not well
explained. The present study indicated that long-term treatment
with LA increases ghrelin mRNA expression, which probably leads to
hyperphagia and a subsequent weight gain with body composition
changes. As previously reported, the present study observed changes
in food intake, body weight and fat mass gain (38). In addition, the mRNA expression of
ghrelin and LPL in the OVX group was even higher than that in the
LA group at the end of the experiment (120 days). Likewise, the
mRNA expression of LPL was higher in the LA group compared with
that in the CTRL group, each group (OVX and LA) had a direct effect
by ovariectomy and LA administration, respectively. These
circumstances may be favorable towards an increase in weight an FM
deposition due to high LPL activity. Studies have reported on the
effect of ghrelin administration and how it modifies food intake,
it has been previously mentioned that suppression of estrogen is
associated with obesity development in mammals and there is
evidence that estrogen blocks the transcription of the LPL gene
(39–41). However, the results of the present
study may indicate that LA had a different effect in ovariectomized
rats, as a downregulation in ghrelin and LPL mRNA expression to
levels similar to those in the LA group were observed at the end of
the experiment, which was probably associated with a decrease in
food consumption and less FM deposition, which in fact corresponded
to the results on body composition. It may be hypothesized that by
LA administration stimulates a replacement of steroidal secretion
in ovariectomized rats in other specialized tissues, which was
supported by previous reports on the GnRH receptor outside of the
hypothalamic-pituitary-reproductive axis (42).
In conclusion, the present study indicated that
long-term administration of LA promoted changes in weight and body
composition in ovariectomized and non-ovariectomized rats. It also
promoted increments in mRNA expression of ghrelin and LPL in
non-ovariectomized animals while a downregulation was present in
ovariectomized animals with the same treatment, which probably
affected food consumption and adipogenesis. To the best of our
knowledge, the present study was one of the first describing the
direct effect of LA on body composition in the long term, taking
into consideration a controlled diet and the absence of
pathophysiological events. LA is a therapeutic drug that is
currently widely used throughout the world, and experimental models
such as that of the present study may serve to elucidate the
mechanisms involved in changes in body composition or for
nutritional prophylaxis in subjects treated with GnRH analogs. To
explain the mechanisms implied to this phenomenon, further
experiments should be performed.
Acknowledgements
The authors would like to thank Moisés
Altamira-Camacho and Irma Hernández-Jasso for their technical
support. The present study was supported by a grant from the
National Council of Science and Technology Mexico (grant no.
467237).
References
|
1
|
Quintanar JL and Salinas E: Neurotrophic
effects of GnRH on neurite outgrowth and neurofilament protein
expression in cultured cerebral cortical neurons of rat embryos.
Neurochem Res. 33:1051–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prange-Kiel J, Jarry H, Schoen M, Kohlmann
P, Lohse C, Zhou L and Rune GM: Gonadotropin-releasing hormone
regulates spine density via its regulatory role in hippocampal
estrogen synthesis. J Cell Biol. 180:417–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conn PM and Crowley WF Jr:
Gonadotropin-releasing hormone and its analogs. Annu Rev Med.
45:391–405. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Periti P, Mazzei T and Mini E: Clinical
pharmacokinetics of depot leuprorelin. Clin Pharmacokinet.
41:485–504. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sennello LT, Finley RA, Chu SY, Jagst C,
Max D, Rollins DE and Tolman KG: Single-dose pharmacokinetics of
leuprolide in humans following intravenous and subcutaneous
administration. J Pharm Sci. 75:158–60. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lønning PE and Lien EA: Pharmacokinetics
of anti-endocrine agents. Cancer Surv. 17:343–370. 1993.PubMed/NCBI
|
|
7
|
Wilson AC, Meethal SV, Bowen RL and Atwood
CS: Leuprolide acetate: A drug of diverse clinical applications.
Expert Opin Investig Drugs. 16:1851–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guzmán-Soto I, Salinas E, Hernández-Jasso
I and Quintanar JL: Leuprolide Acetate, a GnRH Agonist, improves
experimental autoimmune encephalomyelitis: A possible therapy for
multiple sclerosis. Neurochem Res. 37:2190–2197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calderón-Vallejo D and Quintanar JL:
Gonadotropin-releasing hormone treatment improves locomotor
activity, urinary function and neurofilament protein expression
after spinal cord injury in ovariectomized rats. Neurosci Lett.
515:187–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz Galindo C, Gómez-González B, Salinas
E, Calderón-Vallejo D, Hernández-Jasso I, Bautista E and Quintanar
JL: Leuprolide acetate induces structural and functional recovery
of injured spinal cord in rats. Neural Regen Res. 10:1819–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gevers EF, Wit JM and Robinson IC: Effects
of long-term gonadotropin-releasing hormone analog treatment on
growth, growth hormone (GH) Secretion, GH Receptors, and GH-binding
protein in the rat. Pediatr Res. 43:111–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller TD, Nogueiras R, Andermann ML,
Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers
CY, Broglio F, et al: Ghrelin. Mol Metab. 4:437–460. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H and Eckel RH: Lipoprotein lipase:
From gene to obesity. Am J Physiol Endocrinol Metab. 297:E271–E288.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Novelli EL, Diniz YS, Galhardi CM, Ebaid
GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC and Filho JL
Novelli: Anthropometrical parameters and markers of obesity in
rats. Lab Anim. 41:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hohl R, de Oliveira RB, Vaz de Macedo D
and Brenzikofer R: Apparatus for measuring rat body volume: A
methodological proposition. J Appl Physiol (1985). 102:1229–1234.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muscaritoli M, Gleason JR, Meguid MM and
Lukaski HC: Densitometry-based equations for estimating body
composition in Fischer rats. Nutrition. 9:439–445. 1993.PubMed/NCBI
|
|
17
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SJ, Yang EM, Seo JY and Kim CJ:
Effects of gonadotropin-releasing hormone agonist therapy on body
mass index and height in girls with central precocious puberty.
Chonnam Med J. 48:27–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neely EK, Lee PA, Bloch CA, Larsen L, Yang
D, Mattia-Goldberg C and Chwalis K: Leuprolide acetate 1-month
depot for central precocious puberty: Hormonal suppression and
recovery. Int J Pediatr Endocrinol. 2010:3986392010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basaria S, Lieb J II, Tang AM, DeWeese T,
Carducci M, Eisenberger M and Dobs AS: Long-term effects of
androgen deprivation therapy in prostate cancer patients. Clin
Endocrinol (Oxf). 56:779–786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith MR: Changes in fat and lean body
mass during androgen-deprivation therapy for prostate cancer.
Urology. 63:742–745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herbert CA and Trigg TE: Applications of
GnRH in the control and management of fertility in female animals.
Anim Reprod Sci. 88:141–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lessey BA: Medical management of
endometriosis and infertility. Fertil Steril. 73:1089–1096. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei A, Fascetti AJ, Kim K and Ramsey JJ:
Post-castration variations in weight gain in a cohort of young
adult male cats. J Nutr Sci. 3:e372014.PubMed/NCBI
|
|
25
|
Nguyen PG, Dumon HJ, Siliart BS, Martin
LJ, Sergheraert R and Biourge VC: Effects of dietary fat and energy
on body weight and composition after gonadectomy in cats. Am J Vet
Res. 65:1708–1713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang JM, Sacco SM and Ward WE:
Ovariectomy-induced hyperphagia does not modulate bone mineral
density or bone strength in rats. J Nutr. 138:2106–2110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stoch SA, Parker RA, Chen L, Bubley G, Ko
YJ, Vincelette A and Greenspan SL: Bone loss in men with prostate
cancer treated with gonadotropin-releasing hormone agonists. J Clin
Endocrinol Metab. 86:2787–2791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meldrum DR, Chang RJ, Lu J, Vale W, Rivier
J and Judd HL: ‘Medical oophorectomy’ using a long-acting gnrh
agonist-a possible new approach to the treatment of endometriosis.
J Clin Endocrinol Metab. 54:1081–1083. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohtsuka S, Terakawa N, Shimizu I, Sakata
M, Mizutani T, Miyake A, Tanizawa O and Aono T: Studies on GnRH
agonist suppression of estrogen production in patients with
endometriosis. Endocrinol Jpn. 36:611–619. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu H, Ohtani K, Kato Y, Tanaka Y and
Mori M: Withdrawal of [corrected] estrogen increases hypothalamic
neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat.
Neurosci Lett. 204:81–84. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ainslie DA, Morris MJ, Wittert G, Turnbull
H, Proietto J and Thorburn AW: Estrogen deficiency causes central
leptin insensitivity and increased hypothalamic neuropeptide Y. Int
J Obes Relat Metab Disord. 25:1680–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Litwak SA, Wilson JL, Chen W, Garcia-Rudaz
C, Khaksari M, Cowley MA and Enriori PJ: Estradiol prevents fat
accumulation and overcomes leptin resistance in female high-fat
diet mice. Endocrinology. 155:4447–4460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matysková R, Zelezná B, Maixnerová J,
Koutová D, Haluzík M and Maletínská L: Estradiol supplementation
helps overcome central leptin resistance of ovariectomized mice on
a high fat diet. Horm Metab Res. 42:182–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamasaki H, Douchi T, Yamamoto S, Oki T,
Kuwahata R and Nagata Y: Body fat distribution and body composition
during GnRH agonist therapy. Obstet Gynecol. 97:338–342. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boxer RS, Kenny AM, Dowsett R and Taxel P:
The effect of 6 months of androgen deprivation therapy on muscle
and fat mass in older men with localized prostate cancer. Aging
Male. 8:207–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park HK, Lee HS, Ko JH, Hwang IT, Lim JS
and Hwang JS: The effect of gonadotrophin-releasing hormone agonist
treatment over 3 years on bone mineral density and body composition
in girls with central precocious puberty. Clin Endocrinol (Oxf).
77:743–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim EY: Long-term effects of
gonadotropin-releasing hormone analogs in girls with central
precocious puberty. Korean J Pediatr. 58:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Theander-Carrillo C, Wiedmer P,
Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda
TR, Muzzin P, Schürmann A, Szanto I, et al: Ghrelin action in the
brain controls adipocyte metabolism. J Clin Invest. 116:1983–1993.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pedersen SB, Børglum JD, Møller-Pedersen T
and Richelsen B: Effects of in vivo estrogen treatment on adipose
tissue metabolism and nuclear estrogen receptor binding in isolated
rat adipocytes. Mol Cell Endocrinol. 85:13–19. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Homma H, Kurachi H, Nishio Y, Takeda T,
Yamamoto T, Adachi K, Morishige K, Ohmichi M, Matsuzawa Y and
Murata Y: Estrogen suppresses transcription of lipoprotein lipase
gene. Existence of A unique estrogen response element on the
lipoprotein lipase promoter. J Biol Chem. 275:11404–11411. 2009.
View Article : Google Scholar
|
|
41
|
Pedersen SB, Børglum JD, Eriksen EF and
Richelsen B: Nuclear estradiol binding in rat adipocytes. Regional
variations and regulatory influences of hormones. Biochim Biophys
Acta. 1093:80–86. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Skinner DC, Albertson AJ, Navratil A,
Smith A, Mignot M, Talbott H and Scanlan-Blake N: GnRH effects
outside the hypothalamo-pituitary-reproductive axis. J
Neuroendocrinol. 21:282–292. 2009. View Article : Google Scholar : PubMed/NCBI
|