Introduction
Atherosclerosis is the most common lesion that
occurs in the cardiovascular system, with a high incidence
worldwide (1). The occurrence of
atherosclerotic lesions is a chronic and complex process that
involves interactions among various factors, such as local
hemodynamics (2), arterial wall
cells (3), the extracellular matrix
(4), the environment (5) and genetics (6). Vascular endothelial injury, phenotypic
transformation of smooth muscle cells, lipid absorption by
monocytes or macrophages, and inflammatory mediator release are
important processes in the development of atherosclerosis (7–9). The
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
signaling pathway is considered a key signaling pathway that
mediates vascular endothelial cell injury and the release of
inflammatory mediators, such as vascular cell adhesion molecule-1
(VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) (10). An important step in the development
of atherosclerosis is the inflammation of the vascular endothelial
cell layer (11). Importin-α3 is a
key protein that is associated with the nuclear transfer of NF-κB,
which is important in the occurrence of inflammation (12). Importin-α3 was therefore chosen as a
target gene in the present study. In addition to being a
proinflammatory factor, tumor necrosis factor (TNF)-α also
activates NF-κB, which then initiates a cascade reaction of
cytokines, further aggravating inflammatory responses and apoptosis
in tissues (13).
microRNA (miRNA or miR) are a class of endogenous
non-coding RNA that regulate a variety of cellular mechanisms,
including inflammation, cytothesis and lipid metabolism, as well as
participating in the development of atherosclerosis (14). For example, miR-155 regulates
multiple functions of macrophages, including inflammation, lipid
absorption and apoptosis (15,16). In
addition, low-density lipoprotein and mildly oxidized low-density
lipoprotein are able to induce the expression of miR-155 in
macrophages (15,16). miR-342-5p promotes the expression of
miR-155 via V-protein kinase B murine thymoma viral oncogene
homolog 1, and facilitates the expression of inflammatory
mediators, such as nitric oxide, TNF-α and interleukin-6 (17). Furthermore, miR-33 (18), miR-122 (19) and miR-27a (20) affect the development of
atherosclerosis by participating in lipid metabolism. It has been
reported that miR-24 participates in pathophysiological processes,
including tumor formation (21) and
ischemic reperfusion injury (22).
However, the role of miR-24 in atherosclerosis has rarely been
reported. Maegdefessel et al (23) reported that miR-24 limits aortic
vascular inflammation and murine abdominal aneurysm development.
Murata et al (24)
demonstrated that miR-24 in plasma may function as a biomarker for
rheumatoid arthritis. In the present study, serum expression of
miR-24 in elderly patients with atherosclerosis was assessed, and
the effect of miR-24 on importin-α3, TNF-α and the proliferation
and migration ability of vascular endothelial cells was
investigated.
Materials and methods
Patients
A total of 30 patients with atherosclerosis admitted
to Cangzhou Central Hospital between January and June 2016 were
enrolled in the present study, including 20 males and 10 females
(age range, 60–70 years; mean, 64±4.9 years). Inclusion criteria
were as follows: Significantly elevated blood lipids and a positive
diagnosis of atherosclerotic plaque formation using peripheral
vascular ultrasound examinations. A total of 30 healthy subjects
were enrolled as controls, including 20 males and 10 females (age
range, 60–70 years; mean, 63±5.6 years). Healthy subjects had no
history of hypertension, diabetes, atherosclerosis or tumors.
Peripheral blood (10 ml) was collected from all patients and
healthy subjects, and centrifuged at 1,200 × g and 20–22°C for 8
min to separate serum. All procedures were approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China). Written
informed consent was obtained from all participants or their
families.
Cells
Human umbilical vein endothelial cells (HUVECs; Cell
Bank of Chinese Academy of Sciences, Shanghai, China) were cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in an atmosphere containing 5% CO2. Cells
were seeded in culture plates at 3×105 cells/well and
cultured for 24 h. HUVECs were randomly divided into the has-miR-24
mimic group (transfected with has-miR-24 mimic; Guangzhou RiboBio
Co., Ltd., Guangzhou, China) or negative control group
(untransfected). When cells reached 50% confluency they were used
for transfection. In the first vial, 7.5 µl small RNA fragments was
mixed with 125 µl serum-free DMEM. In the second vial, 7.5 µl
liposome (Lipofectamine 2000; Thermo Fisher Scientific, Inc.) was
mixed with 125 µl serum-free DMEM. After standing for 5 min, the
two vials were combined and left to stand at room temperature for
20 min. The mixtures were subsequently added to cells for
incubation at 37°C for 6 h. The medium was subsequently replaced
with DMEM containing 10% fetal bovine serum and cultivated at 37°C
for 48 h, at which point the cells were collected for further
assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Serum (1 ml) was mixed with 1 ml TRIzol (Thermo
Fisher Scientific, Inc.) for lysis and total RNA was extracted
using the phenol chloroform method. The purity of RNA was
determined by A260/A280 using ultraviolet spectrophotometry
(Nanodrop ND1000; Thermo Fisher Scientific, Inc.). cDNA was
obtained by RT using a PrimeScript™ RT reagent kit with
gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China) from 1
µg RNA and stored at −20°C. The temperature protocol was as
follows: 42°C for 2 min, 37°C for 15 min and 85°C for 5 sec.
Expression of miR-24 was determined using a SYBR
PrimeScript miRNA RT-PCR Kit (Takara Biotechnology Co., Ltd.), with
U6 as an internal reference. The reaction system (25 µl) contained
12.5 µl SYBR Premix Ex Taq, 1 µl of each forward primer (miR-24,
5′-GCAGATGGCTCAGTTCAGCAG-3′; U6, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′), 1
µl Uni-miR qPCR primer (cat no. 638315; Takara Biotechnology Co.,
Ltd.), 2 µl template and 8.5 µl ddH2O. The reaction
protocol was as follows: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min (iQ5;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
2−ΔΔCq method was used to calculate the relative
expression of miR-24 against U6 (25). Each sample was tested in
triplicate.
A SYBR-Green RT-qPCR kit (Kapa Biosystems, Inc.,
Wilmington, MA, USA) was used to detect the mRNA expression of
importin-α3 and TNF-α, using GAPDH as an internal reference. The
reaction system (20 µl) was composed of 10 µl SYBR Premix Ex
Taq, 0.5 µl upstream primer (importin-α3,
5′-CTGTGTACGAGAGCGTGGTT-3′; TNF-α, 5′-GGAGAAGGGTGACCGACTCA-3′; and
GAPDH, 5′-AAGGTGAAGGTCGGAGTCA-3′), 0.5 µl downstream primer
(importin-α3, 5′-TATCAGCCCCCTGAAGGTGA-3′; TNF-α,
5′-CTGCCCAGACTCGGCAA-3′; and GAPDH, 5′-GGAAGATGGTGATGGGATTT-3′), 1
µl cDNA and 8 µl ddH2O. Thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 1 min, annealing at 60°C for 40
sec and elongation at 72°C for 30 sec, and final elongation at 72°C
for 1 min. The 2−ΔΔCq method was used to calculate the
relative expression of importin-α3 and TNF-α mRNA against GAPDH
(25). Each sample was tested in
triplicate.
Western blot analysis
HUVECs were seeded into 6-well plates at a density
of 1×106 cells/well. At 48 h after transfection, the
cells were collected and mixed with 100 µl precooled
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 1 mM phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology) for lysis of 15 min
at 4°C. Then, the mixture was centrifuged at 12,000 × g and 4°C for
5 min. The supernatant was used to determine protein concentration
using a bicinchoninic acid protein concentration determination kit
(RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China).
Protein samples (50 µg) were mixed with 2X SDS loading buffer and
denatured in a boiling water bath for 5 min. Following this, the
samples (10 µl) were separated by 10% SDS-PAGE at 100 V. Resolved
proteins were transferred to polyvinylidene difluoride membranes on
ice (300 mA, 1.5 h) and blocked with 5 g/l skimmed milk at room
temperature for 1 h. The membranes were subsequently incubated with
goat anti-human importin-α3 polyclonal primary antibody (1:1,000;
cat no. ab6039), rabbit anti-human TNF-α polyclonal primary
antibody (1:1,000; cat no. ab9635) and rabbit anti-human GAPDH
primary antibody (1:2,000; cat no. ab9485, all Abcam, Cambridge,
UK) at 4°C overnight. Following washing with PBST three times of 15
min, the membranes were incubated with polyclonal goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:1,000; cat
no. ab205718, Abcam) for 1 h at room temperature. Membranes were
washed three times with PBST three times for 15 min and developed
using an enhanced chemiluminescence detection kit (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Image Lab v3.0 software (Bio-Rad
Laboratories, Inc.) was used to acquire and analyze imaging
signals. Expression of importin-α3 and TNF-α protein was calculated
relative to GAPDH.
Cell-Counting Kit 8 (CCK-8) assay
HUVECs were seeded at 5,000 cells/well in 96-well
plates for transfection. At 48 h after transfection, HUVECs were
subjected to CCK-8 assay for the detection of proliferation. At 24,
48 and 72 h, DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) was discarded, the cells were washed with twice with PBS and
10% CCK-8 reaction reagent (Beyotime Institute of Biotechnology)
diluted in DMEM medium was added. Following incubation at 37°C for
2 h, the absorbance of each well was measured at 450 nm using 600
nm as a reference for plotting cell proliferation curves. A total
of five replicate wells were assayed for each group and the mean
values were calculated.
Transwell assay
HUVECs were seeded at 3×105/well into
6-well plates for transfection. At 48 h after transfection,
Transwell chambers (8 µm diameter and 24 wells; Corning Inc.,
Corning, NY, USA) were used to evaluate the migration ability of
HUVECs. Transfected cells were collected by trypsin digestion, and
resuspended at a density of 1×105 cells/ml using DMEM.
The cell suspension (100 µl) was added into the upper chamber. In
the lower chamber, 600 µl DMEM medium supplemented with 10% fetal
bovine serum was added. Following incubation at 37°C for 24 h,
cells in the upper chamber were wiped with a cotton swab. The
chamber was subsequently fixed using 95% ethanol for 10 min at room
temperature. Following staining with 0.1% crystal violet at 22°C
for 30 min, the number of cells in 10 fields were counted under a
light microscope (magnification, ×200).
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool for
studying the functions of miRNA. To elucidate whether importin-α3
(KPNA4) was a target gene of miR-24, TargetScan (targetscan.org) was used to predict miRNA molecules
that may regulate importin-α3, and miR-24 was identified as a
potential regulator of importin-α3. According to the bioinformatics
results, wild-type (WT) and mutant seed regions of miR-24 in the
3′-untranslated region (UTR) of importin-α3 gene were chemically
synthesized in vitro, added to SpeI and
HindIII restriction sites, and cloned into pMIR-REPORT
luciferase reporter plasmids (Promega Corporation, Madison, WI,
USA). Plasmids (0.5 µg) with WT or mutant 3′-UTR DNA sequences were
co-transfected with miR-24 mimic (100 nM; Sangon Biotech Co., Ltd.,
Shanghai, China) into HEK293T cells (ATCC, Manassas, VA, USA).
Following cultivation at 37°C for 24 h, cells were lysed using a
dual luciferase reporter assay kit (Promega Corporation) according
to the manufacturer's manual, and fluorescence intensity was
measured using GloMax 20/20 illuminometer (Promega Corporation).
Using Renilla fluorescence activity as an internal
reference, the fluorescence values of each group of cells were
measured.
Statistical analysis
Statistical analysis was performed using SPSS v16.0
(SPSS, Inc., Chicago, IL, USA). Measurement data were expressed as
the mean ± standard deviation. Two groups of data were compared
using an independent samples t-test. Single factor analysis of
variance was used to compare the means of multiple samples followed
by a Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
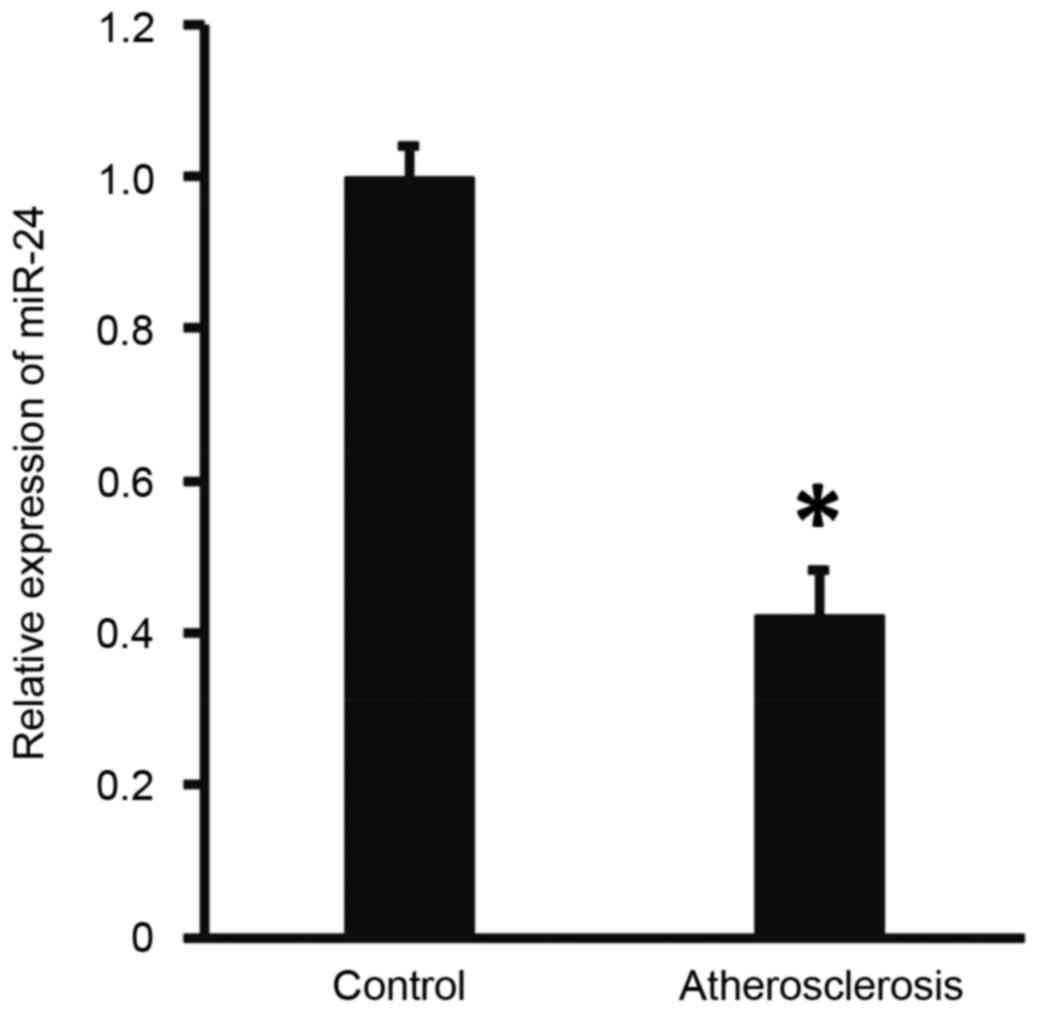
Expression of miR-24 in peripheral
blood from patients with atherosclerosis is abnormal
To measure the expression of miR-24 in the
peripheral blood, RT-qPCR was performed. The results demonstrated
that the level of miR-24 in patients with atherosclerosis was
significantly lower than that of the controls (P<0.05; Fig. 1). These findings suggests that miR-24
expression is abnormal in patients with atherosclerosis.
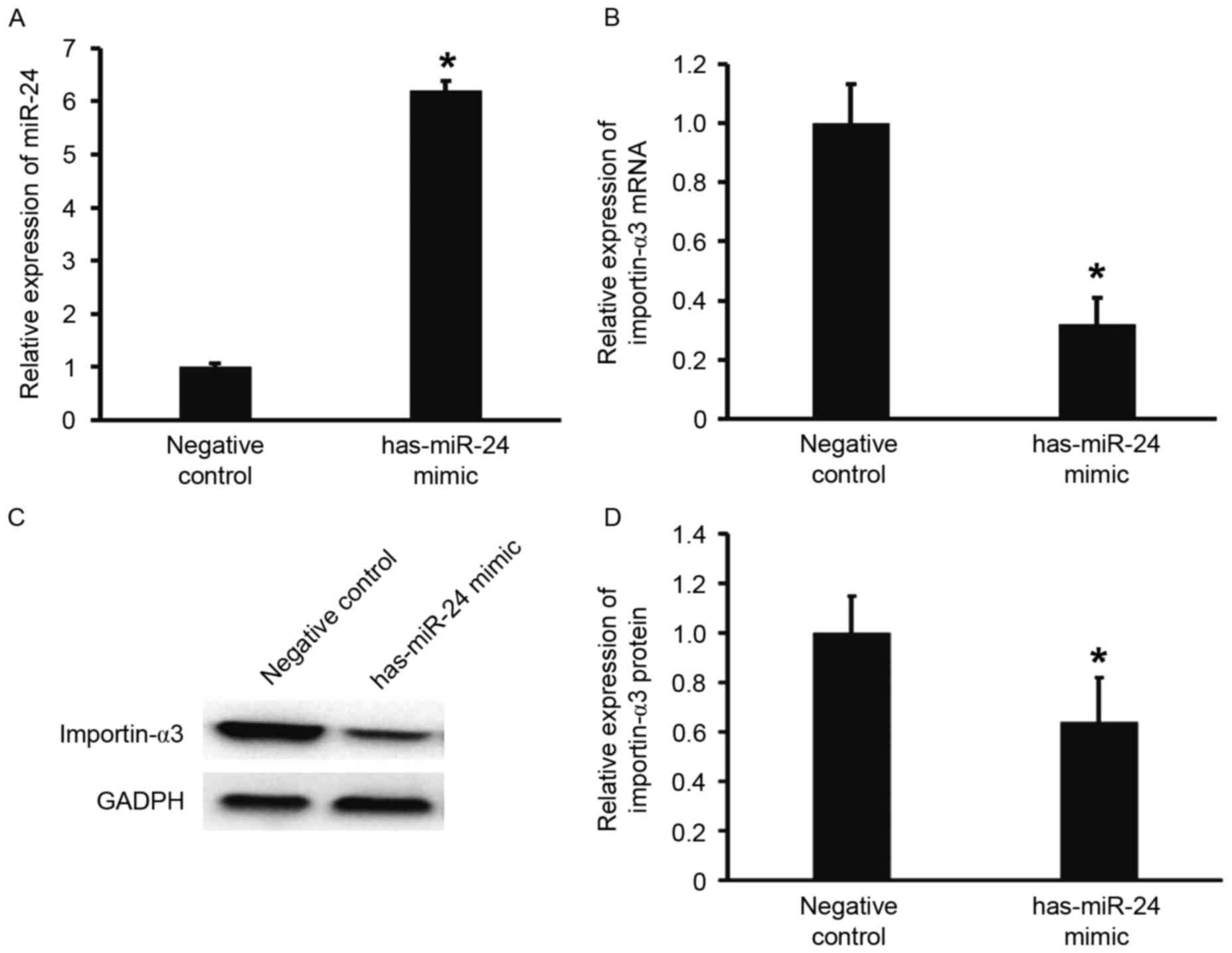
Overexpression of miR-24 inhibits the
transcription and translation of importin-α3 gene
To predict whether miR-24 is able to target
importin-α3, TargetScan was used. The data demonstrated that miR-24
is able to bind with the 3′-UTR of miR-24 (Fig. 2). To test whether miR-24 regulates
the expression of importin-α3, HUVECs were transfected with miR-24
mimic. The results showed that that HUVECs transfected with miR-24
mimics had significantly increased miR-24 levels when compared with
the negative control group (P<0.05; Fig. 3A). In addition, the expression of
importin-α3 mRNA and protein in HUVECs transfected with miR-24
mimics was significantly lower than that of the negative control
(P<0.05; Fig. 3B-D). These
results indicate that overexpression of miR-24 inhibits the
transcription and translation of importin-α3 gene.
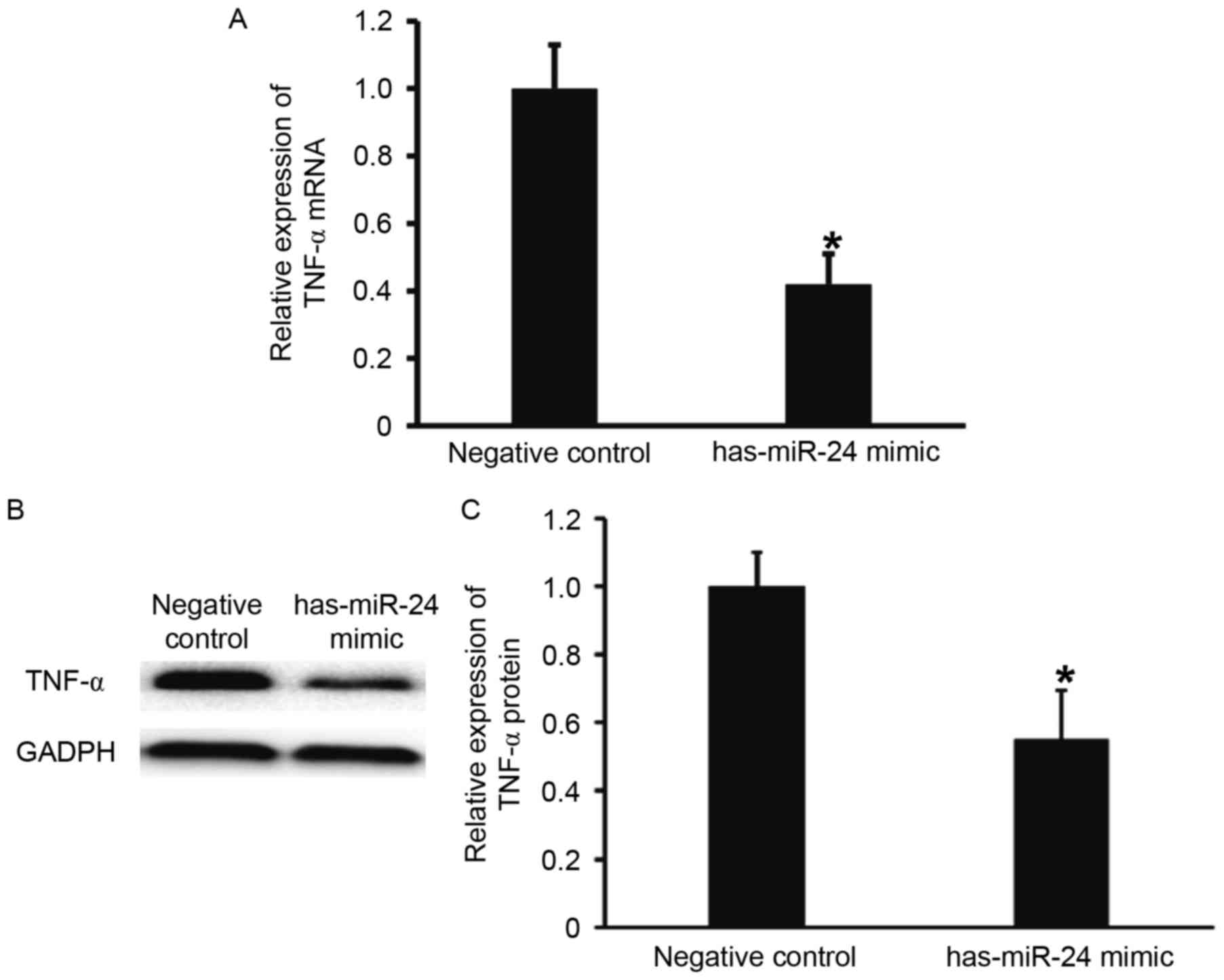
miR-24 negatively regulates the
expression of endothelial inflammatory factor TNF-α
To examine the effect of miR-24 on the expression of
TNF-α, RT-qPCR and western blotting were performed. TNF-α mRNA and
protein expression levels were significantly lower in HUVECs
transfected with miR-24 mimic, when compared with the negative
control group (P<0.05; Fig. 4).
These results suggest that miR-24 negatively regulates the
expression of endothelial inflammatory factor, TNF-α.
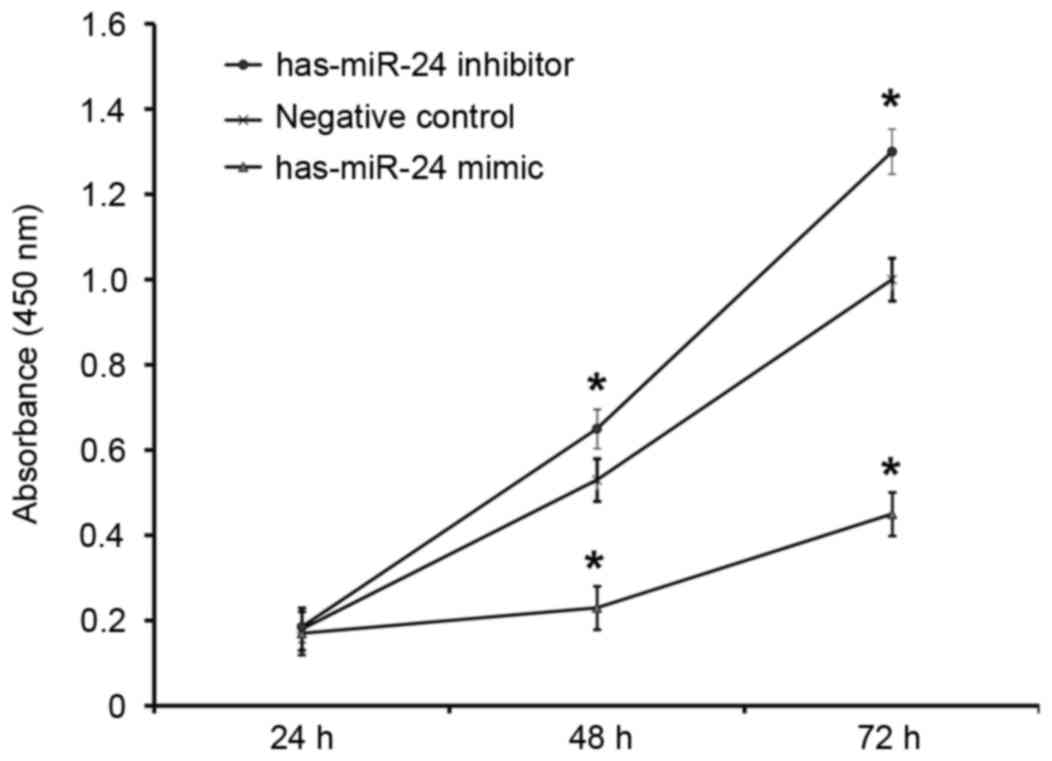
Overexpression of miR-24 inhibits
HUVEC proliferation and miR-24 knockdown promotes it
To determine how miR-24 affects the proliferation of
HUVECs, a CCK-8 assay was employed. The absorbance of HUVECs
transfected with miR-24 mimic was significantly lower than that of
the negative control group at 48 and 72 h (P<0.05; Fig. 5). By contrast, the absorbance of
HUVECs transfected with miR-24 inhibitor was significantly higher
compared with the negative control group at 48 and 72 h (P<0.05;
Fig. 5). This suggests that
overexpression of miR-24 inhibits the proliferation of endothelial
cells, whereas the inhibition of miR-24 expression promotes.
Overexpression of miR-24 reduces the
migration ability of endothelial cells, but inhibition of miR-24
expression promotes it
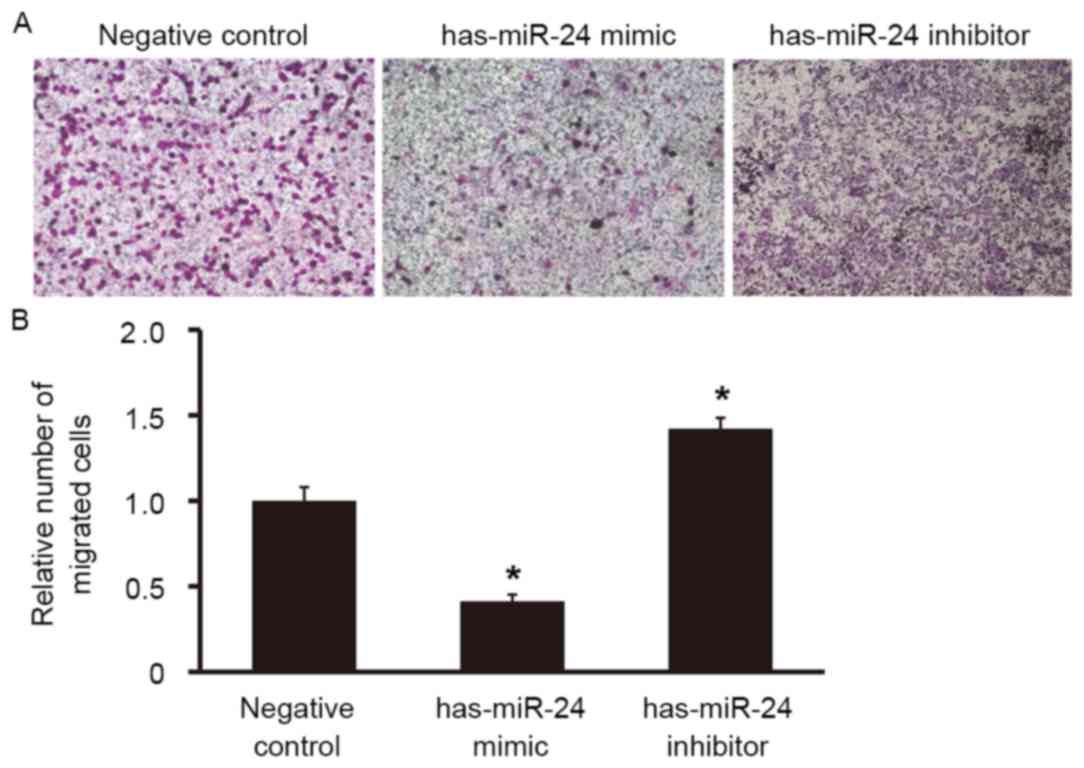
To examine the effect of miR-24 on the migration
ability of HUVECs, a Transwell assay was used. The number of cells
that migrated to the lower chamber was significantly lower in the
miR-24 mimic group compared with the negative control group
(P<0.05; Fig. 6), whereas the
number of cells that migrated to the lower chamber in miR-24
inhibitor group was significantly higher (P<0.05; Fig. 6). This suggests that overexpression
of miR-24 reduces the migration ability of endothelial cells,
whereas miR-24 knockdown promotes it.
miR-24 downregulates the expression of
importin-α3 by binding with the 3′-UTR of importin-α3 gene
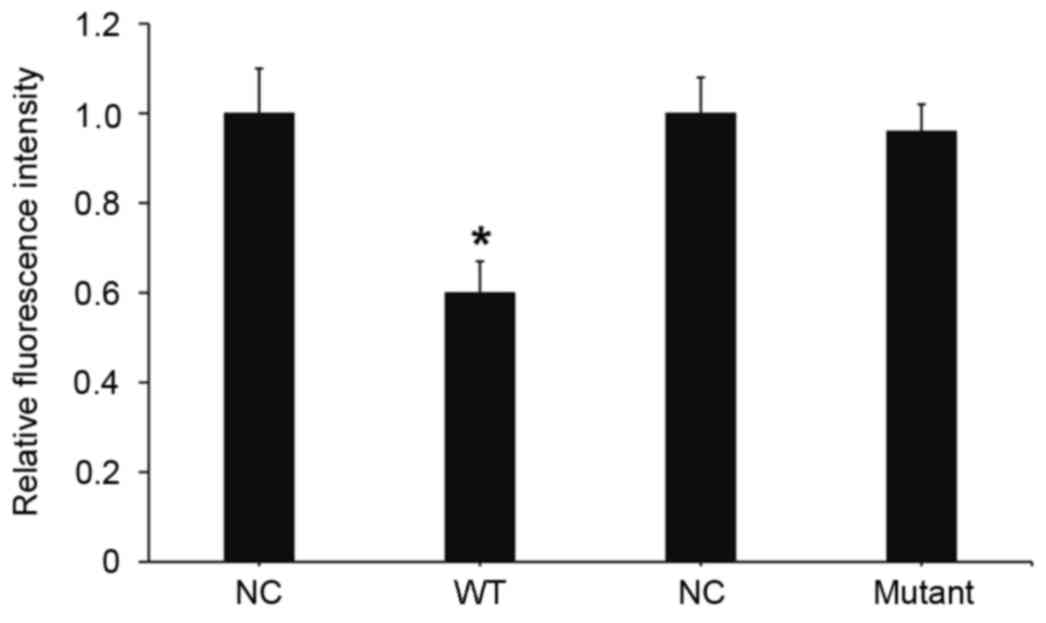
To understand whether miR-24 directly targets
importin-α3, a dual luciferase reporter assay was performed.
Transfection with miR-24 mimics and pMIR-REPORT-wild type
importin-α3 led to a significant decrease in fluorescence
intensity, as compared with the negative control (P<0.05;
Fig. 7). Transfection with miR-24
mimic and pMIR-REPORT-mutant importin-α3 had no significant effect
on fluorescence intensity. These results suggests that miR-24
downregulates the expression of importin-α3 by binding with the
3′-UTR of the importin-α3 gene.
Discussion
Atherosclerosis is the pathological basis of various
types of cardiac and cerebral vascular diseases (26). The most important processes in the
occurrence and development of atherosclerosis include i) vascular
endothelial cell injury and inflammatory activation; ii) the
proliferation and migration of endothelial/smooth muscle cells; and
iii) the release of inflammatory mediators stimulated by macrophage
activation (27–30). It has been reported that miR-24
expression is downregulated in atherosclerotic plaques, and miR-24
participates in the formation of atherosclerosis primarily by
targeting and inhibiting matrix metalloproteinase-14 (31). In the present study, it was
demonstrated that miR-24 expression is significantly lower in
patients with atherosclerosis, as compared with healthy subjects.
Bioinformatics revealed that the importin-α3 gene is a potential
target gene of miR-24. A previous study reported that the importin
protein family (α1, α3, α4, α5, α6 and α7) is an important class of
transport proteins associated with the nuclear transfer of NF-κB,
which is a key process in the activation of the NF-κB signaling
pathway (32). The results of the
present study demonstrate that miR-24 inhibits the expression of
importin-α3 and NF-κB signaling pathway-mediated inflammatory
factor, TNF-α. Naqvi et al (33) demonstrated that TNF-α is also a
target gene of miR-24. Combined with the results of the present
study, this suggests that miR-24 may regulate inflammatory
processes by simultaneously targeting importin-α3 and TNF-α in
respect to the mechanism of atherosclerosis. Therefore, miR-24
reduces the activation of vascular endothelial cells and
inflammatory responses. Maegdefessel et al (23) reported that miR-24 inhibits the
development of aortic vascular inflammation and abdominal aortic
aneurysm in mice by targeting chitinase 3-like 1. Xiang et
al (34) demonstrated that
patients with diabetes have low miR-24 expression, which suggests
potential thrombotic events in these patients. The present study
indicates that endothelial cell activation and inflammatory
responses regulated by miR-24 participate in the development of
atherosclerosis.
miRNAs have previously been reported to participate
in the regulation of vascular endothelial cell functions, and have
important roles in the development of atherosclerosis; for example,
enhanced expression of miR-221 and miR-222 in human arterial
endothelial cells causes reduced levels and activity of nitric
oxide synthase, leading to cell dysfunction (35). In addition, reduced miR-126 and
increased miR-125b expression results in increased endothelial
inflammatory response protein secretion (36). miR-29 affects the production of
collagen and elastin, and has important roles in maintaining
arterial structural integrity (37).
The results of the present study indicate that overexpression of
miR-24 inhibits the proliferation and migration of endothelial
cells. Lal et al (38)
reported that miR-24 targets E2F2 and inhibits the proliferation of
erythroleukemia K562 cells. Similarly, Amelio et al
(39) demonstrated that miR-24
regulates the migration of human keratinocytes and mouse epidermal
cells, suggesting that miR-24 has important roles in the regulation
of proliferation, invasion and migration of cells.
In conclusion, the results of the present study
demonstrate that miR-24 inhibits the proliferation and migration of
endothelial cells by targeting importin-α3 and regulating
inflammatory responses in endothelial cells. Low levels of miR-24
in the peripheral blood are associated with atherosclerosis
progression. Furthermore, miR-24 may provide a novel strategy and
direction for the clinical treatment and research on
atherosclerosis. Further investigation is required to elucidate the
underlying mechanism of inflammation in atherosclerosis and to
explore the mechanism of abnormal expression of miR-24 in the blood
of patients with atherosclerosis.
Acknowledgements
The present study was supported by the Hebei Science
and Technology Support Program Key Projects (grant no.
16277707D).
References
|
1
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology, and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malek AM, Alper SL and Izumo S:
Hemodynamic shear stress and its role in atherosclerosis. JAMA.
282:2035–2042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R and Glomset JA: Atherosclerosis and
the arterial smooth muscle cell: Proliferation of smooth muscle is
a key event in the genesis of the lesions of atherosclerosis.
Science. 180:1332–1339. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsuda S and Kaji T: Atherosclerosis and
extracellular matrix. J Atheroscler Thromb. 10:267–274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morland K, Wing S and Roux A Diez: The
contextual effect of the local food environment on residents'
diets: The atherosclerosis risk in communities study. Am J Public
Health. 92:1761–1767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox CS, Polak JF, Chazaro I, Cupples A,
Wolf PA, D'Agostino RA and O'Donnell CJ: Framingham Heart Study:
Genetic and environmental contributions to atherosclerosis
phenotypes in men and women: Heritability of carotid intima-media
thickness in the Framingham Heart Study. Stroke. 34:397–401. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fenyo IM and Gafencu AV: The involvement
of the monocytes/macrophages in chronic inflammation associated
with atherosclerosis. Immunobiology. 218:1376–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomez D and Owens GK: Smooth muscle cell
phenotypic switching in atherosclerosis. Cardiovasc Res.
95:156–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munro JM and Cotran RS: The pathogenesis
of atherosclerosis: Atherogenesis and inflammation. Lab Invest.
58:249–261. 1988.PubMed/NCBI
|
|
10
|
de Winther MP, Kanters E, Kraal G and
Hofker MH: Nuclear factor kappaB signaling in atherogenesis.
Arterioscler Thromb Vasc Biol. 25:904–914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajendran P, Rengarajan T, Thangavel J,
Nishigaki Y, Sakthisekaran D, Sethi G and Nishigaki I: The vascular
endothelium and human diseases. Int J Biol Sci. 9:1057–1069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fagerlund R, Kinnunen L, Köhler M,
Julkunen I and Melén K: NF-{kappa}B is transported into the nucleus
by importin {alpha}3 and importin {alpha}4. J Biol Chem.
280:15942–15951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouwmeester T, Bauch A, Ruffner H, Angrand
PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J,
Ghidelli S, et al: A physical and functional map of the human
TNF-alpha/NF-kappaB signal transduction pathway. Nat Cell Biol.
6:97–105. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rayner KJ, Fernandez-Hernando C and Moore
KJ: MicroRNAs regulating lipid metabolism in atherogenesis. Thromb
Haemost. 107:642–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koch M, Mollenkopf HJ, Klemm U and Meyer
TF: Induction of microRNA-155 is TLR- and type IV secretion
system-dependent in macrophages and inhibits DNA-damage induced
apoptosis. Proc Natl Acad Sci USA. 109:pp. E1153–E1162. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–4202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M,
Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C and
Schober A: The microRNA-342-5p fosters inflammatory macrophage
activation through an Akt1- and microRNA-155-dependent pathway
during atherosclerosis. Circulation. 127:1609–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramírez CM, Goedeke L, Rotllan N, Yoon JH,
Cirera-Salinas D, Mattison JA, Suárez Y, de Cabo R, Gorospe M and
Fernández-Hernando C: MicroRNA 33 regulates glucose metabolism. Mol
Cell Biol. 33:2891–2902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Xu Y, Hao J, Wang S, Li C and Meng
S: MiR-122 in hepatic function and liver diseases. Protein Cell.
3:364–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shirasaki T, Honda M, Shimakami T, Horii
R, Yamashita T, Sakai Y, Sakai A, Okada H, Watanabe R, Murakami S,
et al: MicroRNA-27a regulates lipid metabolism and inhibits
hepatitis C virus replication in human hepatoma cells. J Virol.
87:5270–5286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie Y, Tobin LA, Camps J, Wangsa D, Yang
J, Rao M, Witasp E, Awad KS, Yoo N, Ried T and Kwong KF:
MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in
cancer cells. Oncogene. 32:2442–2451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meloni M, Marchetti M, Garner K,
Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS,
Madeddu P, Caporali A and Emanueli C: Local inhibition of
microRNA-24 improves reparative angiogenesis and left ventricle
remodeling and function in mice with myocardial infarction. Mol
Ther. 21:1390–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: miR-24 limits aortic vascular inflammation and murine abdominal
aneurysm development. Nat Commun. 5:52142014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murata K, Furu M, Yoshitomi H, Ishikawa M,
Shibuya H, Hashimoto M, Imura Y, Fujii T, Ito H, Mimori T and
Matsuda S: Comprehensive microRNA analysis identifies miR-24 and
miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS
One. 8:e691182013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Libby P, Ridker PM and Hansson GK: Leducq
Transatlantic Network on Atherothrombosis: Inflammation in
atherosclerosis: From pathophysiology to practice. J Am Coll
Cardiol. 54:2129–2138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Packard RR and Libby P: Inflammation in
atherosclerosis: From vascular biology to biomarker discovery and
risk prediction. Clin Chem. 54:24–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chachaj A, Drozdz K and Szuba A: Reverse
cholesterol transport processes and their role in artherosclerosis
regression. Postepy Biochem. 54:301–307. 2008.(In Polish).
PubMed/NCBI
|
|
30
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Gregoli K, Jenkins N, Salter R, White
S, Newby AC and Johnson JL: MicroRNA-24 regulates macrophage
behavior and retards atherosclerosis. Arterioscler Thromb Vasc
Biol. 34:1990–2000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelley JB, Talley AM, Spencer A, Gioeli D
and Paschal BM: Karyopherin alpha7 (KPNA7), a divergent member of
the importin alpha family of nuclear import receptors. BMC Cell
Biol. 11:632010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naqvi AR, Fordham JB, Ganesh B and Nares
S: miR-24, miR-30b and miR-142-3p interfere with antigen processing
and presentation by primary macrophages and dendritic cells. Sci
Rep. 6:329252016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiang Y, Cheng J, Wang D, Hu X, Xie Y,
Stitham J, Atteya G, Du J, Tang WH, Lee SH, et al: Hyperglycemia
repression of miR-24 coordinately upregulates endothelial cell
expression and secretion of von Willebrand factor. Blood.
125:3377–3387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu XD, Wu X, Yin YL, Liu YQ, Geng MM,
Yang HS, Blachier F and Wu GY: Effects of dietary L-arginine or
N-carbamylglutamate supplementation during late gestation of sows
on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in
umbilical vein. Amino Acids. 42:2111–2119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rippe C, Blimline M, Magerko KA, Lawson
BR, LaRocca TJ, Donato AJ and Seals DR: MicroRNA changes in human
arterial endothelial cells with senescence: Relation to apoptosis,
eNOS and inflammation. Exp Gerontol. 47:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boon RA, Seeger T, Heydt S, Fischer A,
Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca
S, Pilato M, et al: MicroRNA-29 in aortic dilation: Implications
for aneurysm formation. Circ Res. 109:1115–1119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lal A, Navarro F, Maher CA, Maliszewski
LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O,
et al: miR-24 Inhibits cell proliferation by targeting E2F2, MYC,
and other cell-cycle genes via binding to ‘seedless’ 3′UTR microRNA
recognition elements. Mol Cell. 35:610–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amelio I, Lena AM, Viticchiè G,
Shalom-Feuerstein R, Terrinoni A, Dinsdale D, Russo G, Fortunato C,
Bonanno E, Spagnoli LG, et al: miR-24 triggers epidermal
differentiation by controlling actin adhesion and cell migration. J
Cell Biol. 199:347–363. 2012. View Article : Google Scholar : PubMed/NCBI
|