Introduction
The incidence of diabetes is currently very high and
shows no decreasing trend. Complications from diabetes can
seriously affect human health. Improvements in living standards and
life expectancy have conversely increased the number of patients
with diabetes. In China, the number of patients with diabetes
account for 2.51% of the population, which indicates a 3-fold
increase since 1980. Additionally, the number of patients with
diabetes is on the increase (1). The
testis is an extremely important part of the male reproductive
organs and contains spermatogenic cells that can maintain the
production of sperm cells. Damage to testicular cells can, not only
cause a decline in male sexual function, but can also lead to
infertility due to abnormal sperm production caused by testicular
cell apoptosis (2). Previous
findings showed that diabetes can cause erectile dysfunction, and
its negative effect on erectile function is next only to senescence
(3). Additionally, sexual
dysfunction and testicular atrophy can be observed in 40–75% of
patients with diabetes, and these patients usually also presented
other complications such as spermatogenesis disorders (4). The incidence rates for these
complications were much higher in patients with diabetes than those
without, and these diseases may become more severe with aging and
prolonged disease course (5–7). Therefore, the prevention and treatment
of testicular disease caused by diabetes has become a focus for
diabetes research.
Chinese medicine has been used in the treatment of
diabetes for a long time. Many studies have shown that Chinese
medicine can significantly improve the symptoms of diabetes and its
related complications, and numerous studies on the pharmacological
activities of Chinese medicines have also provided references for
the use of Chinese medicine in the treatment of diabetes (8). Soybean isoflavones (SI), which are
widely present in legume species, are a group of isoflavone
estrogens. SI includes soy flavonoids, genistein, and legumin
(9). It has been reported that SI
exert a variety of pharmacological activities, including immunity
building, anti-aging effects, anti-tumor effects, and other effects
(10). In this study, SI was
selected as an experimental drug and the aim was to investigate its
inhibitory effects on testicular cell apoptosis in mice with type 2
diabetes.
Materials and methods
Materials and reagents
The following reagents were used in this study: SI
and streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA);
Rabbit anti-mice caspase-3, Bax, and Bcl-2 primary polyclonal
antibodies, and goat anti-rabbit HRP-labeled secondary polyclonal
antibody (cat. no. 19677-0-AP, 23931-1-AP, 12789-1-AP; SA00001-2;
Wuhan SanYing Biotechnology Co., Ltd., Wuhan, China); RNA
extraction kit (Invitrogen Life Technologies, Carlsbad, CA, USA);
primers, reverse transcription kit, and quantitative PCR kit
(Takara, Dalian, China); BCA protein quantitation kit and cell
lysates (Beyotime, Nantong, China); and immunohistochemical
staining kit SP-9001 (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China).
Experimental animals and grouping
Thirty specific pathogen-free (SPF) healthy C57BL/6J
mice were randomly divided into three groups of 10 mice each:
normal controls (control), diabetes (model) and SI-treated. STZ was
dissolved in 0.1 mol/l citrate buffer. After fasting for 24 h, mice
in the diabetes mellitus (DM) model group was administered STZ
solution at a dose of 55 mg/kg via tail vein injection. All mice
were kept in cages with controlled temperature (22–24°C) and light
cycles (24°C and 12/12 light cycles). The humidity was 60±10% with
free access to water. Citrate buffer solution was administered to
mice in the control group. Venous blood was extracted from mice
tail veins on day 3 post-injection to examine blood glucose levels,
which were monitored for 5 days. A blood glucose level of ≥50 mg/dl
indicated the successful establishment of the DM model. Mice with
DM were used for subsequent experimentation. SI was dissolved in
0.5% CMC-Na after stirring for 2 h with a magnetic stirrer. The
solution was kept overnight at room temperature and the supernatant
was used. Intragastric administration of 300 mg/kg/d SI solution to
the SI group was performed at 8 a.m. daily, with 0.5% CMC-Na
solution administered to mice in the control and model groups.
Treatment persisted for 20 weeks, after which mice were
anesthetized with ether and then immediately sacrificed by cervical
dislocation. The bilateral testes were collected after laparotomy
and the epididymis and excess adipose tissue were removed. One
testis was numbered and fixed in freshly prepared 10% formalin
solution for DAPI staining and immunohistochemical analysis. The
other testis was placed in liquid nitrogen and stored in a
refrigerator at −80°C for mRNA and protein extraction. The study
was approved by the Ethics Committee of Qingdao Women and
Children's Hospital.
DAPI staining for detecting testicular
cell apoptosis
After fixation, testicular tissue was
paraffin-embedded and sectioned. After appropriate washing with PBS
to remove the fixative solution, DAPI (1 µg/ml) staining was
performed at room temperature in the dark for 5 min. The DAPI
staining solution was then removed via suction, the sections washed
twice with PBS (5 min each), and staining was observed in the dark
under fluorescence microscopy (Nikon, Tokyo, Japan).
Immunohistochemistry for detecting
caspase-3 protein expression in testicular tissue
The procedure was performed as follows:
Paraffin-embedded mouse testicular tissue sections were dewaxed and
endogenous peroxidases were inactivated with 3%
H2O2. After antigen repair and serum
blocking, the primary antibody (dilution, 1:500) was added and
sections were incubated overnight at 4°C. After washing three times
with PBS, secondary antibody (dilution, 1:1,000) was added and
sections were incubated at room temperature for 30 min. After
washing three times with PBS again, DAB color development was
performed. The slides were sealed with gum and observed under a
microscope (TE2000-U; Nikon, Tokyo, Japan).
Representative regions were selected and staining
results were evaluated according to the degree/intensity of
staining and the percentage of positive cells. Regions were
determined to exhibit no color, faint-yellow, brownish yellow, or
dark brown, which were recorded as 0, 1, 2 and 3 points,
respectively. Under high-power magnification (×400), the percentage
of positive cells was determined to be <5, 5–25, 26–50%, or
>50%, which was recorded as 0, 1, 2, and 3 points, respectively.
The two scores were added, and a total score of ≥3 points was
recorded as a positive expression.
RT-PCR for detecting caspase-3 mRNA
expression in testicular tissue
Approximately 50 mg of testicular tissue for each
mouse was taken from storage at −80°C. Total RNA was extracted and
RNA concentration and purity were detected. Only samples with an
A260/280 ratio between 1.8 and 2.0 were used. Reverse transcription
was performed according to the manufacturers instructions, and cDNA
was subjected to RT-qPCR to detect caspase-3 mRNA expression.
Primer sequences are listed in Table
I. GAPDH expression was used as an endogenous control, and the
reaction conditions were as: 94°C for 5 min, followed by 30 cycles
of 94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec, and
finally 72°C for 5 min. Cq values were processed using the
2−ΔΔCq method according to the following formula: ΔCq
(target gene) = Cq (target gene) – Ct (control gene).
 | Table I.Primer sequences used in RT-PCR. |
Table I.
Primer sequences used in RT-PCR.
| Gene | Primer | Sequence |
|---|
| Caspase-3 | F |
5′-AGAGAACAATGGCGGATA-3′ |
|
| R |
5′-CCAGTTGAGGGATGAAAG-3′ |
| GAPDH | F |
5′-TGTGTCCGTCGTGGATCTGA-3′ |
|
| R |
5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
Western blot analysis
Testicular tissue from each mouse was taken and
total protein was extracted. Protein concentration was measured and
extracted protein samples were processed. Protein (50 µg) from each
sample was subjected to SDS-PAGE electrophoresis, followed by
transfer to a PVDF membrane. After blocking with blocking solution
at room temperature for 1 h, the membranes were incubated with
primary antibody (dilution, 1:1,000) overnight at 4°C. After
washing with TTBS, the membranes were incubated with secondary
antibody (dilution, 1:2,000) at room temperature for 1 h. After
washing with TTBS, color development was performed with a color
developer and images were obtained.
Statistical analysis
Data were presented as mean ± standard deviation,
and the data were processed with SPSS 17.0 software (IBM
Corporation, New York, NY, USA). Comparisons between the two groups
were performed using paired t-tests. Comparisons of countable data
between the groups were performed using χ2 tests.
P<0.05 was considered statistically significant.
Results
Effects of SI on testicular cell
apoptosis in diabetic mice
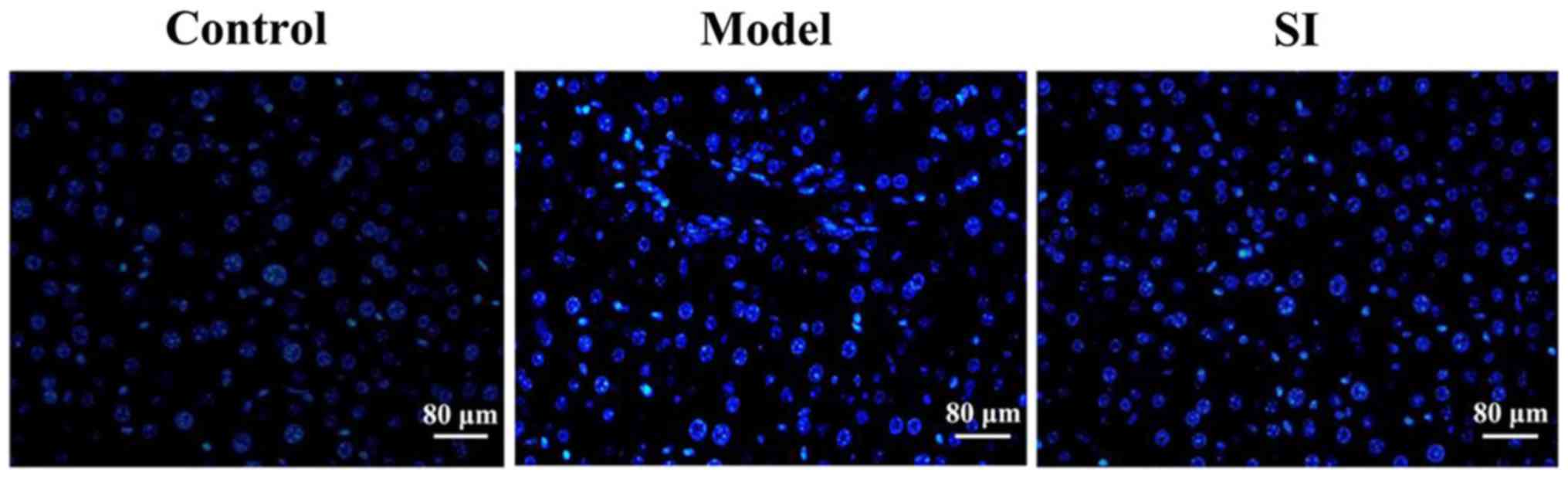
DAPI staining results showed that the testicular
cells in the model group were deformed, with condensed, brightly
stained nuclei. Cell status in the SI group was significantly
improved and the number of apoptotic cells was significantly
reduced (Fig. 1).
Effects of SI on diabetic mouse
testicular tissue caspase-3 expression
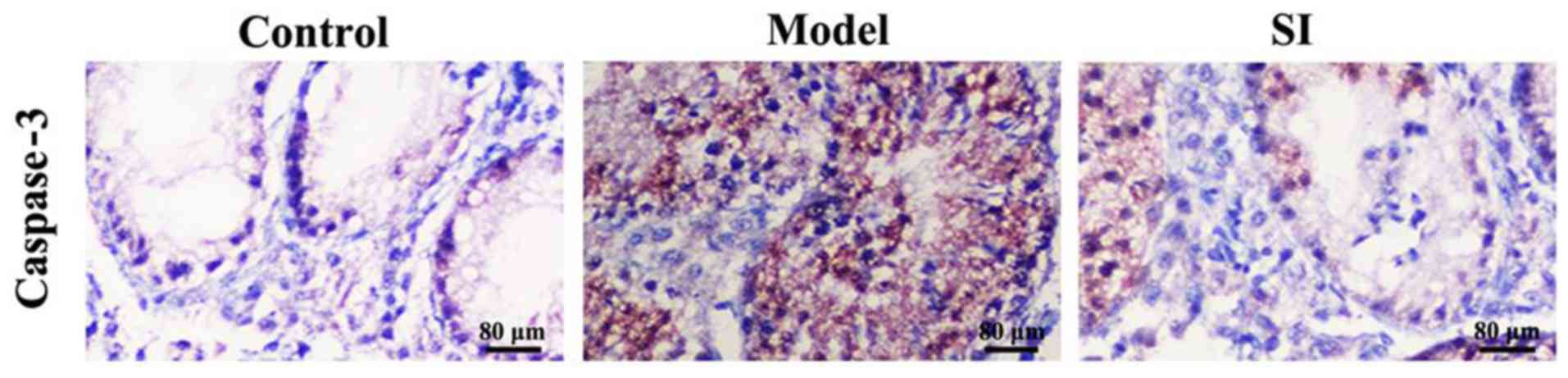
Positive caspase-3 immunohistochemical staining was
indicated by the presence of a brownish yellow color. Caspase-3
protein was found mainly located in the cytoplasm and showed
diffused or scattered distribution patterns (Fig. 2). The positive caspase-3 expression
rate in the control, model, and SI groups was 10, 90, and 20%,
respectively (Table I). SI
significantly reduced caspase-3 expression relative to the model
group.
Effects of SI on caspase-3 mRNA
expression in diabetic mouse testicular tissue
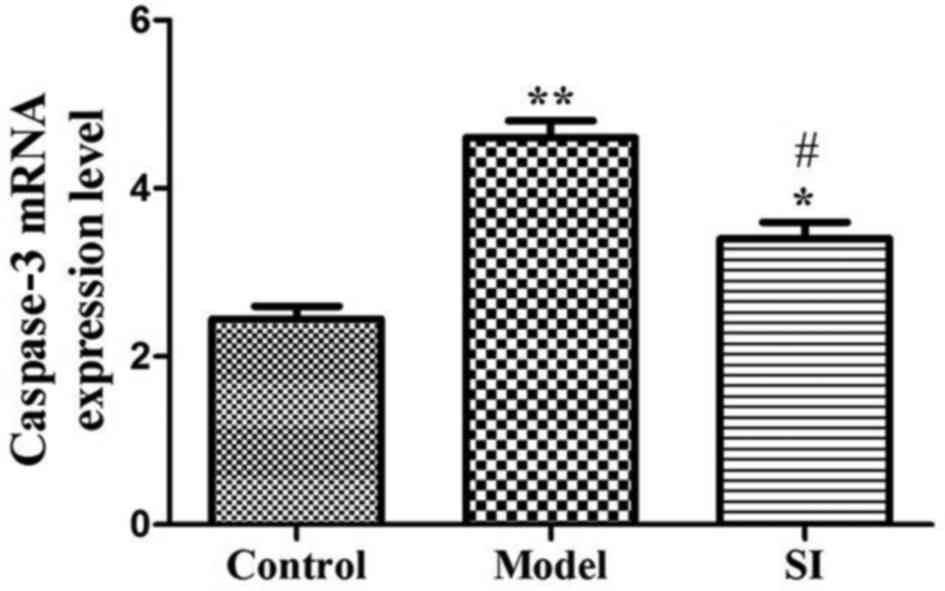
Compared with the control group, caspase-3 mRNA
expression in the model and SI groups were significantly increased
(P<0.01 or P<0.05). Caspase-3 mRNA expression in the SI group
was significantly decreased relative to the model group (P<0.05)
(Fig. 3).
Effects of SI on Bax and Bcl-2 protein
expression in diabetic murine testicular tissue
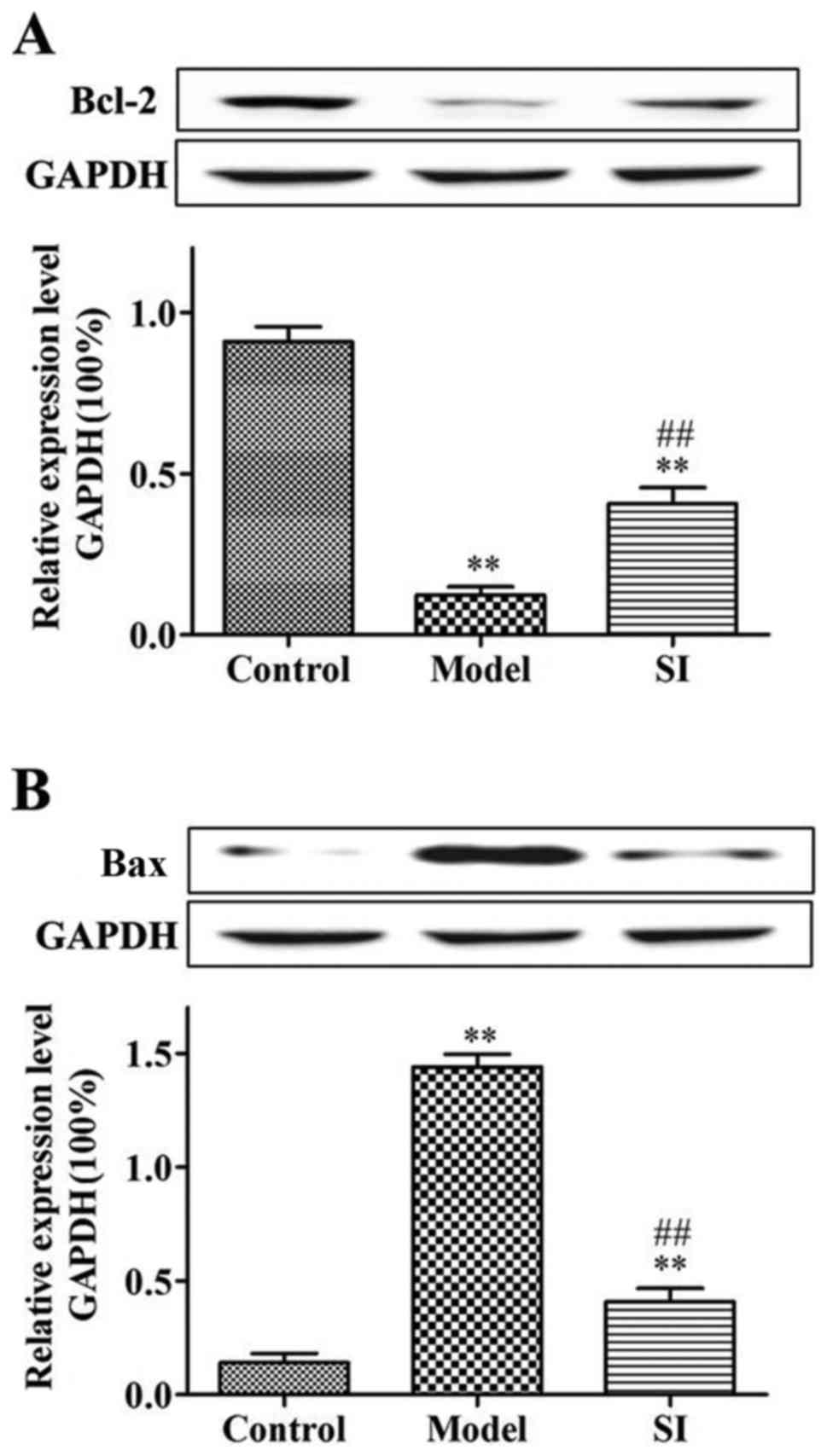
Bcl-2 protein expression was significantly decreased
(P<0.01) and Bax protein expression was significantly increased
(P<0.01) in the model and SI groups relative to the control
group. Bcl-2 protein expression was significantly increased
(P<0.01) and Bax protein expression was significantly decreased
(P<0.01) in the SI group relative to the model group (Fig. 4).
Discussion
In recent years, with the increasing number and
progressively younger profile of diabetes patients, the effect of
diabetes on the reproductive system has attracted increased
attention. The testes are extremely important parts of the male
reproductive organs. Spermatogenic cells in the testis can maintain
sperm cell production. When testicular cells are damaged, cell
apoptosis results, leading to the production of abnormal sperm or
even infertility (11). Diabetes
can, not only lead to the attenuation of spermatogenic cell
function, but also to testicular cell apoptosis (12). Although the mechanism, progression,
and treatment of diabetes have been extensively investigated
(13), cell apoptosis caused by
testicular tissue lesions (a complication of diabetes) is under
investigation, and cannot be effectively corrected with current
available treatments.
Caspase plays an essential role in a variety of cell
apoptotic processes. Previous findings have shown that the
caspase-3 protease plays a key role in apoptosis (14). In apoptotic pathways, a part or all
of the caspase-3 protein can be utilized to hydrolyze protein
substrates. In addition, caspase-3 can also activate other caspase
proteins. Previous studies have demonstrated that caspase-3
inhibition through specific protease inhibitors can significantly
inhibit cell apoptosis (15). Bcl-2,
which plays an important role in apoptosis, has an inhibitory
effect on cell apoptosis (16).
Bcl-2 can protect cells from multiple forms of death and increase
cell survival, resulting in an increase in the number of cells. In
some tumor cells, upregulated Bcl-2 expression can protect tumor
cells from death or at least increase their life span (17), indicating that the Bcl-2 gene is
closely correlated with tumor progression. In contrast to the Bcl-2
gene, the Bax gene can promote apoptosis (18). Bax belongs to the same family as the
Bcl-2 gene, but has the opposite function, and the equilibrium
state of those two determines the degree of cell apoptosis. As a
homologous dimer, Bcl-2 can inhibit cell apoptosis. When Bax
protein levels are increased and Bax protein dimerizes with Bcl-2
or if Bax is present as a homologous dimer, cell apoptosis is
increased (19).
This study aimed to investigate the inhibitory
effect of SI on testicular cell apoptosis in mice with type 2
diabetes, as well as the possible mechanism of action. DAPI
staining was used to detect the mouse testicular cell apoptosis,
and the results showed that SI significantly reduced testicular
apoptosis. The expression and distribution of caspase-3 in mouse
testicular tissue was detected by immunohistochemistry, and
caspase-3 mRNA expression levels were detected using RT-PCR. The
two methods indicated that SI significantly decreased caspase-3
expression. Previous findings have shown that the downregulation of
caspase-3 expression reduced testicular spermatogenic cell
apoptosis in rats with varicocele (20). Bax and Bcl-2 protein expression
levels were detected using western blot analysis, and the results
showed that SI significantly decreased Bax protein expression
levels and increased Bcl-2 protein levels. A similar study showed
that Bcl-2 protein expression level was directly associated with
murine spermatogenic cell apoptosis (21). Tapanainen et al (22) established a mouse model of cell
apoptosis in the reproductive duct, and found that Bax protein
expression was significantly increased and Bcl-2 protein expression
was significantly decreased during cell apoptosis.
In conclusion, SI has an inhibitory effect on
testicular cell apoptosis in mice with type 2 diabetes, and this
effect may be achieved by decreased expression of caspase-3 and Bax
and increased expression of Bcl-2 protein.
References
|
1
|
Funatsu H, Yamashita H, Nakamura S, Mimura
T, Eguchi S, Noma H and Hori S: Vitreous levels of pigment
epithelium-derived factor and vascular endothelial growth factor
are related to diabetic macular edema. Ophthalmology. 113:294–301.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang GP, Han D, Liu G, Gao SG, Cai XQ,
Duan RH and Feng XS: Effects of soy isoflavone and endogenous
oestrogen on breast cancer in MMTV-erbB2 transgenic mice. J Int Med
Res. 40:2073–2082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gautam AK, Agarwal K, Shah BA, Kumar S and
Saiyed HN: Lead induced spermatoxicity in mouse and MPG treatment.
J Environ Biol. 22:287–291. 2001.PubMed/NCBI
|
|
4
|
Montorsi F, Adaikan G, Becher E, Giuliano
F, Khoury S, Lue TF, Sharlip I, Althof SE, Andersson KE, Brock G,
et al: Summary of the recommendations on sexual dysfunctions in
men. J Sex Med. 7:3572–3588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maiorino MI, Bellastella G and Esposito K:
Diabetes and sexual dysfunction: Current perspectives. Diabetes
Metab Syndr Obes. 7:95–105. 2014.PubMed/NCBI
|
|
6
|
Tamler R: Diabetes, obesity, and erectile
dysfunction. Gend Med. 6:4–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thorve VS, Kshirsagar AD, Vyawahare NS,
Joshi VS, Ingale KG and Mohite RJ: Diabetes-induced erectile
dysfunction: Epidemiology, pathophysiology and management. J
Diabetes Complications. 25:129–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang TT and Jiang JG: Active ingredients
of traditional Chinese medicine in the treatment of diabetes and
diabetic complications. Expert Opin Investig Drugs. 21:1625–1642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kevin L: Erectile dysfunction and
testosterone deficiency as gender-specific markers of
cardiometabolic risk in minority and non-minority men: Potential
role of social determinants. Mens Health. 9:139–145. 2012.
View Article : Google Scholar
|
|
10
|
Junuzovic D, Hasanbegovic M and Masic I:
Risk factors for erectile dysfunction in patients with newly
diagnosed diabetes mellitus. Med Arh. 64:345–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bukovsky A: Novel methods of treating
ovarian infertility in older and POF women, testicular infertility,
and other human functional diseases. Reprod Biol Endocrinol.
13:102015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhindsa S, Prabhakar S, Sethi M,
Bandyopadhyay A, Chaudhuri A and Dandona P: Frequent occurrence of
hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol
Metab. 89:5462–5468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pitteloud N, Hardin M, Dwyer AA, Valassi
E, Yialamas M, Elahi D and Hayes FJ: Increasing insulin resistance
is associated with a decrease in Leydig cell testosterone secretion
in men. J Clin Endocrinol Metab. 90:2636–2641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cregan SP, Dawson VL and Slack RS: Role of
AIF in caspase-dependent and caspase-independent cell death.
Oncogene. 23:2785–2796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayashi K, Kojima R and Ito M: Strain
differences in the diabetogenic activity of streptozotocin in mice.
Biol Pharm Bull. 29:1110–1119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cory S, Huang DCS and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song W, Liu MG, Zhang JB, Zhang JJ, Sun MM
and Yu QK: Mechanism of action of EBV, Bcl-2, p53, c-Myc and Rb in
non-Hodgkins lymphoma. Eur Rev Med Pharmacol Sci. 20:1093–1097.
2016.PubMed/NCBI
|
|
18
|
Brady HJ and Gil-Gómez G: Bax. The
pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol.
30:647–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu QL, Abel P, Foster CS and Lalani EN:
bcl-2: Role in epithelial differentiation and oncogenesis. Hum
Pathol. 27:102–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hikim AP Sinha, Lue Y, Diaz-Romero M, Yen
PH, Wang C and Swerdloff RS: Deciphering the pathways of germ cell
apoptosis in the testis. J Steroid Biochem Mol Biol. 85:175–182.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuchi T, Masuko K, Nishimune Y, Obinata
M and Matsui Y: Inhibition of testicular germ cell apoptosis and
differentiation in mice misexpressing Bcl-2 in spermatogonia.
Development. 122:1703–1709. 1996.PubMed/NCBI
|
|
22
|
Tapanainen JS, Tilly JL, Vihko KK and
Hsueh AJ: Hormonal control of apoptotic cell death in the testis:
Gonadotropins and androgens as testicular cell survival factors.
Mol Endocrinol. 7:643–650. 1993. View Article : Google Scholar : PubMed/NCBI
|