Introduction
Pancreatic cancer is one of the most malignant types
of gastrointestinal tract tumor with a 5-year survival rate of
<5% (1). In recent years, the
incidence of pancreatic cancer has been increasing. Early detection
of pancreatic cancer is difficult due to its nonspecific clinical
manifestations and therefore, a large proportion of patients are
diagnosed at the advanced stage beyond the time window for radical
surgery (2). Cancer antigen (CA)19-9
is the main tumor biomarker for diagnosing pancreatic cancer with a
reported sensitivity of 65% and a specificity of 78–94% (3,4).
Considering that CA19-9 cannot be used as an early detection marker
for pancreatic cancer and is also detected in benign
pancreatobiliary diseases, particularly chronic pancreatitis
(5), its specificity and
effectiveness may not be satisfactory. Thus, there is a requirement
for developing novel methodologies to detect pancreatic cancer at
different stages and make earlier diagnoses, which will ultimately
lead to a better prognosis for patients.
With the development of modern molecular biological
techniques, numerous novel and efficient detection methods have
been developed, including those for the detection of
tumor-associated genetic mutations, which is also a current focus
of research on pancreatic cancer. The K-ras gene is closely
associated with the development and progression of pancreatic
cancer. K-ras mutation is an important and early event in
tumorigenesis (6–8). K-ras is able to bind guanine
nucleotides within a growth factor signal transduction pathway,
while pathological mutations of K-ras lead to cell proliferation
(9). The detection of K-ras
mutations in biopsy specimens, pancreatic juice, bile and blood has
been previously reported (10,11).
Genetic mutations detected in biopsy specimens and pancreatic juice
may be a reliable method for diagnosing pancreatic cancer; however,
it is challenging to obtain adequate samples (12). Conversely, K-ras detection in blood
has a relatively low sensitivity of 66–71% and requires combination
with other examinations to increase the diagnostic rate (13–15).
Recent studies have demonstrated that it is possible to extract and
sequence fecal DNA (16,17). However, K-ras mutation has not been
investigated in pancreatic cancer by magnetic nanoprobe. The
present study introduced a nanoparticle trace capture probe, which
is widely applied in detecting trace genetic variants (18,19), to
detect K-ras mutations in the feces of patients with pancreatic
cancer at different stages and further explored the sensitivity and
specificity of the novel K-ras mutation detection method and the
existing CA19-9 examination method as well as their combination
regarding pancreatic cancer diagnosis.
Patients and methods
Patients
Patients with pancreatic diseases admitted to the
Department of Surgery, Jiaxing Second Hospital (Jiaxing, China)
from January 2013 to August 2015 were enrolled in the present
study, including patients with diagnoses of pancreatic cancer
(n=88), chronic pancreatitis (n=35), pancreatic mucinous cyst
(n=10) and pancreatic serous cyst (n=9). Thirty one healthy
individuals were also included as controls. Pancreatic tissue was
obtained from patients after surgical resection at the Department
of Surgery, Jiaxing Second Hospital. Tissue samples were obtained
at the time of resection, during analysis of frozen sections or
both, in accordance with the in-house protocol. Clinicopathological
[i.e., clinical manifestation, tumor location, CA19-9,
carcino-embryonic antigen (CEA)] and demographic data (i.e., age,
sex, histology and tumor stage) were collected and analyzed.
Informed consent was obtained from all the patients and the present
study was approved by the Ethics Committee of Jiaxing Second
Hospital.
DNA extraction from fecal
specimens
Fecal samples were stored at −80°C. DNA was
extracted by phenol-chloroform extraction from 200 mg feces and
purified using a Qiagen purification kit (Qiagen, Hilden, Germany).
DNA concentrations were measured using a ND-1000 NanoDrop (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Establishment of nanoparticle trace
capture probe system
A solution of 1 M NaOH (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 0.1 M salicylic acid (SA, Sigma-Aldrich;
Merck KGaA) with volume at 100 µl each was added to a sterilized
three-necked flask to increase the pH of the whole reaction
solution to ~11.0 under vigorous stirring in an argon gas
atmosphere, followed by the addition of an aqueous solution
comprising a mixture of Fe(III) and Fe(II) oxide salts
(Fe2O3 and Fe3O4; Merck
KGaA) with a molar ratio of 2X Fe(III)/1X Fe(II)/4X SA until a
black suspension was obtained. After refluxing at 90°C for 4 h, a
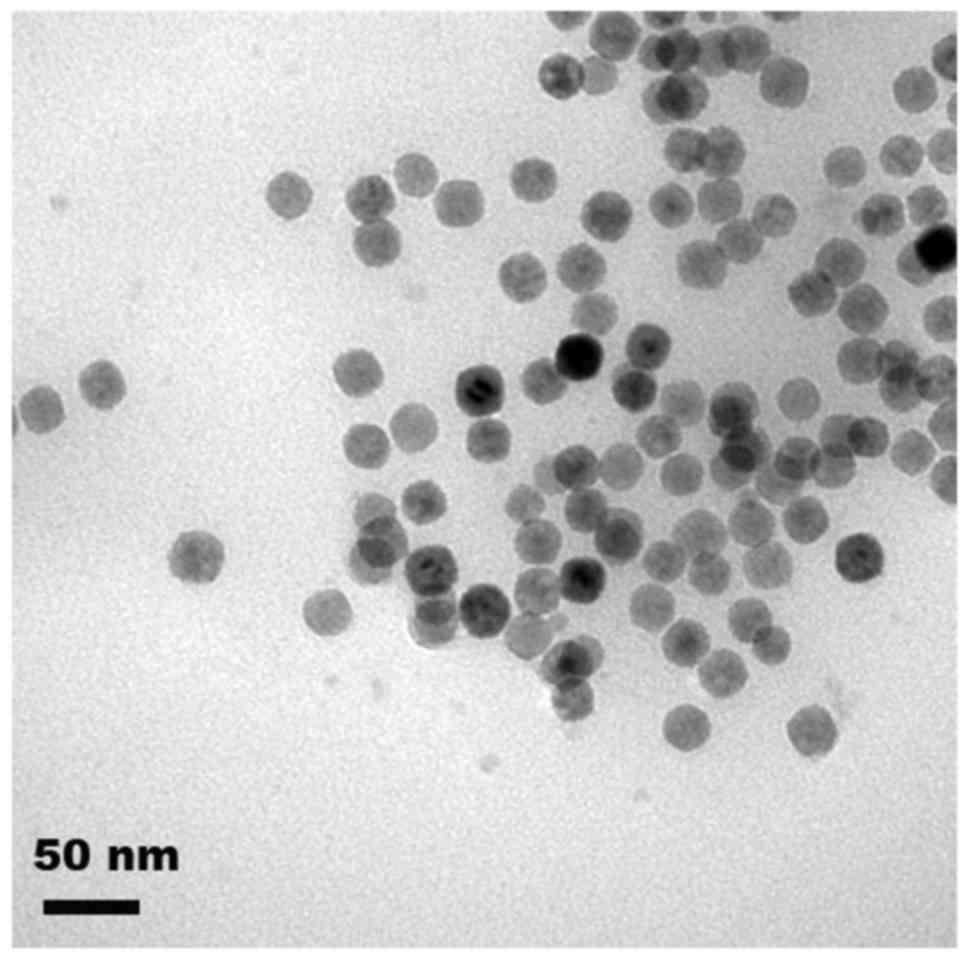
dark-brown suspension was formed. The morphology of the magnetic
nanoparticles was observed by transmission electron microscopy
(Fig. 1).
Detection of K-ras using magnetic nanoparticles. A
capture probe for K-ras (10 µM, Foxgene Co., Ltd., Wuxi, China) was
conjugated with 1 µM magnetic nanoparticles by
1-ethyl-3-(−3-dimethylaminopropyl) carbodiimide hydrochloride
chemistry (Sigma-Aldrich; Merck KGaA) and purified by a magnetic
field, followed by redispersion in Tris-EDTA buffer (20). PCR detection of K-Ras mutation system
contained the final concentrations of each upstream and downstream
primer, blocker (Shanghai Sangon, Shanghai, China) and capture
probe as 0.9, 0.9, 0.9 and 0.20 mmol/l, respectively. The assay was
set up as follows: A total of 20 ng DNA was used and the reaction
was performed in a final volume of 20 ml. Detection sensitivity and
specificity of K-ras (G12V or G13D) were estimated by using serial
dilutions of the corresponding mutant SW480 cell line DNA (Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) mixed with wild-type DNA (5, 1, 0.5 and 0.1% mutant DNA
solutions were prepared). The data were collected and analyzed
using ABI 7500 fast System SDS software v1.4.1 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). PCR data were analyzed with a
manual threshold of 0.1 and baseline from 5 to 15 to obtain cycle
threshold (Cq) values for FAM and VIC channels. The assay was
considered valid when the β-actin CT value was ≤20, the specific
mutant gene CT was 21–37 (w3 copies) and all No Template Controls
had an undetectable CT. PCR aliquots were also analyzed by 0.8%
agorose gel electrophoresis. Relative quantification was performed
using the comparative threshold cycle (2−ΔΔCq) method
(21). The qRT-PCR reaction was
performed at 50°C for 2 min and 95°C for 30 sec, followed by 40
cycles of denaturation at 95°C for 5 sec and annealing at 60°C for
45 sec. All reactions were performed in triplicate. The probe,
primers and blockers used were as follows: Kras capture probe,
5′-CTCTATTGTTGGATCATATTCGTCCACAAAATGATTCTGAATTA-3′; G12V forward
primer, 5′-ACTTGTGGTAGTTGGACCT-3′; G12V reverse primer,
5′-TAACTTGAAACCCAAGGTAC-3′; blocker, CCTACGCCACCAGCT (with 4
pentabases); G13D forward primer,
5′-GTTCTAATATAGTCACATTTTCATTATTTTTATTATAAAGC-3′; G13D reverse
primer, 5′-GTCAAGGCACTCTTGCCTAGG-3′; blocker, CTTGCCTACGCCACCA
(with 4 pentabases); β-actin forward, CTCCATCCTGGCCTCGCTGTβ-actin
reverse primer, GCTGTCACCTTCACCGTTCC.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (International Business Machines, Corp., Armonk, NY,
USA). Continuous and categorical data are shown as the mean ±
standard deviation or the percentage, which were compared by
Student's t-test and the χ2 test, respectively. The
correlation of K-ras and CA19-9 was analyzed by the χ2
test and the specificity, sensitivity, Youden's index (YI),
positive predictive value (PPV) and negative predictive value (NPV)
were also calculated. P<0.05 was considered to indicate a
statistically significant difference.
Results
K-ras mutation detection in fecal DNA
by nanoparticle capture probe has predictive value for pancreatic
cancer
DNA was successfully extracted and validated from
all fecal samples. G12V and G13D mutations in the K-ras gene were
detected using the magnetic s. The CT value was determined and
ranged from 41.71 to 62.61 in patients carrying G12V and/or G13D
mutations. Of the 88 patients with pancreatic cancer, K-ras
mutations were found in 72 (81.8%), including G12V (n=64) and G13D
(n=8) (Table I). The mutation rate
in samples from patients with pancreatic benign diseases was 18.5%
(10/54), including G12V (n=8) and G13D (n=2). Among the 10 cases
with K-ras mutations in the benign group, 7 cases were of chronic
pancreatitis, 2 had a pancreatic mucinous cyst neoplasm and 1 had a
pancreatic serous cyst. Among them, K-ras mutations were also
detected in 9 cases. No mutations were detected in the healthy
controls. Taken together, the K-ras mutation rate in pancreatic
cancer was significantly higher than in that in pancreatic
non-malignant diseases (P<0.05; Table
I).
 | Table I.Fecal K-ras mutations in pancreatic
cancer, pancreatic benign diseases and healthy control group. |
Table I.
Fecal K-ras mutations in pancreatic
cancer, pancreatic benign diseases and healthy control group.
| Group | Cases (n) | Cases carrying K-ras
mutations, n (%) | χ2 | P-value |
|---|
| Pancreatic
cancer | 88 | 72 (81.8) |
|
|
| Pancreatic benign
diseases | 54 | 10 (18.5) | 54.954 |
<0.001a |
| Healthy control | 31 | 0 (0) | 5.043 | 0.025a |
Detection of K-ras mutations in fecal
DNA has high sensitivity and specificity for pancreatic cancer
The sensitivity and specificity of K-ras mutations
in fecal samples for detection of pancreatic cancer was 81.8 and
81.5%, respectively (Table II).
Sixty-eight pancreatic cancer patients had >37 U/ml CA19-9 and
the sensitivity and specificity were 77.3 and 77.8%, respectively,
which were not significantly different from those of the K-ras
mutations (P>0.05; Table II).
Combined detection using fecal K-ras mutations and CA19-9 had a
sensitivity and specificity of 97.7 and 80.9%, respectively,
indicating that this combination significantly increased the
sensitivity of detection of pancreatic cancer to >95%
(P<0.05). The specificity was not enhanced compared with that of
detection by K-ras mutations or CA19-9 alone (P>0.05; Table II).
 | Table II.Fecal K-ras mutation and serum CA19-9
in the diagnosis of pancreatic cancer. |
Table II.
Fecal K-ras mutation and serum CA19-9
in the diagnosis of pancreatic cancer.
| Parameter | Sensitivity (%) | χ2 | P-value | Specificity (%) | χ2 | P-value | YI | PPV (%) | NPV (%) |
|---|
| Serum CA19-9 | 77.3 | – | – | 77.8 | – | – | 0.551 | 85.0 | 67.7 |
| Fecal K-ras | 81.8 | 0.28 | 0.597 | 81.5 | 0.06 | 0.806 | 0.633 | 87.8 | 73.3 |
| K-ras+CA19-9 | 97.7 | 16.06 | <0.001 | 80.9 | 0.68 | 0.513 | 0.786 | 86.1 | 94.4 |
K-ras mutations were not significantly correlated
with any of the clinical features assessed, including age, sex,
clinical manifestations, tumor size, CA19-9 and CEA level, and
tumor-nodes-metastasis stage of pancreatic cancer (Table III). The K-ras mutation rate was
comparable in patients with I+IIA and IIB + III + IV pancreatic
cancer (78.9 vs. 84.0%; P>0.05).
 | Table III.Association of the presence of fecal
K-ras mutations with certain biomarkers for pancreatic cancer. |
Table III.
Association of the presence of fecal
K-ras mutations with certain biomarkers for pancreatic cancer.
|
| Fecal K-ras point
mutations |
|
|---|
|
|
|
|
|---|
| Variable | Yes | No | P-value |
|---|
| Sex |
|
| 0.367 |
| Male | 34 (73.9) | 12 (26.1) |
|
|
Female | 36 (81.8) | 8 (18.2) |
|
| Age (years) | 67.00±10.63 | 69.25±11.33 | 0.451 |
| Clinical
manifestations |
|
| 0.152 |
| Yes | 40 (76.9) | 12 (23.1) |
|
| No | 32 (88.9) | 4 (11.1) |
|
| Location |
|
| 0.396 |
|
Pancreatic head and neck | 46 (79.3) | 12 (20.7) |
|
|
Pancreatic body and tail | 26 (86.7) | 4 (13.3) |
|
| Tumor
diameter (cm) | 4.08±1.22 | 3.50±0.73 | 0.07 |
| Differentiation |
|
| 0.152 |
|
Well | 40 (76.9) | 12 (23.1) |
|
|
Poor | 32 (88.9) | 4 (11.1) |
|
| CA19-9 level
(U/l) |
|
| 0.454 |
|
≥37 | 54 (79.4) | 14 (20.6) |
|
|
<37 | 18 (90.0) | 2 (10.0) |
|
| CEA level
(U/l) |
|
| 0.517 |
| ≥5 | 24 (85.7) | 4 (14.3) |
|
|
<5 | 48 (80.0) | 12 (20.0) |
|
| TNM stage |
|
| 0.543 |
|
I+IIA | 30 (78.9) | 8 (21.1) |
|
|
IIB+III+IV | 42 (84.0) | 8 (16) |
|
Discussion
Pancreatic cancer has a low early detection and
survival rate, and radical resection is the only treatment
available with curative potential. Radical surgery for a pancreatic
cancer sized ≤2 cm has been reported to increase the 5-year
survival rate to 19–52.9% (22,23). The
early diagnosis of pancreatic cancer remains challenging and it is
vital that more sensitive and specific methods are developed to
detect pancreatic cancer. The present study investigated the
feasibility and efficacy of magnetic nanoprobes to detect K-ras
mutations in fecal samples from patients with pancreatic cancer at
various stages. Magnetic nanoparticles were used to extract DNA
from the fecal samples and the probe was able to specifically
capture the point mutation. The results indicated that this
detection was sensitive, reliable, repeatable and cheap.
The novel K-ras mutation detection method used in
the present study had greater sensitivity and specificity than the
previous CA19-9 method. The mutation rate of the K-ras gene in
pancreatic cancer was 81.8%, which is higher than that reported by
previous studies (24,25). Fecal DNA from pancreatic cancer
patients had a higher mutation rate than that of patients with
pancreatic benign diseases and healthy controls (P<0.05). The
nanoparticle capture probe is intended to detect trace DNA content,
which means that smaller clinical samples are required. The present
study also found that the K-ras mutation rate in fecal DNA from
patients with early pancreatic cancer (n=19) was comparable with
that in samples from advanced pancreatic cancer patients (n=25;
78.9 vs. 84.0%; P>0.05). These results supported that K-ras
mutations participate in the initiation of pancreatic cancer, which
is consistent with previous findings. For instance, Wilentz et
al (26) reported that 75–100%
of pancreatic cancer patients had a mutation in K-ras codon 12,
suggesting that K-ras detection may be used as an early detection
marker for pancreatic cancer. In addition, K-ras mutation increased
the risk of pancreatic cancer in patients with chronic pancreatitis
(27), while K-ras mutations were
also observed in certain benign pancreatic diseases (28). In the patient cohort of the present
study, a certain percentage of cases with pancreatic benign
diseases had fecal K-ras mutations. Whether K-ras mutations are an
independent risk factor for pancreatic cancer should be assessed in
future studies.
CA19-9 >37 U/ml is widely acknowledged as an
important serum biomarker for pancreatic cancer (29), but its sensitivity and specificity
are only 70–80%. In the present study, the diagnostic value of
K-ras mutations in fecal samples and serum CA19-9 levels were
compared. The sensitivity and specificity of K-ras mutations for
pancreatic cancer detection were higher than those of CA19-9
(sensitivity, 81.8 vs. 77.3%; specificity, 81.5 vs. 77.8%), but the
differences were not statistically different. Of note, the use of
the two diagnostic markers K-ras and CA19-9 in combination had a
significantly increased sensitivity (97.7%; P<0.05), and
therefore represents a promising early detection method for
pancreatic cancer. Although fecal K-ras mutation detection is of
particular translational significance, its false-negative rate,
which may result from the degradation of DNA, high content of
bilirubin and limited detached tumor cells in feces, should not be
neglected. These issues may be prevented by modifying the fecal DNA
extraction method and multi-genetic multi-locus detection.
In summary, the novel magnetic nanoprobe system used
in the present study was able to detect K-ras mutations in fecal
samples of patients with pancreatic cancer with higher prevalence
than that in samples from patients with benign pancreatic diseases
and healthy controls. Based on these results, fecal K-ras mutation
detection may be recommended for screening high-risk populations,
and its combination with CA19-9 may improve the early detection
rate in pancreatic cancer. The method has the advantage of being
non-invasive. The present study might serve as a pilot analysis of
the clinical significance of fecal K-ras mutation detection in
pancreatic cancer, and its diagnostic potential still requires to
be comprehensively validated in larger cohorts prior to being
introduced into clinical practice. In conclusion, detecting K-ras
mutation in fecal matter by novel magnetic nanoprobe may be used as
a potential tumor marker for diagnosing patients with pancreatic
carcinoma in the future.
Acknowledgements
The present study was supported by NSFC (grant no.
81371682), awarded to Professor Lifeng Qi and the Zhejiang
Provincial Science and Technology Department (grant no.
2014C33139), awarded to Dr Zhengxiang Zhong.
References
|
1
|
Sergeant G, Vankelecom H, Gremeaux L and
Topal B: Role of cancer stem cells in pancreatic ductal
adenocarcinoma. Nat Rev Clin Oncol. 6:580–586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joergensen MT, Brünner N and DeMuckadell
OB: Comparison of circulating MMP-9, TIMP-1 and CA19-9 in the
detection of pancreatic cancer. Anticancer Res. 30:587–592.
2010.PubMed/NCBI
|
|
4
|
Rosty C and Goggins M: Early detection of
pancreatic carcinoma. Hematol Oncol Clin North Am. 16:37–52. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe H, Kawakami H, Yamakawa O,
Satomura Y, Ohta H, Motoo Y, Okai T and Sawabu N: Clinical
usefulness of tumor markers associated with pancreatic cancer.
Rinsho Byori. 42:127–138. 1994.(In Japanese). PubMed/NCBI
|
|
6
|
Huang C, Wang WM, Gong JP and Yang K:
Oncogenesis and the clinical significance of K-ras in pancreatic
adenocarcinoma. Asian Pac J Cancer Prev. 14:2699–2701. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minamoto T: Detection and characterization
of oncogene mutations in preneoplastic and early neoplastic
lesions. Methods Mol Biol. 1105:381–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dabritz J, Preston R, Hänfler J and Oettle
H: Follow-up study of K-ras mutations in the plasma of patients
with pancreatic cancer: Correlation with clinical features and
carbohydrate antigen 19-9. Pancreas. 38:534–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy MJ, Sturgeon C, Lamerz R, Haglund C,
Holubec VL, Klapdor R, Nicolini A, Topolcan O and Heinemann V:
Tumor markers in pancreatic cancer: A European Group on Tumor
Markers (EGTM) status report. Ann Oncol. 21:441–447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin SH, Kim SC, Hong SM, Kim YH, Song KB,
Park KM and Lee YJ: Genetic alterations of K-ras, p53, c-erbB-2,
and DPC4 in pancreatic ductal adenocarcinoma and their correlation
with patient survival. Pancreas. 42:216–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh N, Gupta S, Pandey RM, Chauhan SS
and Saraya A: High levels of cell-free circulating nucleic acids in
pancreatic cancer are associated with vascular encasement,
metastasis and poor survival. Cancer Invest. 33:78–85. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuccio L, Hassan C, Laterza L, Correale L,
Pagano N, Bocus P, Fabbri C, Maimone A, Cennamo V, Repici A, et al:
The role of K-ras gene mutation analysis in EUS-guided FNA cytology
specimens for the differential diagnosis of pancreatic solid
masses: A meta-analysis of prospective studies. Gastrointest
Endosc. 78:596–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teich N and Mossner J: Molecular analysis
of pancreatic juice: A helpful tool to differentiate benign and
malignant pancreatic tumors? Dig Dis. 22:235–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Däbritz J, Preston R, Hänfler J and Oettle
H: Follow-up study of K-ras mutations in the plasma of patients
with pancreatic cancer: Correlation with clinical features and
carbohydrate antigen 19-9. Pancreas. 38:534–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu J, Wang D, Huang Y, Lu Y and Peng C:
Diagnostic value of combining CA 19-9 and K-ras gene mutation in
pancreatic carcinoma: A meta-analysis. Int J Clin Exp Med.
7:3225–3234. 2014.PubMed/NCBI
|
|
16
|
Loktionov A, O'Neill IK, Silvester KR,
Cummings JH, Middleton SJ and Miller R: Quantitation of DNA from
exfoliated colonocytes isolated from human stool surface as a novel
noninvasive screening test for colorectal cancer. Clin Cancer Res.
4:337–342. 1998.PubMed/NCBI
|
|
17
|
Klaassen CH, Jeunink MA, Prinsen CF, Ruers
TJ, Tan AC, Strobbe LJ and Thunnissen FB: Quantification of human
DNA in feces as a diagnostic test for the presence of colorectal
cancer. Clin Chem. 49:1185–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi L, Wu L, Zheng S, Wang Y, Fu H and Cui
D: Cell-penetrating magnetic nanoparticles for highly efficient
delivery and intracellular imaging of siRNA. Biomacromolecules.
13:2723–2730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi L and Gao X: Quantum dot-amphipol
nanocomplex for intracellular delivery and real-time imaging of
siRNA. ACS Nano. 2:1403–1410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan H, Zhang L and Zhang Y: Preparation
of high efficiency and low carry-over immobilized enzymatic reactor
with methacrylic acid-silica hybrid monolith as matrix for on-line
protein digestion. J Chromatogr A. 1371:48–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schnelldorfer T, Ware AL, Sarr MG, Smyrk
TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM and
Farnell MB: Long-term survival after pancreatoduodenectomy for
pancreatic adenocarcinoma: Is cure possible? Ann Surg. 247:456–462.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benassai G, Mastrorilli M, Quarto G,
Cappiello A, Giani U and Mosella G: Survival after
pancreaticoduodenectomy for ductal adenocarcinoma of the head of
the pancreas. Chir Ital. 52:263–270. 2000.PubMed/NCBI
|
|
24
|
Haug U, Wente MN, Seiler CM, Jesenofsky R
and Brenner H: Stool testing for the early detection of pancreatic
cancer: Rationale and current evidence. Expert Rev Mol Diagn.
8:753–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Xu T, Qian J, Wen X and Wu D:
Detecting K-ras and p53 gene mutation from stool and pancreatic
juice for diagnosis of early pancreatic cancer. Chin Med J (Engl).
115:1632–1636. 2002.PubMed/NCBI
|
|
26
|
Wilentz RE, Chung CH, Sturm PD, Musler A,
Sohn TA, Offerhaus GJ, Yeo CJ, Hruban RH and Slebos RJ: K-ras
mutations in the duodenal fluid of patients with pancreatic
carcinoma. Cancer. 82:96–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arvanitakis M, Van Laethem JL, Parma J, De
Maertelaer V, Delhaye M and Devière J: Predictive factors for
pancreatic cancer in patients with chronic pancreatitis in
association with K-ras gene mutation. Endoscopy. 36:535–542. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pugliese V, Pujic N, Saccomanno S,
Gatteschi B, Pera C, Aste H, Ferrara GB and Nicolò G: Pancreatic
intraductal sampling during ERCP in patients with chronic
pancreatitis and pancreatic cancer: Cytologic studies and k-ras-2
codon 12 molecular analysis in 47 cases. Gastrointest Endosc.
54:595–599. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ballehaninna UK and Chamberlain RS: Serum
CA 19-9 as a biomarker for pancreatic cancer-A comprehensive
review. Indian J Surg Oncol. 2:88–100. 2011. View Article : Google Scholar : PubMed/NCBI
|