Introduction
Hypertension is a common, highly prevalent
hemodynamic syndrome characterized by persistently elevated blood
pressure (1). Hypertension may cause
harmful complications in the cardiovascular system and has been
characterized as an independent risk factor for numerous
cardiovascular diseases (2). Current
antihypertensive medications include calcium channel blockers,
phosphodiesterase inhibitors, and nitric oxide (NO), prostaglandin
and endothelial receptor antagonists (3). However, side-effects like affecting the
function of kidney and liver and the occurrence of drug-resistance
after long-term medication have hindered these treatment to achieve
satisfactory therapeutic effects (3), so there is an urgent requirement for
the identification of effective treatments for the management of
hypertension.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that are able to negatively regulate gene expression via binding to
the 3′-untranslated region (UTR) of target mRNAs. Previous studies
have indicated that miRNAs participate in numerous cellular and
molecular events, and the roles served by miRNAs in the
pathogenesis of several diseases have been reported (4,5). miR-145
has been demonstrated to function as a key regulator in the
development of the cardiovascular system. miR-145 is essential for
the differentiation (6) and
phenotype switching (7,8) of vascular smooth muscle cells (VSMCs),
and the upregulation of miR-145 in endothelial cells in response to
shear stress may determine the VSMC phenotype (9). It has also been observed that miR-145
was aberrantly expressed in the serum of patients with coronary
artery disease (10) and acute
myocardial infarction (11),
suggesting that miR-145 is a potential biomarker for the diagnosis
of cardiovascular diseases. Furthermore, several studies have
indicated that the abnormal expression of miR-145 may be associated
with the pathogenesis of cardiovascular diseases, including
pulmonary arterial hypertension (12), myocardial infarction (13) and atherosclerosis (14). The association between miR-145 and
hypertension has also been discussed previously (15); however, further investigation is
required to determine the underlying molecular mechanisms of this
association.
NO is considered to be a long-term regulator of
arterial pressure (16). Arginine is
a rate-limiting substrate of endothelial NO synthase (eNOS), an
enzyme that catalyzes the production of NO in the vascular
endothelium (17–19). Solute carrier family 7 member 1
(SLC7A1) is an amino acid transporter that regulates arginine
metabolism (20). Previous studies
have indicated that an extracellular deficiency of SLC7A1 has been
demonstrated to be associated with reduced endothelial function and
NO production (21), which may lead
to hypertension.
The present study aimed to investigate the role of
miR-145 and SLC7A1 in endothelial cells and a rat model of
spontaneous hypertension. It was hypothesized that miR-145 serves
an important role in the pathogenesis of hypertension via targeting
SLC7A1.
Materials and methods
Animals
A total of 10 26-week-old male spontaneously
hypertensive rats (SHRs; weight, 400±20 g) were used in the present
study, and 10 age-matched normotensive male Wistar-Kyoto rats
(WKYs) served as the control group. All animals were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). All rats were housed in cages and kept under
standard laboratory conditions (12-h light/dark cycle; temperature
of 22±2°C; relative humidity of 55±5% and access to food and tap
water ad libitum). Rats were habituated for 7 days before
the experiment. Animal experiments were performed according to the
Guide for the Care and Use of Laboratory Animals (Ministry of
Health, Beijing, China). The present study was approved by the
Animal Care Committee of Henan College of Traditional Chinese
Medicine (Zhengzhou, China). The systolic and diastolic blood
pressure of the rats was measured using the tail-cuff method every
week for 4 weeks. On day 28 the rats were sacrificed, and the
thoracic aortas were collected and stored at −80°C until
required.
Cell culture
Rat vascular endothelial cells (RVECs) were isolated
from the thoracic aortas of the WKY rats as previously described
(22). Cells were cultured at 37°C
in an atmosphere containing 5% CO2 in RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.).
Cell transfection
The miR-145 inhibitor (sequence,
5′-GUCCAGUUUUCCCAGGAAUCCCU-3′), mimic (sequence,
5′-GGAUUCCUGGGAAAACUGGACUU-3′) and negative control (sequence,
5′-AGGUAGUGUAAUCGCCUUGTT-3′) were purchased from GenePharma
(Shanghai, China), and cell transfection was performed using
Lipofectamine® RNAiMAX (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. Cells were harvested 48 h
after transfection for analysis.
NO measurement
The level of NO in the thoracic aortas of the rats
was determined using an NO assay kit (S0021; Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol.
Dual-luciferase reporter assay
The SLC7A1 3′-UTR complementary DNA fragment
containing the putative binding sites for miR-145 and a mutated
3′-UTR of SLC7A1 were amplified and subcloned into a
pGL3-luciferase promoter vector (Promega Corporation, Madison, WI,
USA). RVECs were seeded into 6-well plates at a density of
1×105 cells/well, and were cotransfected with the
intended pGL3-luciferase promoter vector and an miR-145 mimic or
inhibitor. Following culture for 24 h at room temperature, the
cells were harvested and lysed using radioimmunoprecipitation assay
buffer (cat. no. P0013E; Beyotime Institute of Biotechnology), and
the relative activity of luciferase was determined using a
dual-luciferase reporter assay kit (cat. no. RG028; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. The relative Renilla luciferase activities were
normalized to firefly luciferase activities, which was used as an
internal control for transfection efficiency.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to extract total RNA according to the manufacturer's protocol.
RT-qPCR was performed using the One Step SYBR®
PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China) on an Applied Biosystems 7500 Real-Time PCR system
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The thermocycling conditions were as follows: 95°C for 30
sec; and cycles of 95°C for 5 sec and 60°C for 30 sec. Primers were
synthesized by Genscript Nanjing, Inc. (Nanjing, China), and the
sequences were as follows: GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′; miR-145 forward,
5′-AAGGGAGTCCAGTTTTCCCAGGAATCC-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGAC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
and SLC7A1 forward, 5′-CTGGAGTGCGACTTTTGACG-3′ and reverse,
5′-TGTTGACCATGGCTGACTCC-3′. The relative expression of SLC7A1 and
miR-154 was analyzed using the 2−ΔΔCq method (23) with GAPDH and U6 as the internal
references, respectively.
Western blotting
RVECs were lysed using radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Total protein concentration in the
supernatant was measured using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. A total of 25 µg of protein samples were
separated by SDS-PAGE on an 8% gel and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked at 4°C using 5% non-fat milk
for 1 h and incubated at 4°C overnight with the following primary
antibodies: Anti-SLC7A1 (1:2,000; 14195-1-AP; Proteintech Group,
Inc., Chicago, IL, USA), anti-phosphorylated (p)-eNOS (1:1,000;
ab199956; Abcam, Cambridge, MA, USA) and anti-β-actin (1:2,000;
ab3280; Abcam). The membranes were subsequently washed and
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:10,000; ab131368; Abcam) for 1 h at room temperature.
The protein bands were then visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology) on a
ChemiDoc™ XRS+imaging system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) according to the manufacturer's
protocol.
Bioinformatics
Bioinformatics analysis was performed to predict the
target of miR-145. Targetscan (http://www.targetscan.org/) and miRWalk websites
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html)
were used.
Statistical analysis
Data are presented as the mean ± standard deviation.
The statistical significance of differences between groups were
analyzed with SPSS software (version 17.0; SPSS, Inc., Chicago, IL,
USA) using one-way analysis of variance and least significant
difference post hoc test. All tests were two-tailed and P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-145 is upregulated in the thoracic
aorta of SHRs
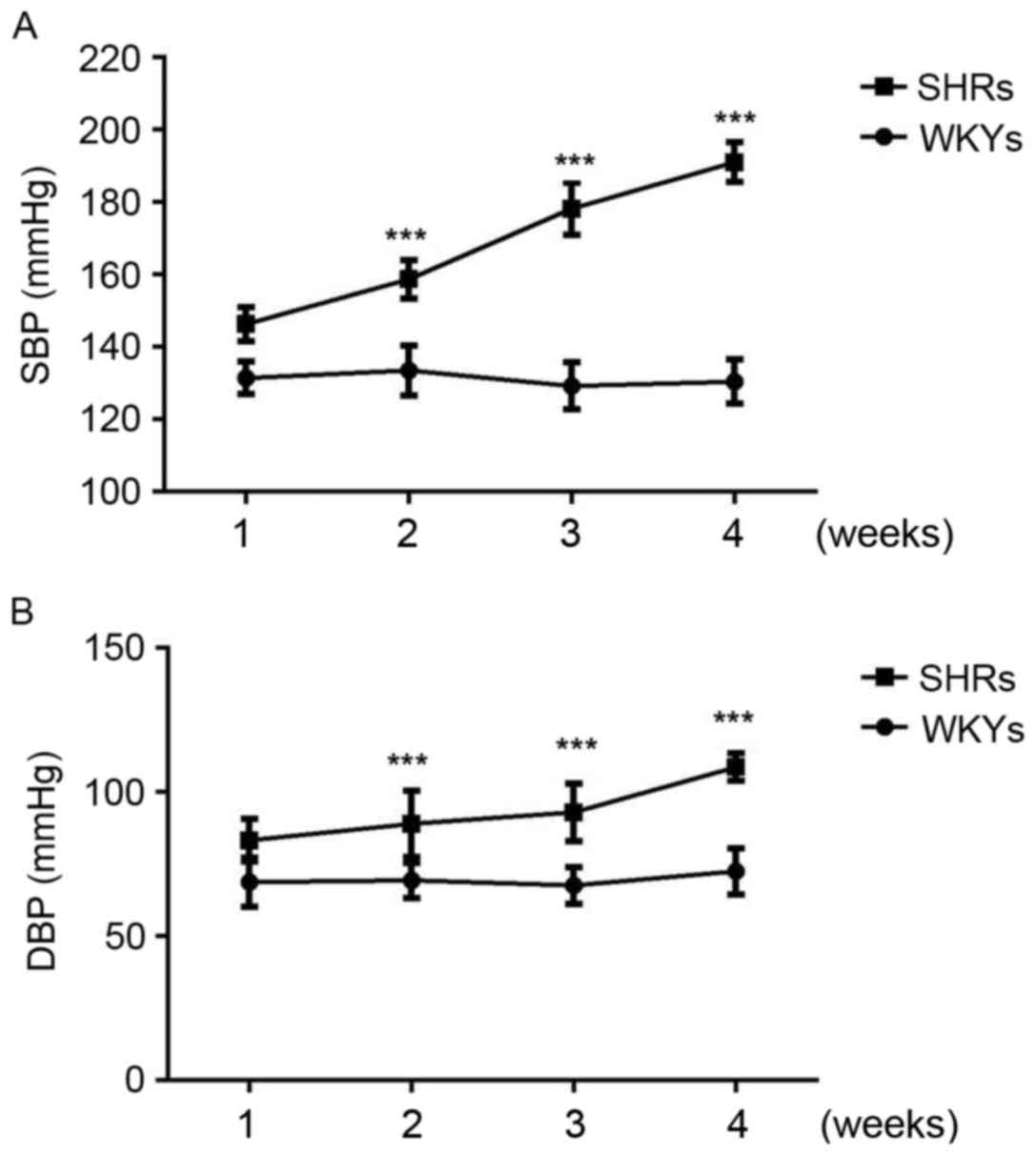
As expected, systolic and diastolic blood pressure
was significantly increased in SHRs compared with WKYs at 2, 3 and
4 weeks (P<0.001; Fig. 1).
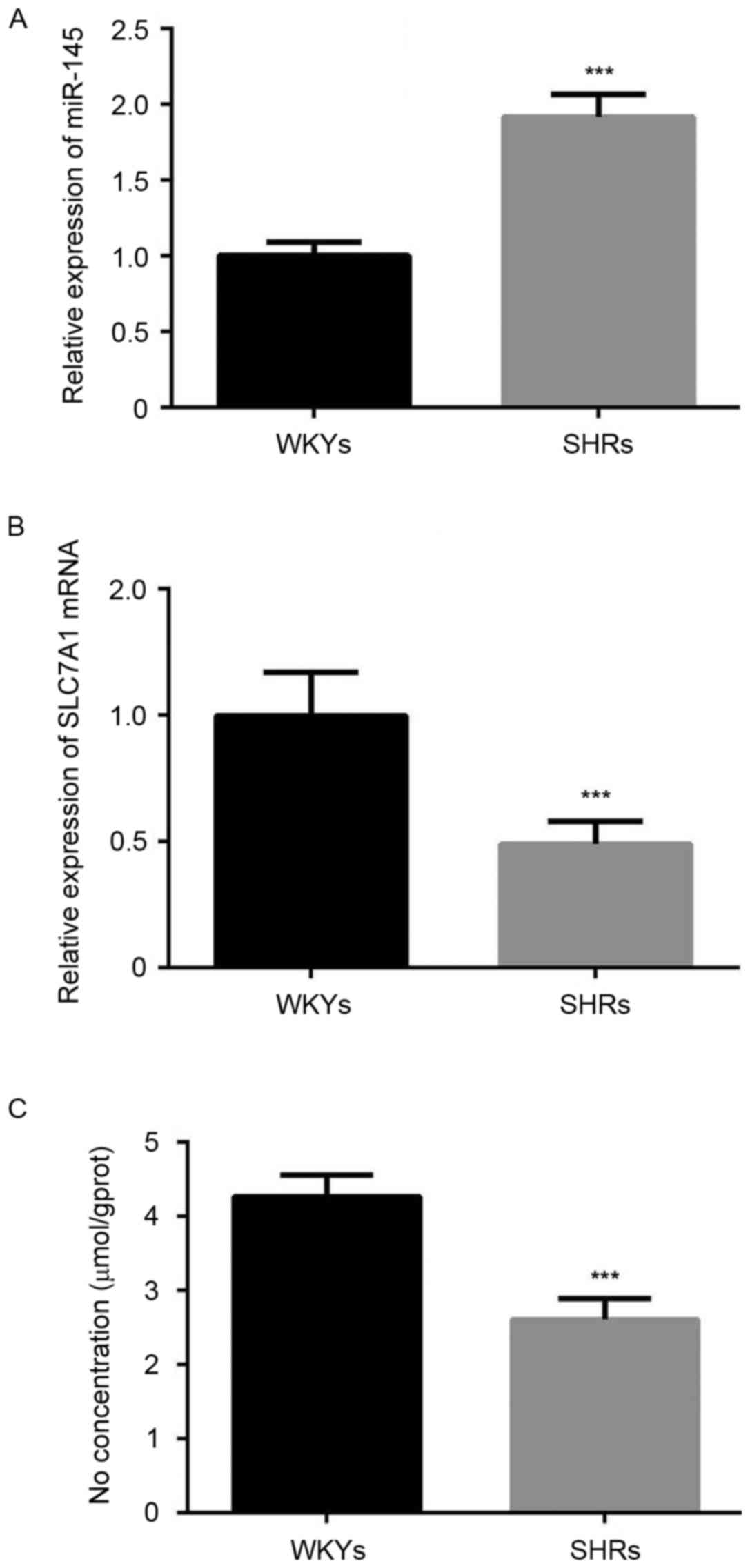
Furthermore, the results of RT-qPCR analysis demonstrated that
miR-145 was significantly overexpressed in the thoracic aorta of
SHRs compared with WKYs (P<0.001; Fig. 2A). A significant decrease in the
expression of SLC7A1 (P<0.001; Fig.
2B), and the content of NO (P<0.001; Fig. 2C) was also observed in the thoracic
aorta of SHRs compared with WKYs.
Silencing miR-145 increases the
expression of SLC7A1 in RVECs
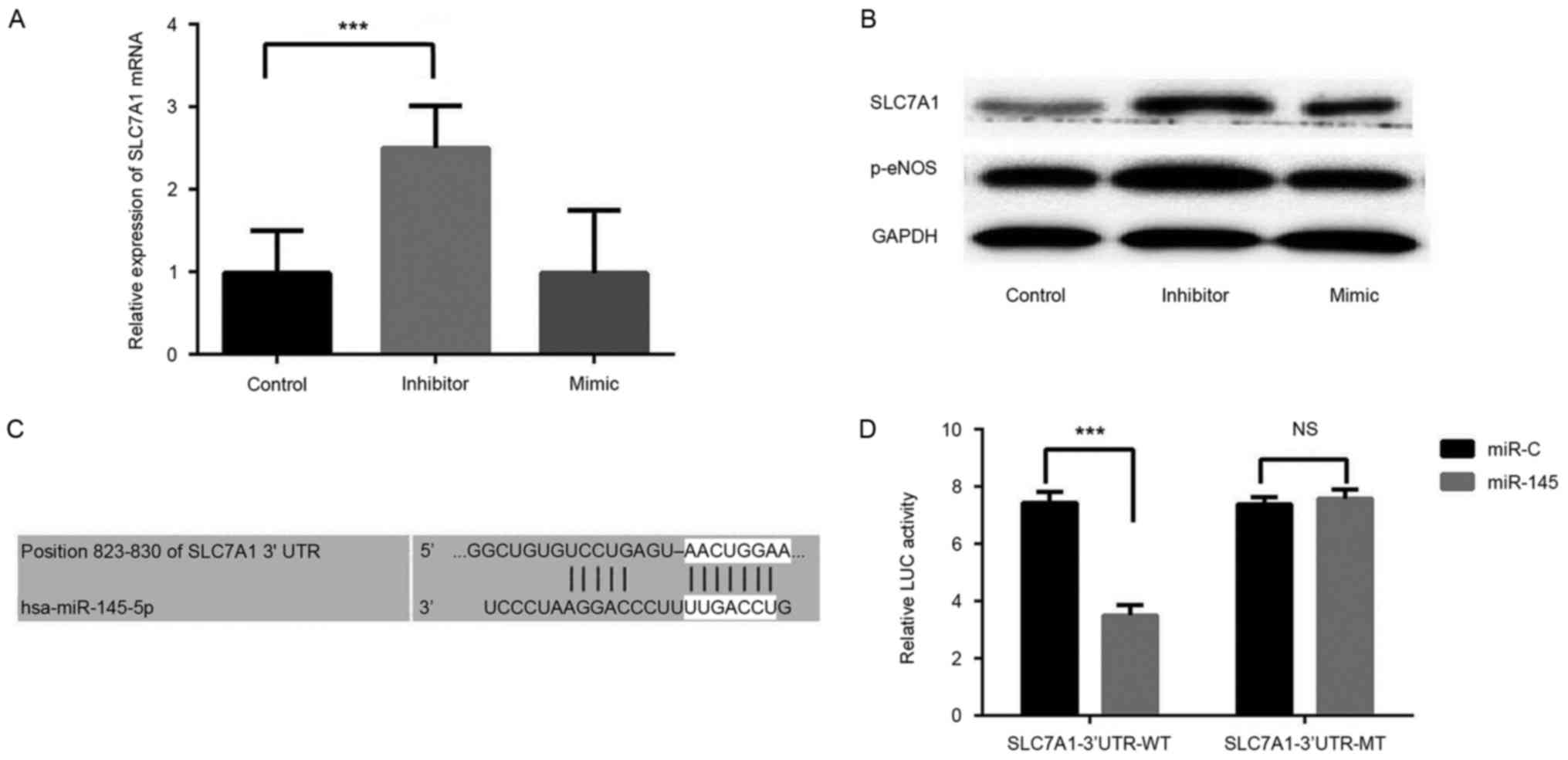
RVECs were transfected with a miR-145 inhibitor or
mimic, and RT-qPCR and western blot analyses were performed to
determine the role of miR-145 in RVECs. A significant increase in
SLC7A1 expression was observed in RVECs transfected with the
miR-145 inhibitor compared with control group (P<0.001), whereas
transfection with the miR-145 mimic did not significantly affect
SLC7A1 expression (Fig. 3A).
Silencing miR-145 also induced a marked increase in the expression
of p-eNOS (Fig. 3B), an enzyme that
controls the production of NO in the vascular endothelium.
SLC7A1 is a direct target of
miR-145
The results of the present study indicated that the
expression of miR-145 negatively affects the expression of SLC7A1
in the thoracic aorta of rats, thus a dual-luciferase reporter
assay was performed to confirm that SLC7A1 is a target gene of
miR-145. We performed bioinformatics analysis in Targetscan and
miRWalk websites and revealed that SLC7A1 is a predicted target of
miR-145 (Fig. 3C). The results of
the dual-luciferase reporter assay demonstrated that miR-145 mimic
transfection significantly decreased the luciferase activity of
pGL3-wild type SLC7A1 3′-UTR-transfected RVECs (P<0.001 vs.
miR-145 mimic group). miR-145 mimic transfection had no significant
effect on pGL3-mutated SLC7A1 3′-UTR luciferase activity (Fig. 3D). These data indicate that miR-145
directly targets SLC7A1 to negatively regulate the expression of
SLC7A1 in vitro.
Discussion
In recent years, increasing evidence has indicated
that miRNAs serve important roles in the pathogenesis of
hypertension. miR-21 has been demonstrated to reduce blood pressure
and alleviate cardiac hypertrophy in SHR models (24), and miR-27a had been identified to be
downregulated in the aortas of SHRs compared with WKYs (25). In the serum of patients with
hypertension, the expression of miR-150 and miR-192 was identified
to be significantly downregulated, and the levels of miR-130a, −195
and −92a were markedly upregulated (26). Furthermore, serum levels of miR-130a
and miR-195 have been reported to be positively correlated with
blood pressure (26). The role of
miR-145 in hypertension has also been reported; Santovito et
al (27) examined the expression
of miR-145 in the atherosclerotic plaques of patients with and
without essential hypertension, and identified that miR-145 was
overexpressed in patients with hypertension. Sala et al
(14) investigated a miR-143/miR-145
knockout mouse model and observed that these mice had thinner
arteries with reduced vascular tone and smooth muscle layer width,
which led to a significantly lower blood pressure. These results
suggest that miR-143 and miR-145 serve key roles in regulating VSMC
function and blood pressure. In the present study, it was
demonstrated that miR-145 was significantly upregulated in the
thoracic aorta of SHR compared with WKY rats, which was consistent
with the observations of previous studies in humans and rats.
However, the expression of miR-145 in the peripheral blood of
patients with hypertension may have different patterns to that
observed in tissue samples. Kontaraki et al (28) observed a decreased expression of
miR-145 in the peripheral blood mononuclear cells of patients with
essential hypertension compared with healthy volunteers, and that
the mean 24 h diastolic blood pressure was negatively correlated
with the expression of miR-145 in these cells. These results
indicate that the expression of miR-145 is inconsistent between the
blood and blood vessels of patients and rats with hypertension.
Certain target genes of miR-145 have been identified
and demonstrated to serve important regulatory roles in a number of
cellular processes in previous studies. In colorectal cancer,
miR-145 has been identified to suppress the migration and invasion
of cancerous cells via targeting erythroblast
transformation-specific-related gene (29). In addition, in nasopharyngeal
carcinoma miR-145 has been demonstrated to directly target
disintegrin and metalloprotease domain-containing protein 17, and
inhibit cancer cell invasion and migration (30). Furthermore, in osteogenesis miR-145
suppresses osteogenic differentiation by targeting transcription
factor Sp7 (31). In
isoproterenol-induced cardiomyocyte hypertrophy, miR-145 has been
reported to serve a protective role through targeting transcription
factor GATA-6 (32). In the present
study, it was observed that the expression of miR-145 was
significantly increased in the thoracic aorta of SHRs, whereas the
expression of SLC7A1 was significantly downregulated. Silencing of
miR-145 in RVECs induced a significant increase in SLC7A1
expression, and a dual-luciferase reporter assay confirmed that
SLC7A1 is a direct target of miR-145. These data indicate that
miR-145 serves an important role in the pathogenesis of
hypertension via targeting SLC7A1, an L-arginine transporter that
affects the production of NO and the function of endothelial cells
(33).
NO serves a key role in the cardiovascular system,
including dilating blood vessels to relieve hypertension. In the
present study, the production of NO was identified to be decreased
in the thoracic aorta of the SHR group compared with the control
group. Silencing miR-145 was demonstrated to increase the
expression of p-eNOS in the SHRs. These results indicate that
miR-145 negatively regulates the production of NO through targeting
SLC7A1.
In conclusion, the results of the present study
indicate that miR-145 participates in the pathogenesis of
hypertension via targeting SLC7A1 in vitro and in
vivo. Thus, the results of the current study provide novel
evidence that miR-145 has a potential application as a therapeutic
target for the treatment of hypertension.
References
|
1
|
Munroe PB, Barnes MR and Caulfield MJ:
Advances in blood pressure genomics. Circ Res. 112:1365–1379. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lackland DT and Weber MA: Global burden of
cardiovascular disease and stroke: Hypertension at the core. Can J
Cardiol. 31:569–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tykarski A, Narkiewicz K, Gacing Z,
Januszewicz A, Litwin M, Kostka-Jeziorny K, Adamczak M,
Szczepaniak-Chicheł L, Chrostowska M, Czarnecka D, et al: 2015
Guidelines for the management of hypertension. recommendations of
the polish society of hypertension-short version. Kardiol Pol.
73:676–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harries LW: Long non-coding RNAs and human
disease. Biochem Soc Trans. 40:902–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun
L, Davis K, Weise RD and Li RK: Role of miR-145 in cardiac
myofibroblast differentiation. J Mol Cell Cardiol. 66:94–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rangrez AY, Massy ZA, Metzinger-Le Meuth V
and Metzinger L: miR-143 and miR-145: Molecular keys to switch the
phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet.
4:197–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YN, Xie BD, Sun L, Chen W, Jiang SL,
Liu W, Bian F, Tian H and Li RK: Phenotypic switching of vascular
smooth muscle cells in the ‘normal region’ of aorta from
atherosclerosis patients is regulated by miR-145. J Cell Mol Med.
20:1049–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hergenreider E, Heydt S, Treguer K,
Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS,
Yin X and Mayr M: Atheroprotective communication between
endothelial cells and smooth muscle cells through miRNAs. Nat Cell
Biol. 14:249–256. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehta R, Otgonsuren M, Younoszai Z, Allawi
H, Raybuck B and Younossi Z: Circulating miRNA in patients with
non-alcoholic fatty liver disease and coronary artery disease. BMJ
Open Gastroenterol. 3:e0000962016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meder B, Keller A, Vogel B, Haas J,
Sedaghat-Hamedani F, Kayvanpour E, Just S, Borries A, Rudloff J,
Leidinger P, et al: MicroRNA signatures in total peripheral blood
as novel biomarkers for acute myocardial infarction. Basic Res
Cardiol. 106:13–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caruso P, Dempsie Y, Stevens HC, McDonald
RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al:
A role for miR-145 in pulmonary arterial hypertension: Evidence
from mouse models and patient samples. Circ Res. 111:290–300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higashi K, Yamada Y, Minatoguchi S, Baba
S, Iwasa M, Kanamori H, Kawasaki M, Nishigaki K, Takemura G,
Kumazaki M, et al: MicroRNA-145 repairs infarcted myocardium by
accelerating cardiomyocyte autophagy. Am J Physiol Heart Circ
Physiol. 309:H1813–H1826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sala F, Aranda JF, Rotllan N, Ramírez CM,
Aryal B, Elia L, Condorelli G, Catapano AL, Fernández-Hernando C
and Norata GD: MiR-143/145 deficiency attenuates the progression of
atherosclerosis in Ldlr-/-mice. Thromb Haemost. 112:796–802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xin M, Small EM, Sutherland LB, Qi X,
McAnally J, Plato CF, Richardson JA, Bassel-Duby R and Olson EN:
MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and
responsiveness of smooth muscle cells to injury. Genes Dev.
23:2166–2178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordon SS: Management of hypertension in
the elderly patient. Clin Interv Aging. 4:379–389. 2009.PubMed/NCBI
|
|
17
|
Cheng WH, Lu PJ, Hsiao M, Hsiao CH, Ho WY,
Cheng PW, Lin CT, Hong LZ and Tseng CJ: Renin activates
PI3K-Akt-eNOS signalling through the angiotensin AT(1) and Mas
receptors to modulate central blood pressure control in the nucleus
tractus solitarii. Br J Pharmacol. 166:2024–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurihara N, Alfie ME, Sigmon DH, Rhaleb
NE, Shesely EG and Carretero OA: Role of nNOS in blood pressure
regulation in eNOS null mutant mice. Hypertension. 32:856–861.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Q, Yang J, Santulli G, Reiken SR,
Wronska A, Kim MM, Osborne BW, Lacampagne A, Yin Y and Marks AR:
Maintenance of normal blood pressure is dependent on IP3R1-mediated
regulation of eNOS. Proc Natl Acad Sci USA. 113:pp. 8532–8537.
2016; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Frank JW, Little DR, Dunlap KA,
Satterfield MC, Burghardt RC, Hansen TR, Wu G and Bazer FW:
Functional role of arginine during the peri-implantation period of
pregnancy. I. Consequences of loss of function of arginine
transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J.
28:2852–2863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diaz-Perez F, Radojkovic C, Aguilera V,
Veas C, González M and Lamperti L: Escudero C and Aguayo:
L-arginine transport and nitric oxide synthesis in human
endothelial progenitor cells. J Cardiovasc Pharmacol. 60:439–449.
2012. View Article : Google Scholar PubMed/NCBI
|
|
22
|
Kwan HY, Leung PC, Huang Y and Yao X:
Depletion of intracellular Ca2+ stores sensitizes the flow-induced
Ca2+ influx in rat endothelial cells. Circ Res. 92:286–292. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang X, Wang F, Zhou L, Yin Z, Fan
J, Nie X, Wang P, Fu XD, Chen C and Wang DW: MicroRNA-21 lowers
blood pressure in spontaneous hypertensive rats by upregulating
mitochondrial translation. Circulation. 134:734–751. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu Q, Wang B, Zhang XF, Ma YP, Liu JD and
Wang XZ: Contribution of renin-angiotensin system to
exercise-induced attenuation of aortic remodeling and improvement
of endothelial function in spontaneously hypertensive rats.
Cardiovasc Pathol. 23:298–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karolina DS, Tavintharan S, Armugam A,
Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF and Jeyaseelan K:
Circulating miRNA profiles in patients with metabolic syndrome. J
Clin Endocrinol Metab. 97:E2271–E2276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Santovito D, Mandolini C, Marcantonio P,
De Nardis V, Bucci M, Paganelli C, Magnacca F, Ucchino S,
Mastroiacovo D, Desideri G, et al: Overexpression of microRNA-145
in atherosclerotic plaques from hypertensive patients. Expert Opin
Ther Targets. 17:217–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kontaraki JE, Marketou ME, Zacharis EA,
Parthenakis FI and Vardas PE: Differential expression of vascular
smooth muscle-modulating microRNAs in human peripheral blood
mononuclear cells: Novel targets in essential hypertension. J Hum
Hypertens. 28:510–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Wu X, Xu Y, Wu S, Li Z, Chen R,
Huang N, Zhu Z and Xu X: miR-145 suppresses colorectal cancer cell
migration and invasion by targeting an ETS-related gene. Oncol Rep.
36:1917–1926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J, Yin L, Jiang N, Guo WJ, Gu JJ, Chen
M, Xia YY, Wu JZ, Chen D, Wu JF, et al: MiR-145, a microRNA
targeting ADAM17, inhibits the invasion and migration of
nasopharyngeal carcinoma cells. Exp Cell Res. 338:232–238. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia J, Tian Q, Ling S, Liu Y, Yang S and
Shao Z: miR-145 suppresses osteogenic differentiation by targeting
Sp7. FEBS Lett. 587:3027–3031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li R, Yan G, Zhang Q, Jiang Y, Sun H, Hu
Y, Sun J and Xu B: miR-145 inhibits isoproterenol-induced
cardiomyocyte hypertrophy by targeting the expression and
localization of GATA6. FEBS Lett. 587:1754–1761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bethany KR, Kimberly JT, Lee DS, Clifton
NM and Randall SP: Arginine increases development of in vitro
produced porcine embryos and affects the PRMT-DDAH-NO Axis. Reprod
Fertil Dev. 27:655–666. 2015. View
Article : Google Scholar : PubMed/NCBI
|