Introduction
Cilostazol (CLZ) has anti-platelet aggregation and
vasodilatory effects with minimal cardiac effects (1). In clinical studies, CLZ has been used
as a therapeutic agent for improving symptoms in conditions such as
cancer (2) and pain accompanied by
chronic arterial obstruction (3) and
for ameliorating (4) and preventing
cerebral infarction (5). In
addition, recent studies indicated that CLZ reduced the degree of
neuronal cell death after transient cerebral ischemia (6). However, CLZ is poorly water-soluble
(the solubility is 4.83 µg/ml in the water at 37°C) and its
bioavailability is low (7).
Therefore, the development of a strategy for increasing the
bioavailability of CLZ is needed.
Recently, many methods using poly (lactic-coglycolic
acid) (PLGA) nanospheres (8,9), redox nanoparticles (RNPO)
(10), emulsions (11), and polymer micelles (12) have been evaluated for improving the
bioavailability of poorly water-soluble drugs. We also reported a
method for producing drug nanoparticles using the ball and bead
mill (13–18) and showed that indomethacin solid
nanoparticles exhibited enhanced drug bioavailability after oral
administration (17). We
hypothesized that an oral formulation of CLZ nanoparticles would
provide high drug absorption and that enhancing the bioavailability
of CLZ would increase its effectiveness for preventing neuronal
cell death after transient cerebral ischemia. In addition, we
expected this formulation would lead to a decrease in the required
amount of orally administered CLZ.
It is important to carefully select a method for
preparing the drug nanoparticles. Conventional milling methods,
such as planetary ball milling, have been widely used in the drug
nanoparticle processing field (19,20). In
addition, a combination of recrystallization and ball milling can
be used to prepare large amounts of fine drug nanoparticles at a
low price (21). In this study, we
designed new oral formulations containing CLZ solid nanoparticles
using a combination of recrystallization and ball mill methods.
Moreover, we investigated the usefulness of these formulations by
evaluating drug bioavailability and their protective effect in
ischemic stroke using a cerebral ischemia/reperfusion-induced
injury model (MCAO/reperfusion mice).
Materials and methods
Materials
CLZ was kindly provided by Otsuka Pharmaceutical
Co., Ltd. (Tokyo, Japan). 2-Hydoxypropyl-β-cyclodextrin (HPβCD) was
purchased from Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan).
Low-substituted methylcellulose (MC) was provided by Shin-Etsu
Chemical Co., Ltd. (Tokyo, Japan). Docusate sodium (DS) was
obtained from Sigma Co., Inc. (Tokyo, Japan). All other chemicals
used were of the highest purity commercially available.
Animals
Wistar rats (7 weeks, male) and ICR mice (6 weeks,
male) were housed under standard conditions (fluorescent light
07:00 a.m.-07:00 p.m., 25±1°C) and allowed free access to a
commercial diet (CR-3; Clea Japan Inc., Tokyo, Japan) and water.
All procedures were performed in accordance with the Kindai
University School of Pharmacy Committee for the Care and Use of
Laboratory Animals, and the study received ethical approval form
the Kindai University School of Pharmacy Ethics Committee (Osaka,
Japan). In this study, we administered 3 mg/kg CLZ following the
dose in clinic.
Preparation of oral formulations
containing CLZ
CLZ nanoparticles were prepared using the ball mill
method. Zirconia balls (diameter: 10 mm), DS, and MC were added to
a zirconia cup (diameter: 45 mm) containing CLZ or recrystallized
CLZ (CLZ/R) and the mixture was crushed using a Pulverisette 7 for
24 h (400 rpm, room temperature, milled-CLZ). In addition, CLZ/R
was prepared as follows: 0.5 g of CLZ was dissolved in 50 ml of 50%
ethanol at 120°C, then the extracted CLZ was treated with
sonication for 5 s using a sonicator (Yamato Science Co., Ltd,
Tokyo, Japan) and allowed to stand for 24 h. Thereafter, CLZ/R was
collected by filtration (recovery rate: 92.3%). These micro- and
nanoparticles of CLZ and CLZ/R were dispersed using 5% HPβCD
solution containing gum arabic (solubility at room temperature is
approximately 500 mg/ml in water), polyvinylpyrrolidone (PVP,
solubility at room temperature is approximately >100 mg/ml in
water), and D-mannitol (mannitol, solubility at room temperature is
approximately 200 mg/ml in water) and the mixture was freeze-dried
for 3 days (CLZ tablet, major axis 11.91±0.02 mm, minor axis
7.05±0.01 mm, thickness 6.87±0.02 mm, weight 153.0±2.5 mg, CLZ
content 22.3±0.7 mg/tablet, disintegration time 30±6 sec, mean ±
standard error, n=10). The oral CLZ formulations (tablets) are
presented in Table I. The CLZ
tablets were suspended in purified water (re-dispersion of CLZ
tablet) and used for the in vitro, in situ, and in
vivo studies. The particle size was measured using a
nanoparticle size analyzer (SALD-7100, Shimadzu Corp., Kyoto,
Japan; refractive index 1.60–0.10i).
 | Table I.Formulations of CLZ tablets. |
Table I.
Formulations of CLZ tablets.
|
| Content (w/w%) |
|
|---|
|
|
|
|
|---|
| Formulation | CLZ | DS | MC | HPβCD | Gum arabic | PVP | Mannitol | Treatment |
|---|
| CLZmicro
tablet | 17 | 4 | 17 | 2.5 | 47 | 2.5 | 10 | – |
|
CLZ/Rmicro tablet | 17 | 4 | 17 | 2.5 | 47 | 2.5 | 10 |
Recrystallization |
| CLZnano
tablet | 17 | 4 | 17 | 2.5 | 47 | 2.5 | 10 | Ball mill |
|
CLZ/Rnano tablet | 17 | 4 | 17 | 2.5 | 47 | 2.5 | 10 | Recrystallization,
Ball mill |
Characterization of CLZ
The morphology of CLZ was characterized using a
scanning electron microscope (SEM) and powder X-ray diffraction
(XRD). The SEM (JSM-5200, JEOL, Japan) was operated at an
excitation voltage of 20 kV. The samples were fixed on a brass stub
using carbon double-sided tape and deposited and gold-plated prior
to recording images. After the degree of the vacuum reached
0.2–0.02 Torr, the gold plating was performed for 100–120 sec to
form a 10–20 nm gold layer. The XRD patterns of the samples were
recorded using a SmartLab 2 kW (Rigaku, Co., Tokyo, Japan) with a
Cu-Ka (λ=1.54 Å) target. The X-rays were done at 40 kV and 40 mA
Philips. Data were obtained from 5° to 40° diffraction angles with
a scanning rate of 3°/min and a step size of 0.02.
In situ intestinal absorption of
CLZ
The experiment was performed according to our
previous reports (22). Wistar rats
were fasted for 10 h before dosing and allowed free access to water
throughout the experiment. Rats were anesthetized with isoflurane
and placed on a heating mat to maintain a body temperature of
approximately 37°C. A target portion of intestine (4–5 cm) was
exposed through a midline abdominal incision and silicon tubing
(TERUMO Corp., Tokyo, Japan) was inserted in one end of the
intestine. The opposite end was tied and 1.5–2.0 ml of 3 mg/kg CLZ
suspension (CLZ tablet in purified water) was injected through the
tube. Heparin (10 mg/kg) was injected into the femoral vein. The
mesenteric vein was cannulated with an appropriate size of
polyethylene tubing (Hibiki Co., Tokyo, Japan) and blood samples
(0.1 ml) were collected from a portal vein at 0 (pre-dose), 5, 10,
15, 20, 25, and 30 min after dosing. Plasma samples were obtained
by centrifugation (20,400 × g, 20 min, 4°C). The amount of CLZ in
the filtrates was determined using a Shimadzu LC-10AD system
equipped with a CTO-6A column oven (Shimadzu Corp., Kyoto, Japan;
HPLC method). The conditions were as follows: Internal standard,
benzophenone; column, Inertsil ODS-3 (3 µm, column size: 2.0×50 mm;
Shimadzu Co., Inc., Tokyo, Japan); mobile phase,
acetonitrile/methanol/water (35/15/50, v/v/v); flow rate, 0.25
ml/min; column temperature, 35°C; wavelength, 254 nm.
Analysis of CLZ concentration in the
stomach and small intestine after oral administration
The experiment was performed according to our
previous studies (17). CLZ tablets
were suspended using purified water and orally administered to
Wistar rats at a dose of 3 mg/kg. The rats were fasted for 10 h
before oral administration of CLZ, but had free access to water.
The rats were killed under deep ether anesthesia 3, 6, and 9 h
later. The stomach and small intestine (40% of the upper part of
total small intestine, approximately 28 cm) were excised and these
mucous membrane samples (0.1 mg) were homogenized in 300 µl of
methanol and centrifuged (20,400 × g, 20 min, 4°C). The amount of
CLZ in the supernatant was analyzed using the HPLC method described
above.
Analysis of plasma CLZ
concentration
CLZ tablets were suspended using purified water and
orally administered to Wistar rats at a dose of 3 mg/kg. The rats
were fasted for 10 h before dosing and allowed free access to water
throughout the experiment. Blood samples (0.1 ml) were collected
from a jugular vein at 0 (pre-dose), 0.5, 1, 2, 4, 6, and 8 h after
oral administration of CLZ and centrifuged (20,400 × g, 20 min,
4°C). The amount of CLZ in the supernatant was determined using the
HPLC method described above.
The CLZ concentration in the plasma after a single
injection of 0.3 ml of CLZ solution (100 µg/kg) into the femoral
vein was analyzed according to equation 1:
CCLZ=C0·e–ke·t
where CCLZ was the plasma CLZ
concentration, C0 was the initial concentration
of CLZ in the plasma (4.10±0.13 µg/ml), ke was
the elimination rate constant for CLZ from the plasma (2.05±0.31
h−1), and Vd was the distribution
volume (30.5±1.52 µl/kg). These data were obtained from three
experiments.
The absorption of CLZ after the single
administration of a CLZ tablet was calculated as the apparent
absorption rate constant (ka, h−1)
according to equation 2:
CCLZ=ka·F·DVd(ka–ke)(e–ke·(t–tlag)+e–ka·(t–tlag))
where ka was the absorption rate
constant, t was the time after CLZ administration (0–8 h),
F was the fraction of CLZ absorbed, D was the dose of
CLZ administered (3 mg/kg), and tlag was the lag
time (h). The area under the CLZ concentration-time curve (AUC) was
determined according to the trapezoidal rule up to 8 h. The mean
residence time (MRT) was calculated from the area under the first
moment curve (AUMC)/AUC. In addition, the bioavailability was
calculated as the ratio of the AUC after oral administration to the
AUC after intravenous administration.
Induction of focal cerebral
ischemia/reperfusion model
Focal cerebral ischemia/reperfusion was caused by
middle cerebral artery occlusion (MCAO), which was completed
following the method described in our previous reports (15). MCAO was induced by the insertion of a
silicone-coated 8–0 nylon monofilament into the left middle
cerebral artery under isoflurane anesthesia. Two h after this
procedure, the mice were briefly reanesthetized with isoflurane and
middle cerebral artery blood flow was restored by withdrawing the
nylon monofilament. Three h after reperfusion, CLZ suspension (CLZ
tablet in purified water) was orally administered to the MCAO mice
at a dose of 3 mg/kg. Three days after reperfusion, the brain was
removed and three slices were removed for analysis: i) from the
bregma (2 mm, AreaA), ii) 1 mm anterior to the bregma (2
mm, AreaB), and iii) 1 mm posterior to the bregma (2 mm,
AreaC), using Brain Matrices (Brain ScienceIdea Co.,
Ltd., Osaka, Japan). The slices of brain tissue were dyed with 1.5%
2,3,5-triphenyl tetrazolium chloride (TTC) for 15 min and monitored
under a digital camera. The images obtained were analyzed using
Image J software and the infarct area was calculated
(mm2). The infarct volume (mm3) was estimated
according to the following equation 3:
Infarct
volume(mm3)=AreaA×2+AreaB×2+AreaC×2
Neurological deficit mice were tested for
neurological deficits 72 h after MCAO followed by reperfusion
(MCAO/reperfusion). Each mouse was masked for the investigator.
Statistical Analysis
Unpaired Student's t-tests and Dunnett's
multiple comparison tests were used for statistical analysis and
P-values less than 0.05 were considered significant. All
data are expressed as the mean ± standard deviation or standard
error of the mean.
Results
Preparation of tablets containing CLZ
nanoparticles
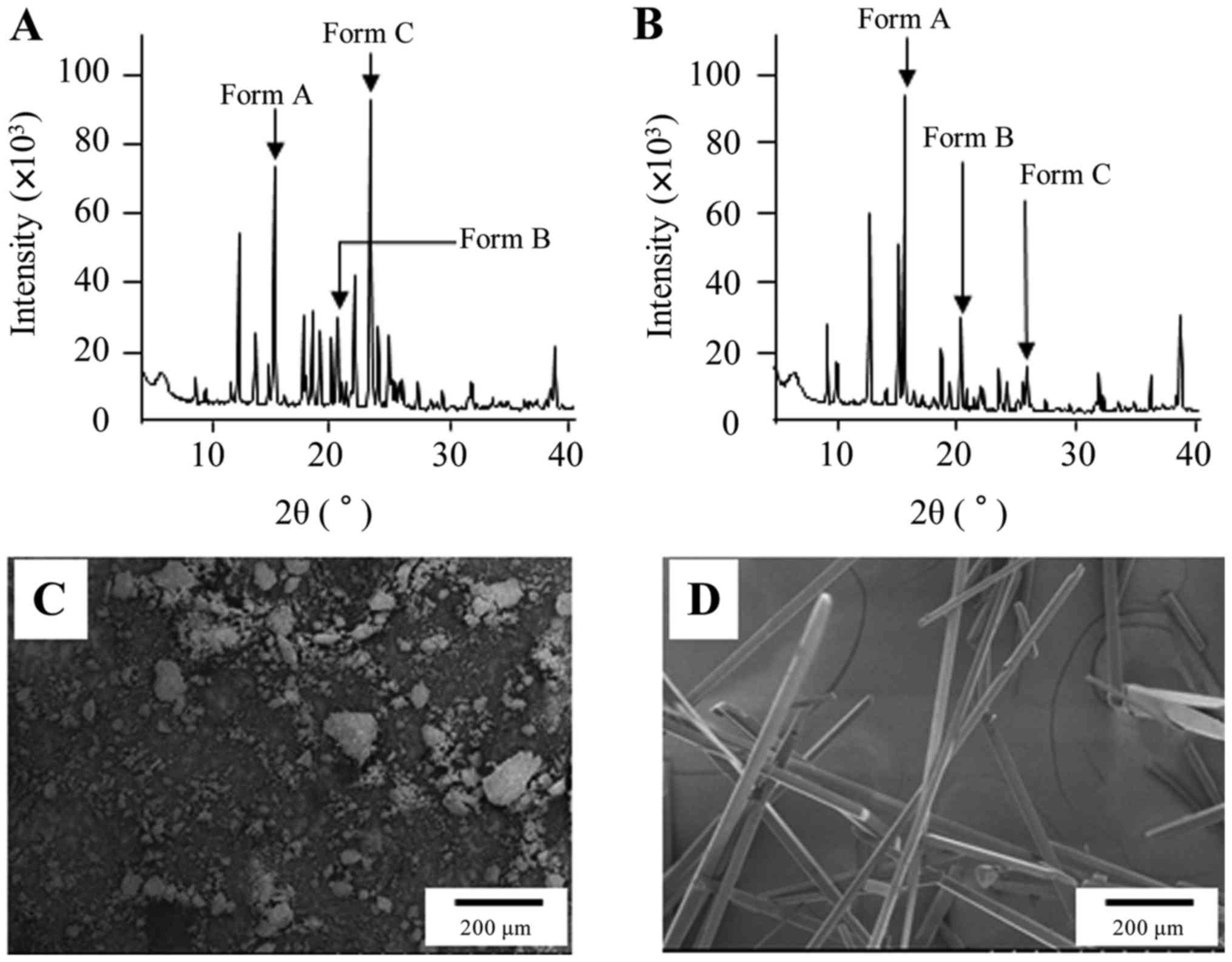
Fig. 1 shows the XRD
patterns (A, B) and SEM images (C, D) of CLZ and CLZ/R. The
transparent acicular crystal appeared after recrystallization. In
the XRD patterns, the indicated CLZ peak forms of polymorphs were
observed and CLZ consisted of Forms A, B, and C (Fig. 1A). However, the indicated peak of
Form C decreased and the indicated peak of Form A was enhanced
(Fig. 1B). Thereafter, we prepared
CLZ tablets containing CLZ micro- and nanoparticles using the ball
mill method and evaluated them for re-dispersibility. The mean
particle sizes of CLZ, CLZ/R, milled-CLZ, and milled-CLZ/R were
65.4±26.7, 48.3±34.4, 0.067±0.018, and 0.073±0.016 µm, respectively
(means ± standard deviation). The tablets containing CLZ as
described in Table I were prepared
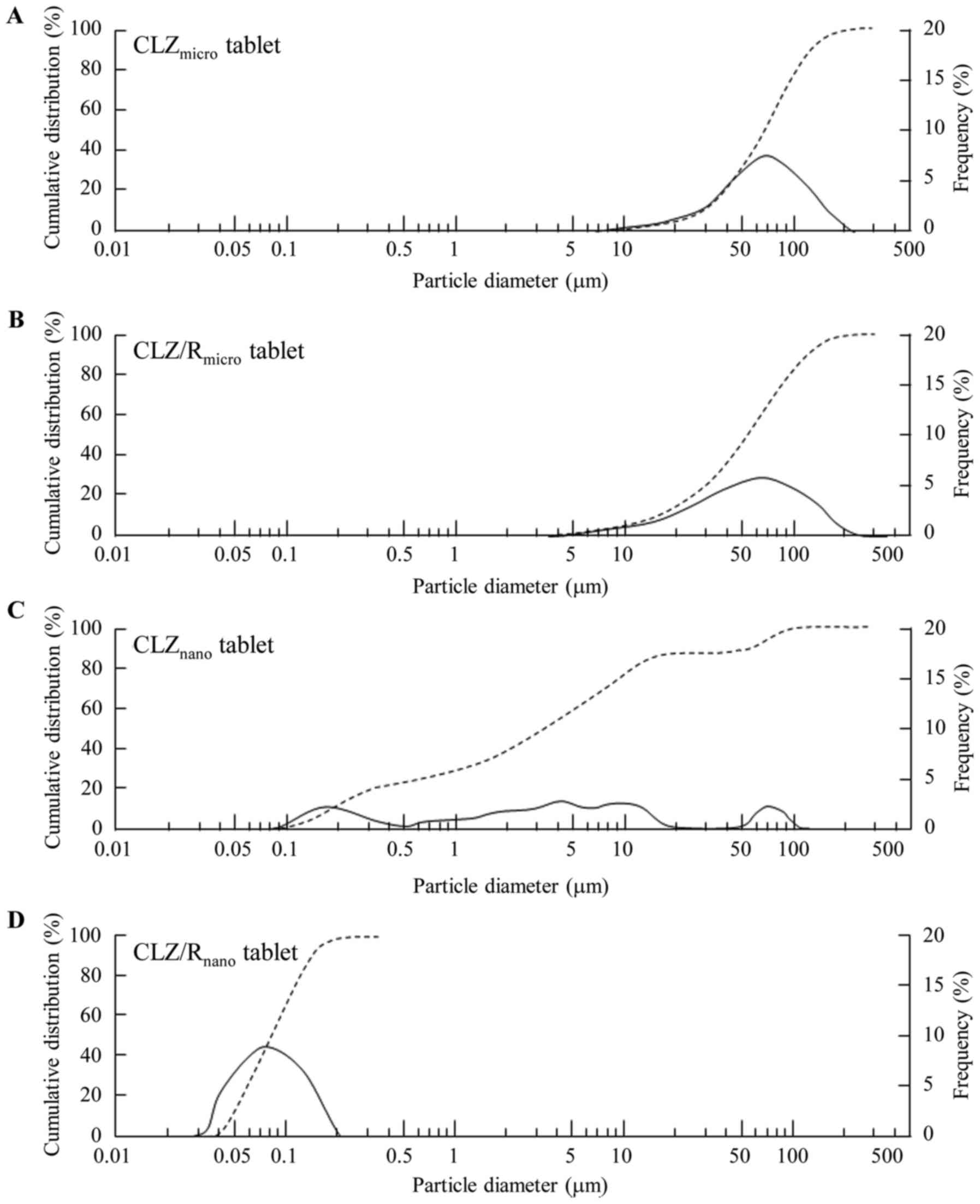
using CLZ and CLZ/R with or without the ball mill. Fig. 2 shows the particle size distribution
of the CLZmicro (A), CLZ/Rmicro (B),
CLZnano (C), and CLZ/Rnano (D) tablets. Even
though the particles in CLZnano tablets (2.79±82.1 µm)
aggregated after re-dispersion, the mean particle size of the
CLZ/Rnano tablets (0.068±0.029 µm) was similar to that
of the milled-CLZ/Rnano tablets (means ± standard
deviation).
Pharmacokinetics of the oral
formulation containing CLZ nanoparticles
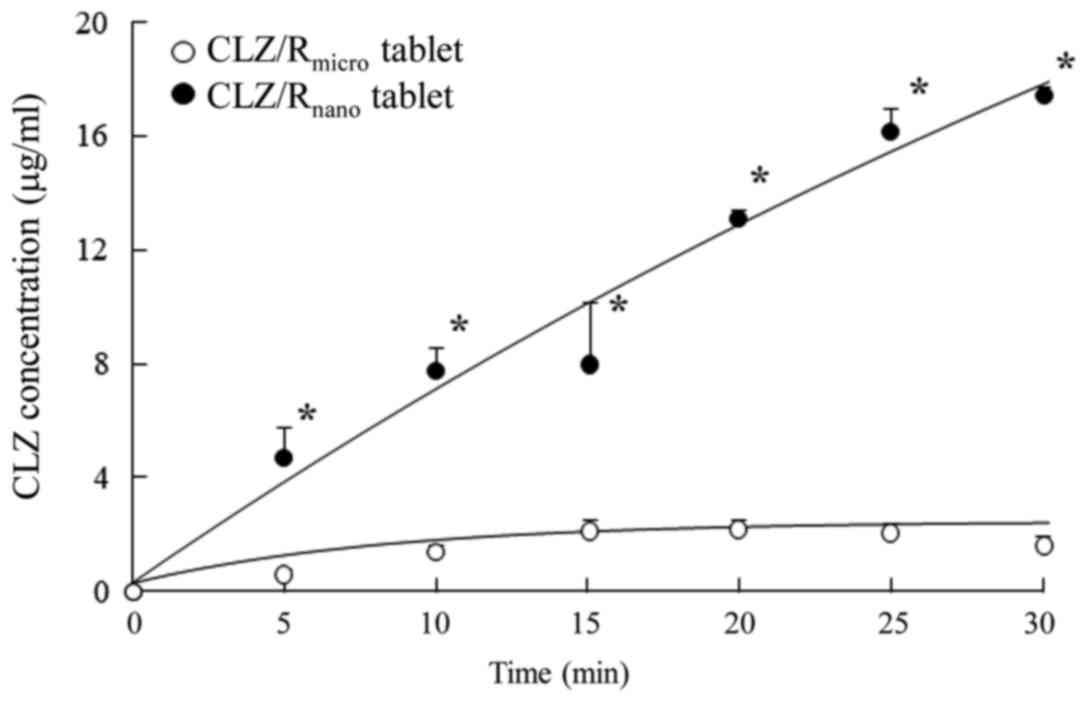
The in situ loop technique was used to
evaluate the drug absorption of the CLZ/R tablet and Fig. 3 shows the difference in drug
absorption between the CLZ/Rmicro and
CLZ/Rnano tablets. The absorption of
CLZ/Rnano tablets was significantly higher than that of
CLZ/Rmicro tablets. Fig.
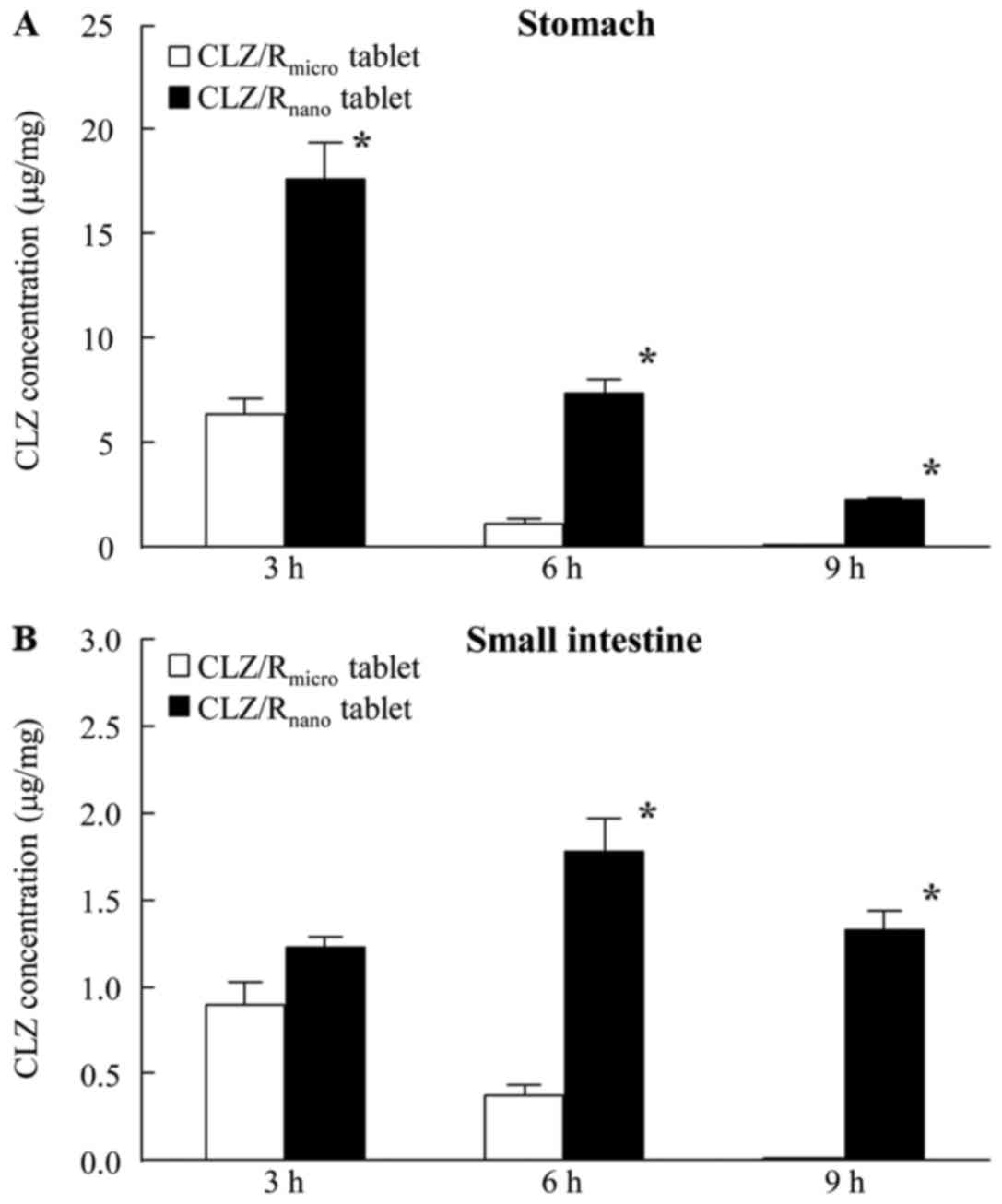
4 shows the amounts of CLZ in the mucosal membrane of the rat
stomach and small intestine after oral administration of
CLZ/Rmicro and CLZ/Rnano tablets. The
residence amount and time after CLZ/Rnano tablet
administration were significantly greater than those after
CLZ/Rmicro tablet administration. The amount of CLZ in
mucosal membrane of the stomach reached a maximum 3 h after
administration of CLZ/Rmicro and CLZ/Rnano
tablets, then decreased with time. After administration of the
CLZmicro tablet, the amount of CLZ in the mucosal
membrane of the small intestine also reached a maximum after 3 h.
However, the amount of CLZ in the mucosal membrane of the small
intestine remained constant during the period 3–12 h after
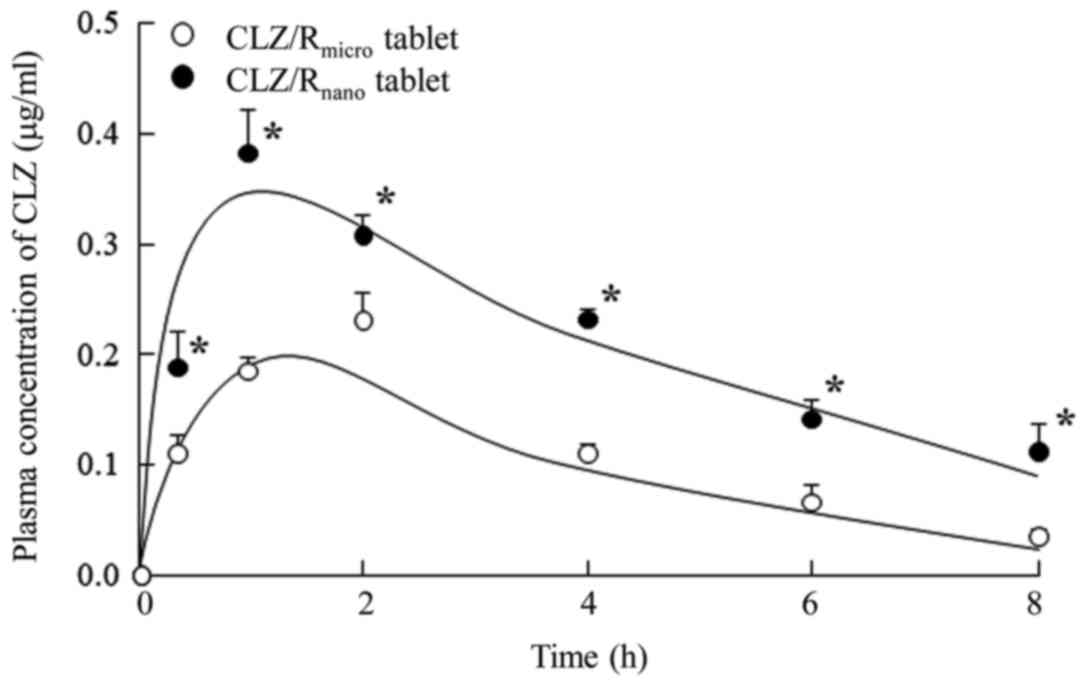
administration of CLZ/Rnano tablets. Fig. 5 shows the plasma CLZ concentrations
after oral administration of CLZ/Rmicro and
CLZ/Rnano tablets in rats and Table II summarizes the pharmacokinetic
parameters calculated from the in vivo intestinal
penetration data. The plasma CLZ levels, AUC, and MRT
in rats administered CLZ/Rnano tablets were all
significantly higher than those in rats administered
CLZ/Rmicro tablets. Moreover, the bioavailability of the
CLZ/Rnano tablets was 2.1-fold higher than that of the
CLZ/Rmicro tablets.
 | Table II.Pharmacokinetic parameters for plasma
CLZ concentration after oral administration of CLZ/R tablet. |
Table II.
Pharmacokinetic parameters for plasma
CLZ concentration after oral administration of CLZ/R tablet.
| Preparation | ka
(/h) | tlag
(h)x10−2 |
Fx10−2 | AUC (µg●h/ml) | MRT (h) |
|---|
|
CLZ/Rmicro tablet | 0.31±0.07 | 7.61±4.12 | 1.41±0.19 | 0.94±0.11 | 2.85±0.21 |
|
CLZ/Rnano tablet | 0.25±0.03 | 6.04±2.70 |
2.81±0.26a |
1.71±0.08a |
3.32±0.18a |
Therapeutic effect of the oral
formulation containing CLZ/R nanoparticles on ischemic stroke
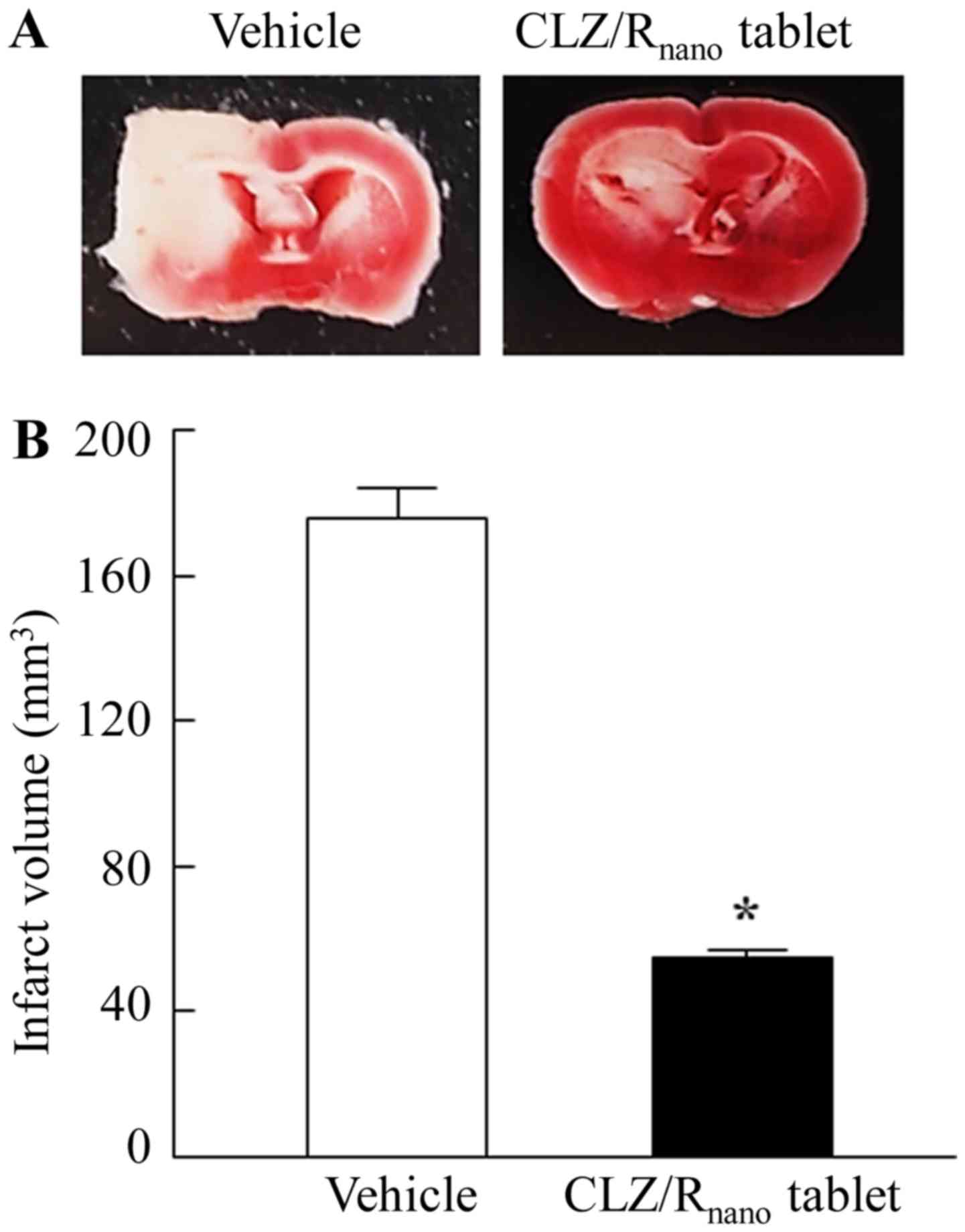
Fig. 6 shows the
effects on ischemic stroke in MCAO/reperfusion mice administered
CLZ/Rnano tablets. Treatment with CLZ/Rnano
tablets attenuated ischemic stroke and the infarct volume of the
MCAO/reperfusion mice administered CLZ/Rnano tablets was
31% of that of the vehicle-administered mice (vehicle). The
neurological deficits in MCAO/reperfusion mice were also protected
after the oral administration of CLZ/Rmicro and
CLZ/Rnano tablets (vehicle, 2.7±0.3;
CLZ/Rnano tablet, 1.2±0.3, means ± standard error,
n=3–9).
Discussion
Poorly water-soluble drugs are poorly absorbed when
administered orally. We previously designed drug nanoparticles by
the breakdown method using a ball and bead mill (13–18) and
showed that indomethacin solid nanoparticles enhanced drug
bioavailability in the small intestine of rats (17). In this study, we designed new oral
formulations containing CLZ solid nanoparticles using a combination
of recrystallization and breakdown methods. Moreover, we
investigated the therapeutic effect of the oral formulations
containing CLZ nanoparticles on ischemic stroke in MCAO/reperfusion
mice.
First, we attempted to prepare an oral formulation
(tablet) containing CLZ nanoparticles. Stowell et al
(23)and Whittall et al
(24) reported that three forms of
CLZ polymorphs exist (Form A, B and C). Forms A, B, and C were
characterized by XRD and had the indicated peaks at 2θ=13.0±0.2
(Form A), 2θ=21.5±0.2 (Form B), and 2θ=25.0±0.2° (Form C). In this
study, CLZ that was not recrystallized (original CLZ powder)
consisted of a mix of Forms A, B and C. However, the peak of Form B
was decreased after recrystallization and that of Form A was
decreased in recrystallized CLZ (CLZ/R) (Fig. 1). Furthermore, we prepared an oral
formulation containing CLZ nanoparticles using the ball mill method
and evaluated the changes in CLZ particle size after the
re-dispersion of the tablets (Fig.
2). The particles aggregated after the CLZnano
tablets were re-dispersed; however, the stability of the
re-dispersed oral formulation was improved when recrystallized CLZ
was used. In addition, the particle size after re-dispersion of the
CLZ/Rnano tablets was similar to that of CLZ/R prepared
with the ball mill. The stability of the nanoparticles was improved
by changing the ratio of the forms of polymorphs in the
recrystallized CLZ. The relationship between the polymorph forms
and their stability after re-dispersion will be examined in a
future study.
Thereafter, we evaluated drug absorption and
residence time in the small intestine after the oral administration
of CLZ/Rmicro and CLZ/Rnano tablets. The
absorption, residence amount, and residence time of the
CLZ/Rnano tablets were significantly greater than those
of the CLZ/Rmicro tablets (Figs. 3–5).
Furthermore, we investigated the pharmacokinetics after the oral
administration of CLZ/Rmicro and CLZ/Rnano
tablets. The plasma CLZ levels, AUC, and MRT in rats administered
CLZ/Rnano tablets were also increased compared with
those in rats administered CLZ/Rmicro tablets (Table II and Fig. 5). In addition, the absorption of
CLZ/Rnano tablets was higher than that of the
commercially available CLZ OD tablets (AUC, 0.55±0.05, means ±
standard error, n=5). These results indicated that bioavailability
was enhanced after administration of CLZ solid nanoparticles.
It is important to clarify the mechanism of the
enhancement of bioavailability that is exhibited by the
administration of nanoparticles. The residue of CLZ in the stomach
and small intestine after oral administration of the
CLZ/Rnano tablets was significantly increased compared
to that of the CLZ/Rmicro tablets (Fig. 4). In addition, we previously reported
that the nanoparticle formulation of indomethacin enhanced the
permeability of the corneal and intestinal membrane compared with
the microparticle formulation (17,18).
Taking these findings together, we hypothesized that the character
of the solid nanoparticles caused the enhancement of the
bioavailability of CLZ/Rnano tablets.
CLZ is widely used for the secondary prevention of
cerebral infarction. Recently, Kasahara et al (25) reported that treatment with CLZ
suppressed the disruption of the microvasculature in ischemic
areas. In addition, we reported that the intravenous administration
of CLZ attenuated brain damage when administered immediately after
the onset of ischemic stroke symptoms in MCAO/reperfusion mice
(15). From these findings, we
investigated whether the oral administration of CLZ/R tablets
prevented ischemic stroke in MCAO/reperfusion mice and found that
CLZ/Rnano tablets attenuated ischemic stroke (Fig. 6). This result indicated that CLZ/R,
in which the crystal structure was changed, also had a protective
effect on ischemic stroke. Further studies are needed to elucidate
the usefulness of the oral formulation containing CLZ nanoparticles
in the treatment of ischemic stroke. In addition, it is important
to clarify the relationships between crystal structure and
stability after the re-dispersion of CLZ tablets. Therefore, we are
now investigating the effect of each of the CLZ polymorphic forms
(Form A-C) in the CLZ/Rnano tablet on stability in
re-dispersion, drug absorption, and treatment of ischemic stroke.
In addition, we demonstrated the difference in the preventive
effects on ischemic stroke between CLZ micro and nanoparticles with
or without recrystallization using MCAO/reperfusion mice.
In conclusion, we prepared a novel oral formulation
containing CLZ nanoparticles (CLZ/Rnano tablet) that
exhibited a high stability after re-dispersion and a high drug
bioavailability. In addition, the CLZ/Rnano tablets
ameliorated neurological deficits caused by ischemic stroke in
MCAO/reperfusion mice. It is possible that the oral formulation
containing CLZ/R nanoparticles may be useful for effectively
treating ischemic stroke patients. These findings provide
significant information that can be used to design further studies
aimed at developing treatments for ischemic stroke patients and to
improve drug bioavailability.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
concentration-time curve
|
|
AUMC
|
area under the first moment curve
|
|
CLZ
|
cilostazol
|
|
CLZ/R
|
recrystallized cilostazol
|
|
DS
|
docusate sodium
|
|
HPβCD
|
2-hydoxypropyl-β-cyclodextrin
|
|
ka
|
absorption rate constant
|
|
MC
|
methylcellulose
|
|
MCAO/reperfusion
|
middle cerebral arterial occlusion
followed by reperfusion
|
|
MRT
|
mean residence time
|
|
PLGA
|
poly (lactic-coglycolic acid)
|
|
PVP
|
polyvinylpyrrolidone
|
|
RNPO
|
redox nanoparticles
|
|
SEM
|
scanning electron microscope
|
|
TTC
|
2,3.5-triphenyl tetrazolium
chloride
|
|
XRD
|
powder X-ray diffraction
|
References
|
1
|
Kimura Y, Tani T, Kanbe T and Watanabe K:
Effect of cilostazol on platelet aggregation and experimental
thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.PubMed/NCBI
|
|
2
|
Kanbayashi J, Liu Y, Sun B, Shakur Y,
Yoshitake M and Czerwiec F: Cilostazol as a unique antithrombotic
agent. Curr Pharm Des. 9:2289–2302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bramer SL and Forbes WP: Relative
bioavailability and effects of a high fat meal on single dose
cilostazol pharmacokinetics. Clin Pharmacokinet. 37 Suppl
2:S13–S23. 1999. View Article : Google Scholar
|
|
4
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
Effect of particle size reduction on dissolution and oral
absorption of a poorly water-soluble drug, cilostazol, in beagle
dogs. J Control Release. 111:56–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
In vitro-in vivo correlation for wet-milled tablet of poorly
water-soluble cilostazol. J Control Release. 130:29–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Park SY, Shin YW, Hong KW, Kim CD,
Sung SM, Kim KY and Lee WS: Neuroprotection by CLZ, a
phosphodiesterase type 3 inhibitor, against apoptotic white matter
changes in rat after chronic cerebral hypoperfusion. Brain Res.
1082:182–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rasenack N and Müller BW: Micron-size drug
particles: Common and novel micronization techniques. Pharm Dev
Technol. 9:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami H, Kobayashi M, Takeuchi H and
Kawashima Y: Further application of a modified spontaneous
emulsification solvent diffusion method to various types of PLGA
and PLA polymers for preparation of nanoparticles. Powder Technol.
107:137–143. 2000. View Article : Google Scholar
|
|
9
|
Kawashima Y: Design of poly
(lactic-co-glycolic acid) (PLGA) nanosphere for developing to DDS.
J Pharm Sci Technol Jpn. 66:224–238. 2006.
|
|
10
|
Sha S, Vong LB, Chonpathompikunlert P,
Yoshitomi T, Matsui H and Nagasaki Y: Suppression of NSAID-induced
small intestinal inflammation by orally administered redox
nanoparticles. Biomaterials. 34:8393–8400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igarashi R, Takenaga M, Takeuchi J,
Kitagawa A, Matsumoto K and Mizushima Y: Marked hypotensive and
blood flow-increasing effects of a new lipo-PGE(1) (lipo-AS013) due
to vascular wall targeting. J Control Release. 71:157–164. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kataoka K, Harada A and Nagasaki Y: Block
copolymer micelles for drug delivery design, characterization and
biological significance. Adv Drug Deliv Rev. 47:113–131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai N, Nakazawa Y, Ito Y, Kanai K,
Okamoto N and Shimomura Y: A nanoparticle-based ophthalmic
formulation of dexamethasone enhances corneal permeability of the
drug and prolongs its corneal residence time. Biol Pharm Bull.
40:1055–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai N, Ito Y, Okamoto N and Shimomura Y:
Size effect of rebamipide ophthalmic nanodispersions on its
therapeutic efficacy for corneal wound healing. Exp Eye Res.
151:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagai N, Yoshioka C, Ito Y, Funakami Y,
Nishikawa H and Kawabata A: Intravenous administration of
cilostazol nanoparticles ameliorates acute ischemic stroke in a
cerebral ischemia/reperfusion-induced injury model. Int J Mol Sci.
16:29329–29344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagai N, Yoshioka C, Tanabe W, Tanino T,
Ito Y, Okamoto N and Shimomura Y: Effects of ophthalmic
formulations containing cilostazol nanoparticles on retinal
vasoconstriction in rats injected with endothelin-1. Pharm Anal
Acta. 6:42015.
|
|
17
|
Nagai N and Ito Y: Effect of solid
nanoparticle of indomethacin on therapy for rheumatoid arthritis in
adjuvant-induced arthritis rat. Biol Pharm Bull. 37:1109–1118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N, Ito Y, Okamoto N and Shimomura Y:
A nanoparticle formulation reduces the corneal toxicity of
indomethacin eye drops and enhances its corneal permeability.
Toxicology. 319:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi M, Na K, Kim J, Sakamoto Y, Terasaki
O and Ryoo R: Stable single-unit-cell nanosheets of zeolite MFI as
active and long-lived catalysts. Nature. 461:246–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mintova S, Olson NH, Valtchev V and Bein
T: Mechanism of zeolite A nanocrystal growth from colloids at room
temperature. Science. 283:958–960. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tosheva L and Valtchev VP: Nanozeolites:
Synthesis, crytallisation mechanism and applications. Chem Mater.
17:24942005. View Article : Google Scholar
|
|
22
|
Nagai N and Ito Y: Delay of cataract
development in the Shumiya cataract rat by water containing
enhanced concentrations of magnesium and calcium. Curr Eye Res.
32:439–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stowell GW, Behme RJ, Denton SM, Pfeiffer
I, Sancilio FD, Whittall LB and Whittle RR: Thermally-prepared
polymorphic forms of cilostazol. J Pharm Sci. 91:2481–2488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Whittall LB, Whittle RR and Stowell GW:
Polymorphic forms of cilostazol. Acta Cryst C. 58:0525–0527. 2002.
View Article : Google Scholar
|
|
25
|
Kasahara Y, Nakagomi T, Matsuyama T, Stern
D and Taguchi A: Cilostazol reduces the risk of hemorrhagic
infarction after administration of tissue-type plasminogen
activator in a murine stroke model. Stroke. 43:499–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|