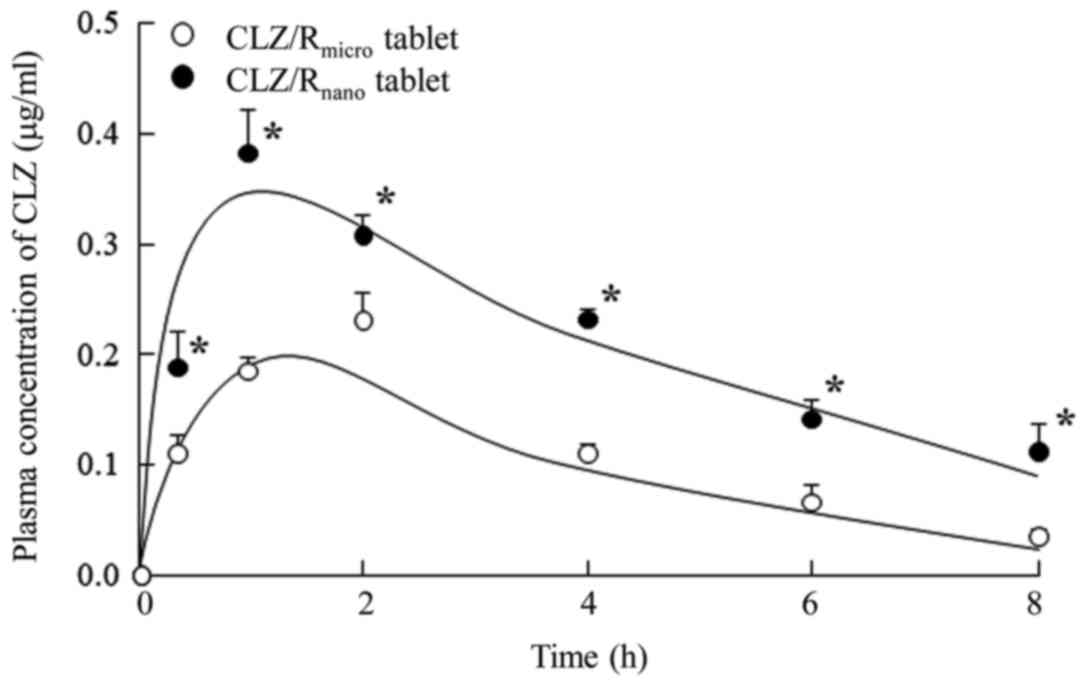
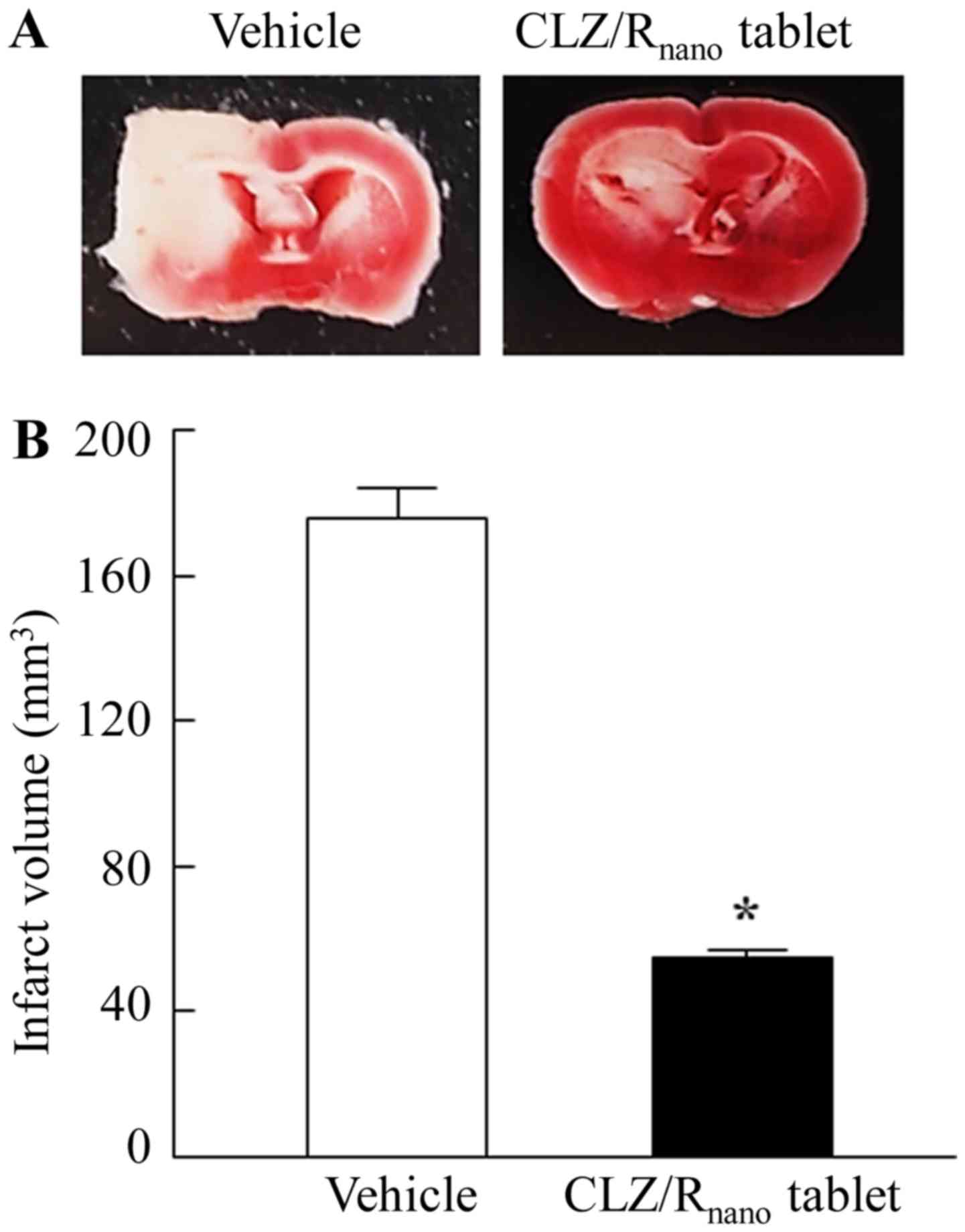
|
1
|
Kimura Y, Tani T, Kanbe T and Watanabe K:
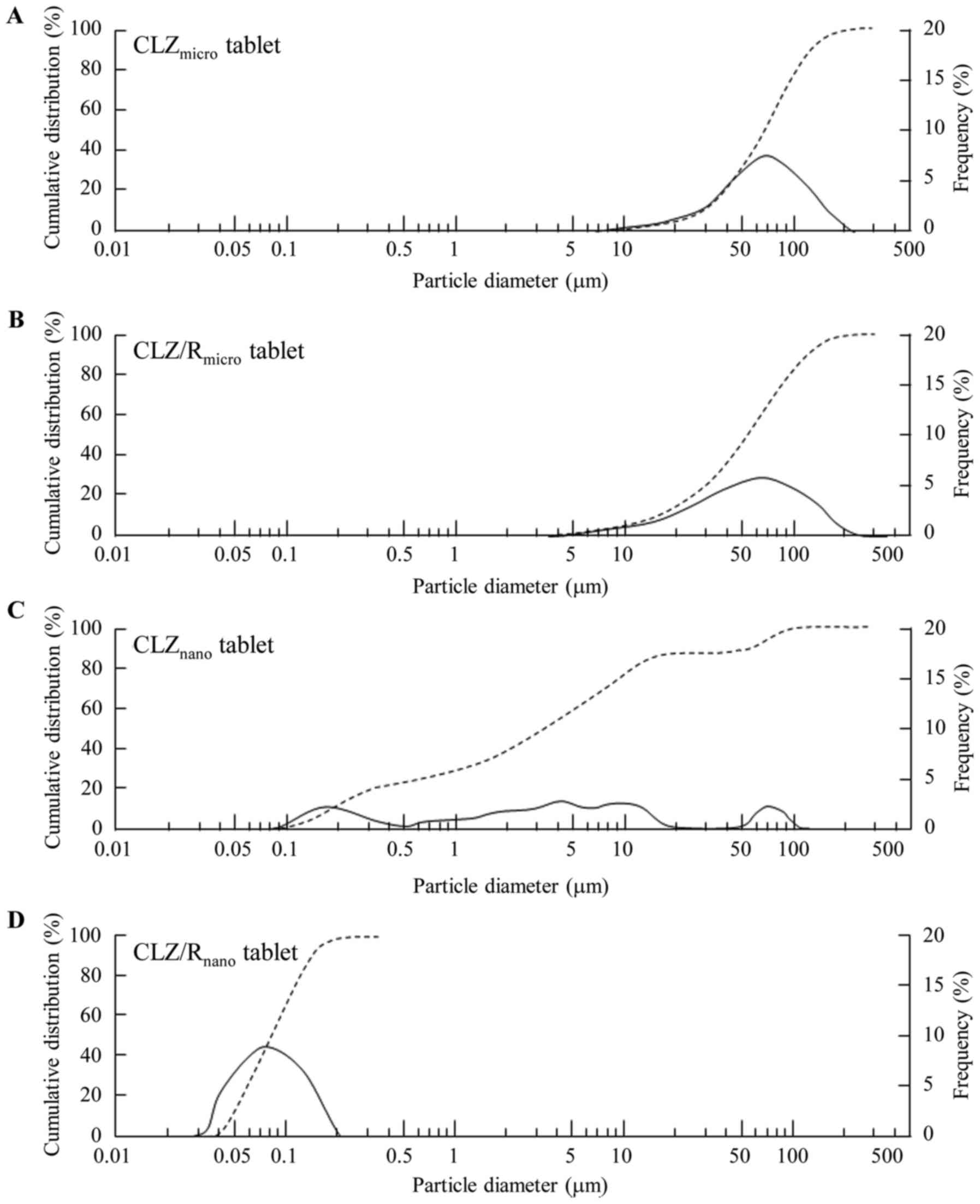
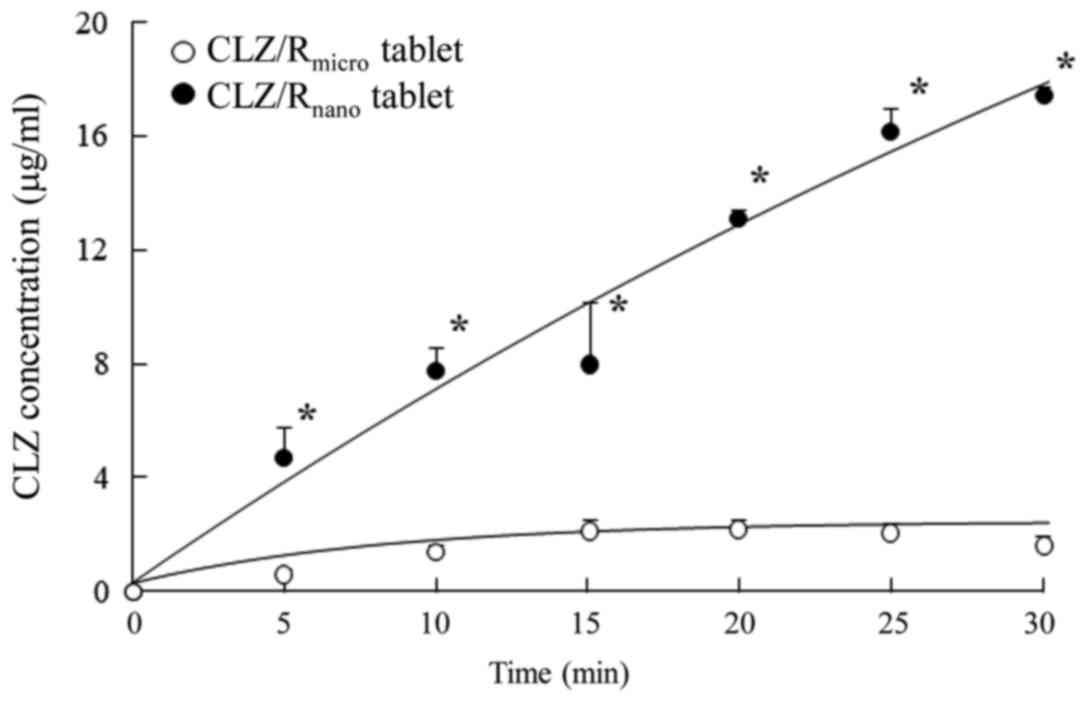
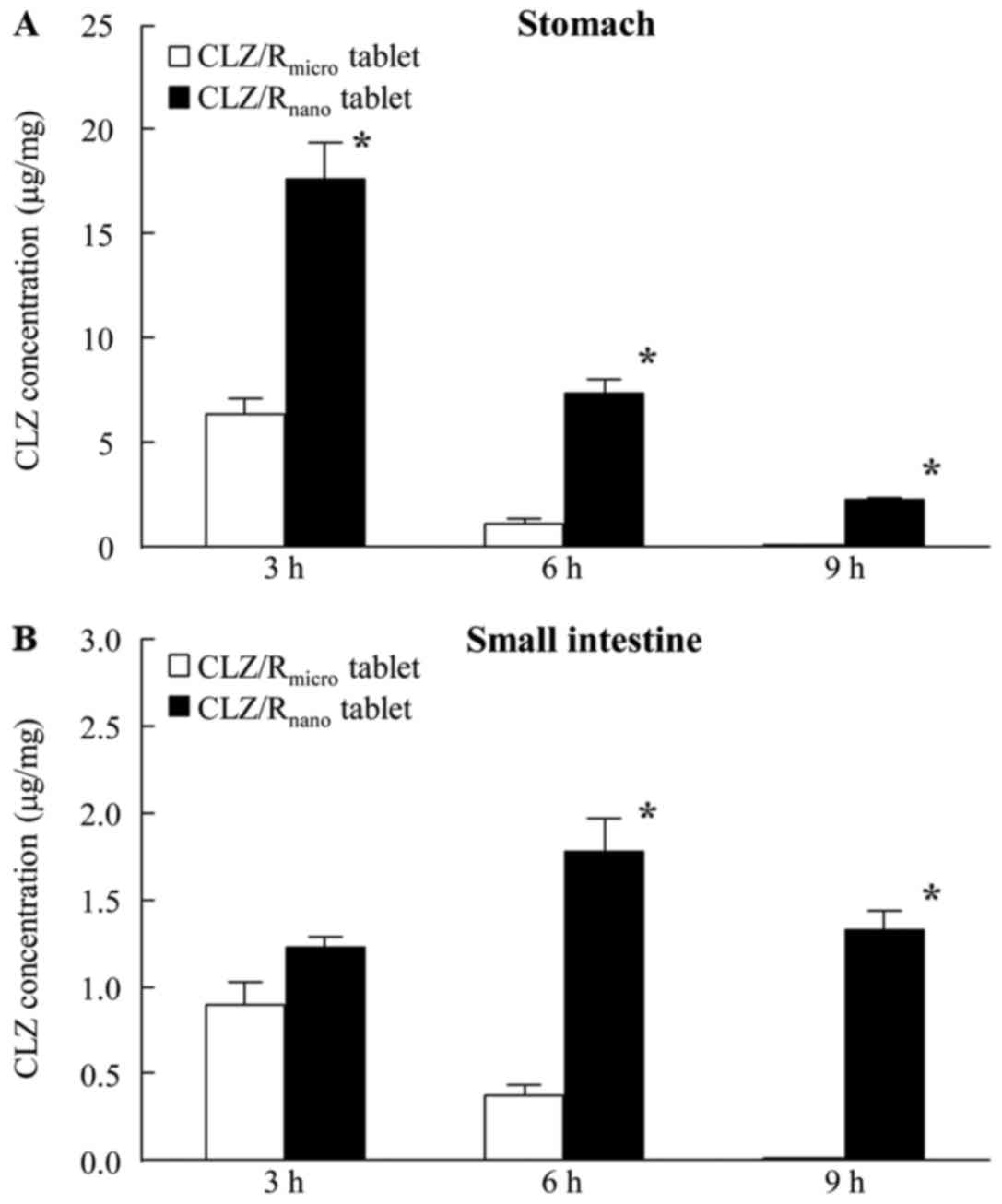
Effect of cilostazol on platelet aggregation and experimental
thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.PubMed/NCBI
|
|
2
|
Kanbayashi J, Liu Y, Sun B, Shakur Y,
Yoshitake M and Czerwiec F: Cilostazol as a unique antithrombotic
agent. Curr Pharm Des. 9:2289–2302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bramer SL and Forbes WP: Relative
bioavailability and effects of a high fat meal on single dose
cilostazol pharmacokinetics. Clin Pharmacokinet. 37 Suppl
2:S13–S23. 1999. View Article : Google Scholar
|
|
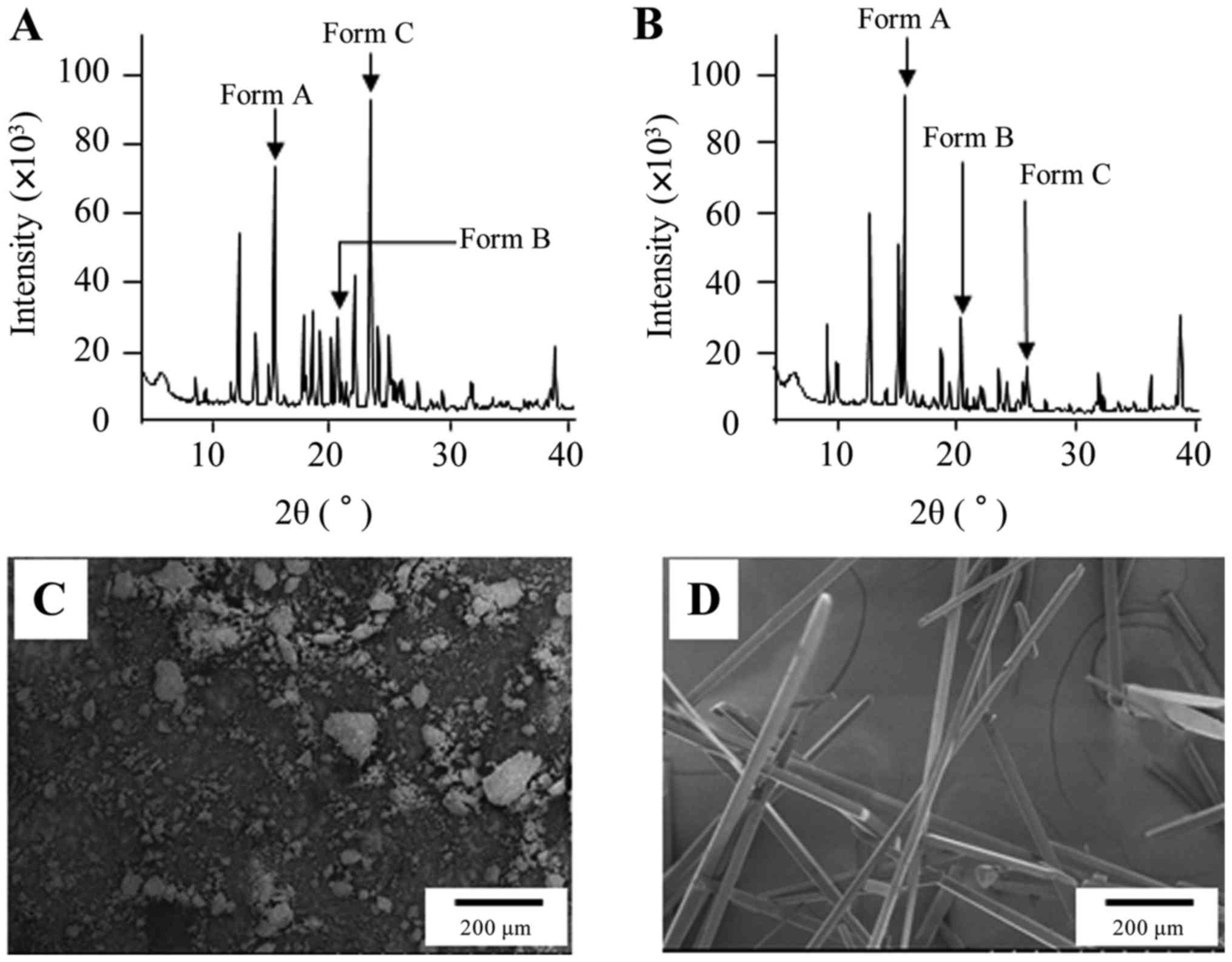
4
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
Effect of particle size reduction on dissolution and oral
absorption of a poorly water-soluble drug, cilostazol, in beagle
dogs. J Control Release. 111:56–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
In vitro-in vivo correlation for wet-milled tablet of poorly
water-soluble cilostazol. J Control Release. 130:29–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Park SY, Shin YW, Hong KW, Kim CD,
Sung SM, Kim KY and Lee WS: Neuroprotection by CLZ, a
phosphodiesterase type 3 inhibitor, against apoptotic white matter
changes in rat after chronic cerebral hypoperfusion. Brain Res.
1082:182–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rasenack N and Müller BW: Micron-size drug
particles: Common and novel micronization techniques. Pharm Dev
Technol. 9:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami H, Kobayashi M, Takeuchi H and
Kawashima Y: Further application of a modified spontaneous
emulsification solvent diffusion method to various types of PLGA
and PLA polymers for preparation of nanoparticles. Powder Technol.
107:137–143. 2000. View Article : Google Scholar
|
|
9
|
Kawashima Y: Design of poly
(lactic-co-glycolic acid) (PLGA) nanosphere for developing to DDS.
J Pharm Sci Technol Jpn. 66:224–238. 2006.
|
|
10
|
Sha S, Vong LB, Chonpathompikunlert P,
Yoshitomi T, Matsui H and Nagasaki Y: Suppression of NSAID-induced
small intestinal inflammation by orally administered redox
nanoparticles. Biomaterials. 34:8393–8400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igarashi R, Takenaga M, Takeuchi J,
Kitagawa A, Matsumoto K and Mizushima Y: Marked hypotensive and
blood flow-increasing effects of a new lipo-PGE(1) (lipo-AS013) due
to vascular wall targeting. J Control Release. 71:157–164. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kataoka K, Harada A and Nagasaki Y: Block
copolymer micelles for drug delivery design, characterization and
biological significance. Adv Drug Deliv Rev. 47:113–131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai N, Nakazawa Y, Ito Y, Kanai K,
Okamoto N and Shimomura Y: A nanoparticle-based ophthalmic
formulation of dexamethasone enhances corneal permeability of the
drug and prolongs its corneal residence time. Biol Pharm Bull.
40:1055–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai N, Ito Y, Okamoto N and Shimomura Y:
Size effect of rebamipide ophthalmic nanodispersions on its
therapeutic efficacy for corneal wound healing. Exp Eye Res.
151:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagai N, Yoshioka C, Ito Y, Funakami Y,
Nishikawa H and Kawabata A: Intravenous administration of
cilostazol nanoparticles ameliorates acute ischemic stroke in a
cerebral ischemia/reperfusion-induced injury model. Int J Mol Sci.
16:29329–29344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagai N, Yoshioka C, Tanabe W, Tanino T,
Ito Y, Okamoto N and Shimomura Y: Effects of ophthalmic
formulations containing cilostazol nanoparticles on retinal
vasoconstriction in rats injected with endothelin-1. Pharm Anal
Acta. 6:42015.
|
|
17
|
Nagai N and Ito Y: Effect of solid
nanoparticle of indomethacin on therapy for rheumatoid arthritis in
adjuvant-induced arthritis rat. Biol Pharm Bull. 37:1109–1118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N, Ito Y, Okamoto N and Shimomura Y:
A nanoparticle formulation reduces the corneal toxicity of
indomethacin eye drops and enhances its corneal permeability.
Toxicology. 319:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi M, Na K, Kim J, Sakamoto Y, Terasaki
O and Ryoo R: Stable single-unit-cell nanosheets of zeolite MFI as
active and long-lived catalysts. Nature. 461:246–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mintova S, Olson NH, Valtchev V and Bein
T: Mechanism of zeolite A nanocrystal growth from colloids at room
temperature. Science. 283:958–960. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tosheva L and Valtchev VP: Nanozeolites:
Synthesis, crytallisation mechanism and applications. Chem Mater.
17:24942005. View Article : Google Scholar
|
|
22
|
Nagai N and Ito Y: Delay of cataract
development in the Shumiya cataract rat by water containing
enhanced concentrations of magnesium and calcium. Curr Eye Res.
32:439–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stowell GW, Behme RJ, Denton SM, Pfeiffer
I, Sancilio FD, Whittall LB and Whittle RR: Thermally-prepared
polymorphic forms of cilostazol. J Pharm Sci. 91:2481–2488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Whittall LB, Whittle RR and Stowell GW:
Polymorphic forms of cilostazol. Acta Cryst C. 58:0525–0527. 2002.
View Article : Google Scholar
|
|
25
|
Kasahara Y, Nakagomi T, Matsuyama T, Stern
D and Taguchi A: Cilostazol reduces the risk of hemorrhagic
infarction after administration of tissue-type plasminogen
activator in a murine stroke model. Stroke. 43:499–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|