|
1
|
Golpour S, Rafie N, Safavi SM and
Miraghajani M: Dietary isoflavones and gastric cancer: A brief
review of current studies. J Res Med Sci. 20:893–900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hossain MM and Chen Z: Comments on
‘Application of an adaptive design to a randomized phase II
selection trial in gastric cancer: A report of the study design’ by
Satoshi Morita and Junichi Sakamoto. Pharmaceutical Statistics.
Pharm Stat. 11:267–268. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khayatzadeh S, Feizi A, Saneei P and
Esmaillzadeh A: Vitamin D intake, serum Vitamin D levels, and risk
of gastric cancer: A systematic review and meta-analysis. J Res Med
Sci. 20:790–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pimenta-Melo AR, Monteiro-Soares M,
Libânio D and Dinis-Ribeiro M: Missing rate for gastric cancer
during upper gastrointestinal endoscopy: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1041–1049. 2016.
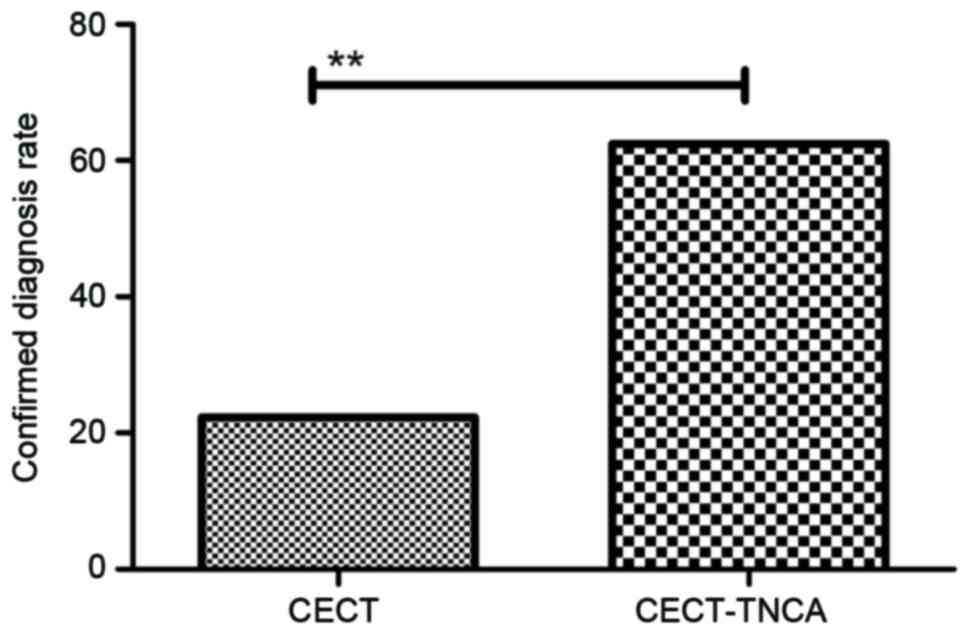
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veisani Y and Delpisheh A: Survival rate
of gastric cancer in Iran; a systematic review and meta-analysis.
Gastroenterol Hepatol Bed Bench. 9:78–86. 2016.PubMed/NCBI
|
|
6
|
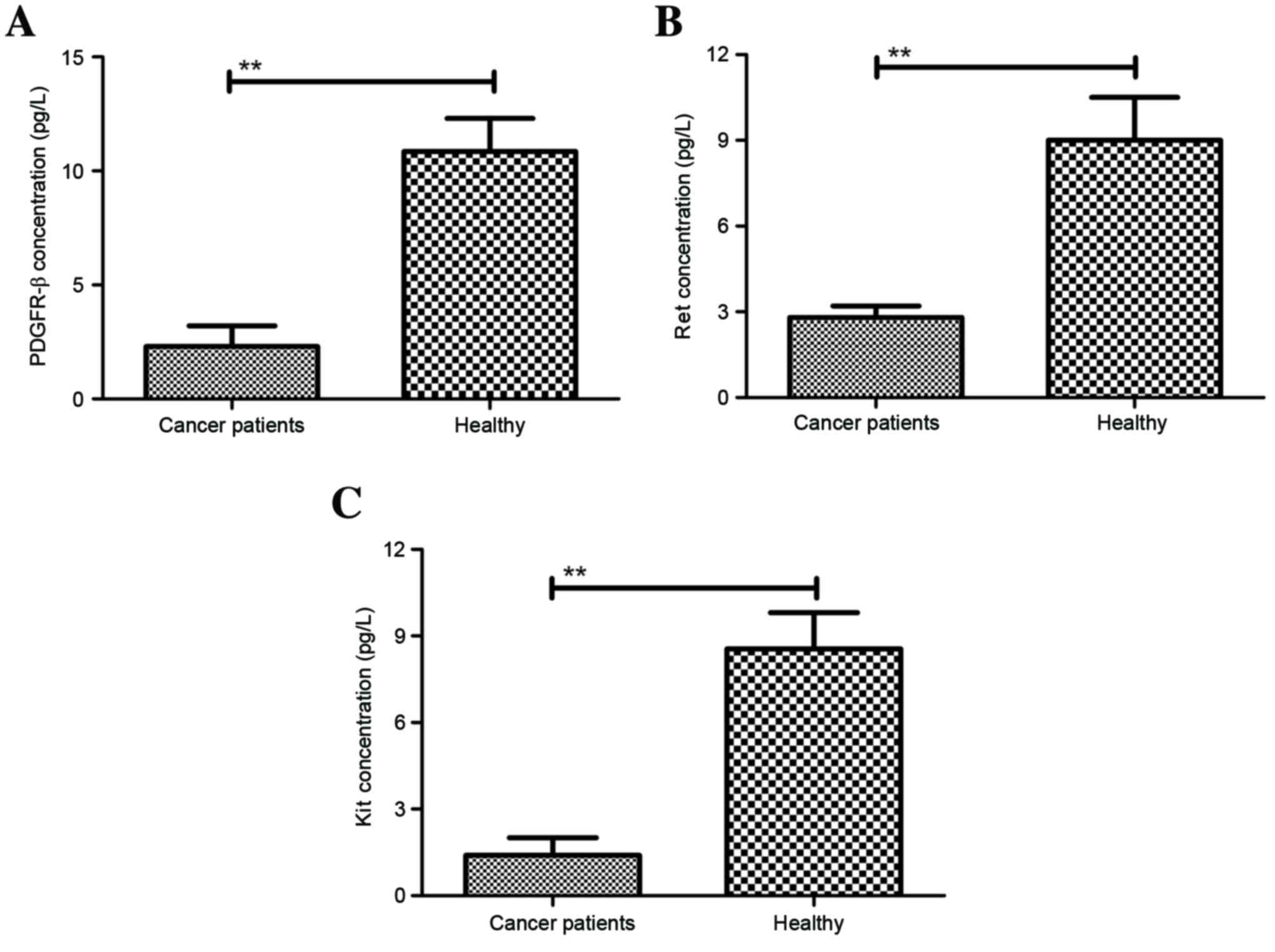
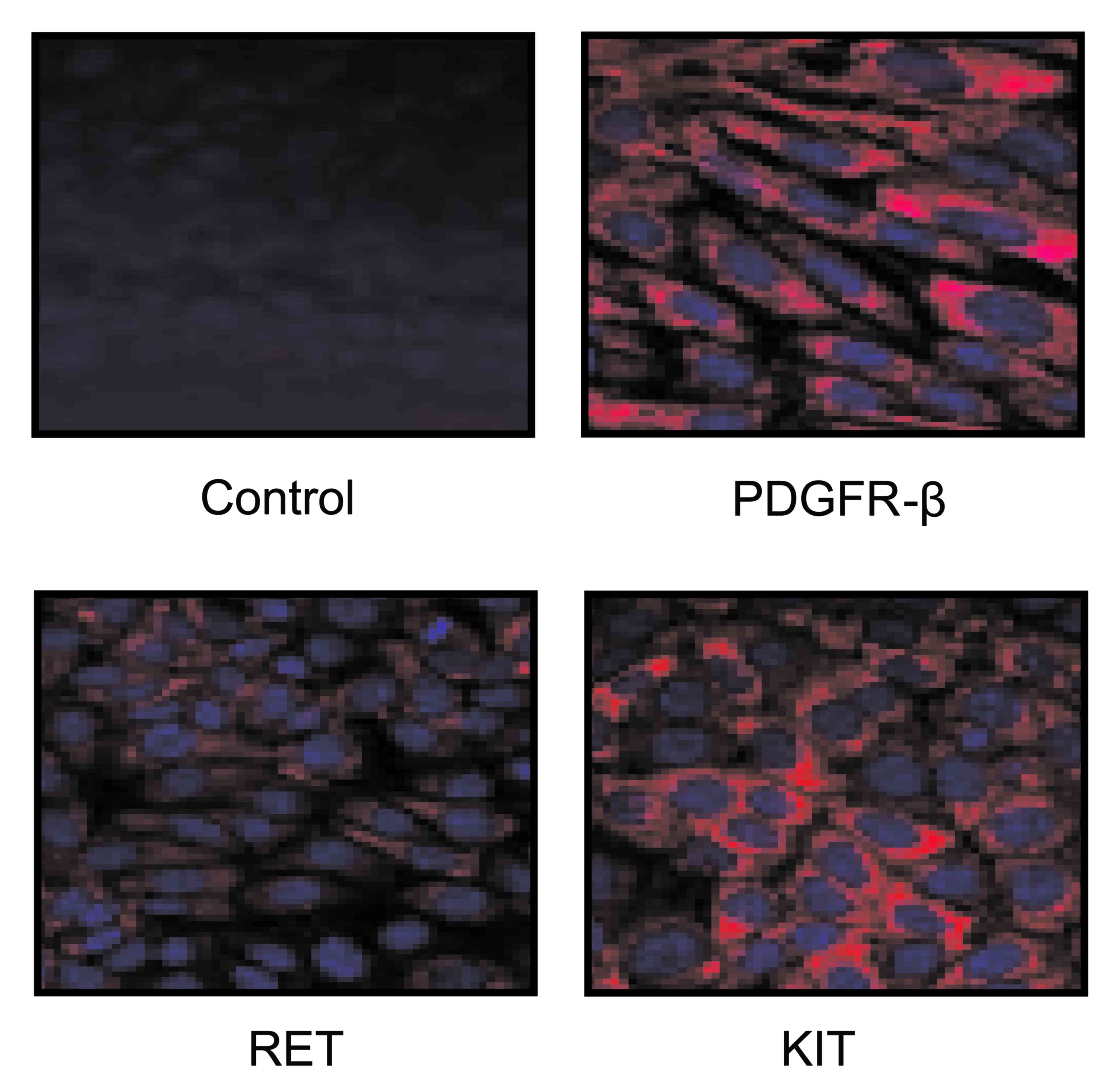
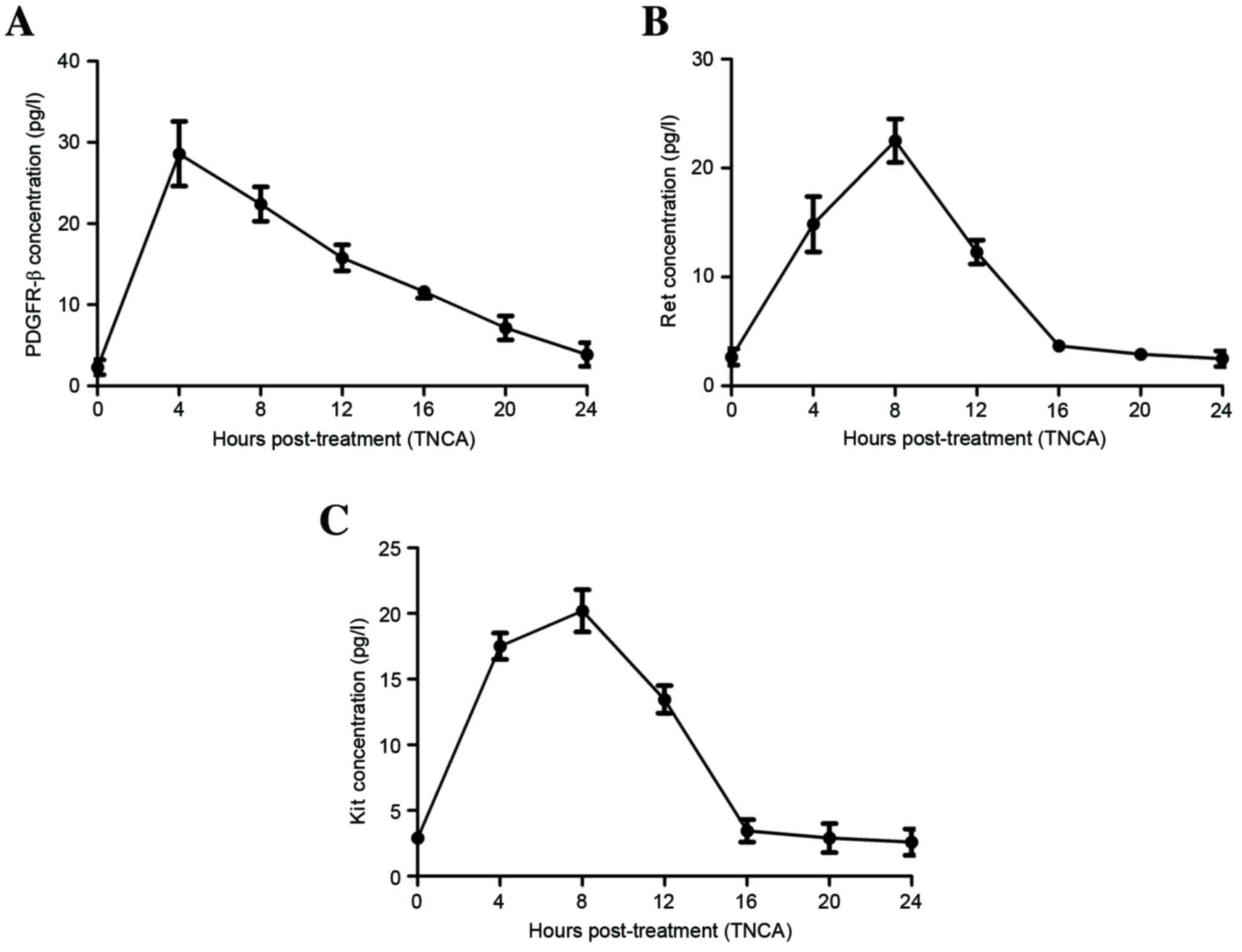
Nakajima T, Inokuchi K, Hattori T, Inoue
K, Taguchi T, Kondou T, Abe O, Kikuchi K, Tanabe T and Ogawa N:
Multi-institutional cooperative study of adjuvant
immunochemotherapy in gastric cancer-five-year survival rate. Gan
To Kagaku Ryoho. 16:799–806. 1989.(Article in Japanese). PubMed/NCBI
|
|
7
|
Jung SA, Park YM, Hong SW, Moon JH, Shin
JS, Lee HR, Ha SH, Lee DH, Kim JH, Kim SM, et al: Cellular
inhibitor of apoptosis protein 1 (cIAP1) stability contributes to
YM155 resistance in human gastric cancer cells. J Biol Chem.
290:9974–9985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izawa M, Mori T, Satoh T, Teramachi K and
Sairenji T: Identification of an alternative form of caspase-9 in
human gastric cancer cell lines: A role of a caspase-9 variant in
apoptosis resistance. Apoptosis. 4:321–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moody SE, Schinzel AC, Singh S, Izzo F,
Strickland MR, Luo L, Thomas SR, Boehm JS, Kim SY, Wang ZC and Hahn
WC: PRKACA mediates resistance to HER2-targeted therapy in breast
cancer cells and restores anti-apoptotic signaling. Oncogene.
34:2061–2071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiota M, Yokomizo A and Naito S:
Pro-survival and anti-apoptotic properties of androgen receptor
signaling by oxidative stress promote treatment resistance in
prostate cancer. Endocr Relat Cancer. 19:R243–R253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamato I, Sho M, Shimada K, Hotta K, Ueda
Y, Yasuda S, Shigi N, Konishi N, Tsujikawa K and Nakajima Y:
PCA-1/ALKBH3 contributes to pancreatic cancer by supporting
apoptotic resistance and angiogenesis. Cancer Res. 72:4829–4839.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanat O, O'Neil B and Shahda S: Targeted
therapy for advanced gastric cancer: A review of current status and
future prospects. World J Gastrointest Oncol. 7:401–410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebell MH, Culp MB and Radke TJ: A
systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazzei MA, Guerrini S, Mazzei FG,
Squitieri N Cioffi, Notaro D, de Donato G, Galzerano G, Sacco P,
Setacci F, Volterrani L and Setacci C: Follow-up of endovascular
aortic aneurysm repair: Preliminary validation of digital
tomosynthesis and contrast enhanced ultrasound in detection of
medium- to long-term complications. World J Radiol. 8:530–536.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schalk SG, Demi L, Bouhouch N, Kuenen MPJ,
Postema AW, de la Rosette JJMCH, Wijkstra H, Tjalkens TJ and Mischi
M: Contrast-enhanced ultrasound angiogenesis imaging by mutual
information analysis for prostate cancer localization. IEEE Trans
Biomed Eng. 64:661–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markic D, Krpina K, Ahel J, et al:
Different presentations of renal cell cancer on ultrasound and
computerized tomography. Urologia. 81:228–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Wang Z, Huang C, Gu X, Jia T, Zhang
A, Wu Z, Zhu L, Luo X, Zhao X, et al: A functional CT contrast
agent for in vivo imaging of tumor hypoxia. Small. 12:3995–4006.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krylova NS, Demkina AE, Poteshkina NG and
Khashieva FM: Tissue Doppler imaging and ultrasonic methods for
evaluating myocardial deformation in the diagnosis of hypertrophic
cardiomyopathy. Kardiologiia. 54:79–84. 2014.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
20
|
Shan L: Polyethylene glycol-coated and
folic acid-conjugated superparamagnetic iron oxide
nanoparticlesMolecular Imaging and Contrast Agent Database (MICAD).
Bethesda (MD): 2004
|
|
21
|
Nakamoto Y, Ishimori T, Sano K, Temma T,
Ueda M, Saji H and Togashi K: Clinical efficacy of dual-phase
scanning using Ga-DOTATOC-PET/CT in the detection of neuroendocrine
tumours. Clin Radiol. 71:1069.e1–5. 2016. View Article : Google Scholar
|
|
22
|
Angelov KG, Vasileva MB, Grozdev KS,
Sokolov MB and Todorov G: Clinical and pathological
characteristics, and prognostic factors for gastric cancer survival
in 155 patients in Bulgaria. Hepatogastroenterology. 61:2421–2424.
2014.PubMed/NCBI
|
|
23
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kargahi N, Razavi SM, Deyhimi P and
Homayouni S: Comparative evaluation of eosinophils in normal
mucosa, dysplastic mucosa and oral squamous cell carcinoma with
hematoxylin-eosin, Congo red, and EMR1 immunohistochemical staining
techniques. Electron Physician. 7:1019–1026. 2015.PubMed/NCBI
|
|
25
|
Soler M, Estevez MC, Villar-Vazquez R,
Casal JI and Lechuga LM: Label-free nanoplasmonic sensing of
tumor-associate autoantibodies for early diagnosis of colorectal
cancer. Anal Chim Acta. 930:31–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hopper AD and Campbell JA: Early diagnosis
of oesophageal cancer improves outcomes. Practitioner. 260:23–28,
3. 2016.PubMed/NCBI
|
|
27
|
Shah RB, Leandro G, Romerocaces G, Bentley
J, Yoon J, Mendrinos S, Tadros Y, Tian W and Lash R: Improvement of
Diagnostic Agreement among Pathologists in Resolving an ‘Atypical
Glands Suspicious for Cancer’ diagnosis in prostate biopsies
utilizing a novel ‘Disease-Focused Diagnostic Review’ quality
improvement process. Hum Pathol. 56:155–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haider MA, Yao X, Loblaw A and Finelli A:
Multiparametric magnetic resonance imaging in the diagnosis of
prostate cancer: A Systematic Review. Clin Oncol (R Coll Radiol).
28:550–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen WG, Yang CM, Xu LH, Zhang N, Liu XY,
Ma YG, Huo XL, Han YS, Tian DA and Zheng Y: Gene chip technology
used in the detection of HPV infection in esophageal cancer of
Kazakh Chinese in Xinjiang Province. J Huazhong Univ Sci Technol
Med Sci. 34:343–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li RZ, Shi JF, Zhou QZ, Wu RF, Li N, Wu
LN, Zhou YQ, Wang Q, Liu ZH, Liu B and Qiao YL: Evaluation of gene
chip technology for high risk type human papillomavirus in cervical
cancer screening. Zhonghua Yi Xue Za Zhi. 86:307–311. 2006.(In
Chinese). PubMed/NCBI
|
|
31
|
Hampton T: Breast cancer gene chip study
under way: Can new technology help predict treatment success? Jama.
291:2927–2930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watson RW: Gene-chip technology and
prostate cancer: The identification of new genes regulating tumour
progression. BJU Int. 91:3072003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kooiman J, Sijpkens YW, de Vries JP,
Brulez HF, Hamming JF, van der Molen AJ, Aarts NJ, Cannegieter SC,
Putter H, Swarts R, et al: A randomized comparison of 1-h sodium
bicarbonate hydration versus standard peri-procedural saline
hydration in patients with chronic kidney disease undergoing
intravenous contrast-enhanced computerized tomography. Nephrol Dial
Transplant. 29:1029–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mir MA, Bali BS, Mir RA and Wani H:
Assessment of the severity of acute pancreatitis by
contrast-enhanced computerized tomography in 350 patients. Ulus
Travma Acil Cerrahi Derg. 19:103–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kakimoto N, Chindasombatjaroen J, Tomita
S, Shimamoto H, Uchiyama Y, Hasegawa Y, Kishino M, Murakami S and
Furukawa S: Contrast-enhanced multidetector computerized tomography
for odontogenic cysts and cystic-appearing tumors of the jaws: Is
it useful? Oral Surg Oral Med Oral Pathol Oral Radiol. 115:104–113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Freling N, Roele E, Schaefer-Prokop C and
Fokkens W: Prediction of deep neck abscesses by contrast-enhanced
computerized tomography in 76 clinically suspect consecutive
patients. Laryngoscope. 119:1745–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Konduru N, Keller J, Ma-Hock L, Gröters S,
Landsiedel R, Donaghey TC, Brain JD, Wohlleben W and Molina RM:
Biokinetics and effects of barium sulfate nanoparticles. Part Fibre
Toxicol. 11:552014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liss P, Hansell P, Fasching A and Palm F:
Iodinated contrast media inhibit oxygen consumption in freshly
isolated proximal tubular cells from elderly humans and diabetic
rats: Influence of nitric oxide. Ups J Med Sci. 121:12–16. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mishra SK, Kumar BS, Khushu S, Tripathi RP
and Gangenahalli G: Increased transverse relaxivity in ultrasmall
superparamagnetic iron oxide nanoparticles used as MRI contrast
agent for biomedical imaging. Contrast Media Mol Imaging.
11:350–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kitoh Y: 5. Inspection of hepatocellular
carcinoma 3-Contrast for the diagnosis of hepatocellular carcinoma:
Techniques of image contrast and the choice of MR contrast agent.
Nihon Hoshasen Gijutsu Gakkai Zasshi. 72:441–451. 2016.(In
Japanese).
|
|
41
|
Zhang J, Liu J, Wang LM, Li ZY and Yuan Z:
Retroreflective-type Janus microspheres as a novel contrast agent
for enhanced optical coherence tomography. J Biophotonics.
10:878–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal-cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eichelberg C, Junker K, Ljungberg B and
Moch H: Diagnostic and prognostic molecular markers for renal cell
carcinoma: A critical appraisal of the current state of research
and clinical applicability. Eur Urol. 55:851–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hutson TE: Targeted therapies for the
treatment of metastatic renal cell carcinoma: Clinical evidence.
Oncologist. 16 Suppl 2:S14–S22. 2011. View Article : Google Scholar
|
|
45
|
Pinto RP, Lima FK, Kulkzynski JM and
Moreira LF: Expression of P16 and PDGFR-beta in gastric
adenocarcinoma. Rev Col Bras Cir. 36:199–203. 2009.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang F, Tang JM, Wang L, Wu PP and Zhang
M: Immunohistochemical detection of RET proto-oncogene product in
tumoral and nontumoral mucosae of gastric cancer. Anal Quant
Cytopathol Histopathol. 36:128–136. 2014.
|
|
47
|
Bdo N Borges, Eda S Santos, Bastos CE,
Pinto LC, Anselmo NP, Quaresma JA, Calcagno DQ, Burbano RM and
Harada ML: Promoter polymorphisms and methylation of E-cadherin
(CDH1) and KIT in gastric cancer patients from northern Brazil.
Anticancer Res. 30:2225–2233. 2010.PubMed/NCBI
|
|
48
|
Kingston MJ, Perriman DM, Neeman T, Smith
PN and Webb AL: Contrast agent comparison for three-dimensional
micro-CT angiography: A cadaveric study. Contrast Media Mol
Imaging. 11:319–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lakin BA, Patel H, Holland C, Freedman JD,
Shelofsky JS, Snyder BD, Stok KS and Grinstaff MW:
Contrast-enhanced CT using a cationic contrast agent enables
non-destructive assessment of the biochemical and biomechanical
properties of mouse tibial plateau cartilage. J Orthop Res.
34:1130–1138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sudarski S, Henzler T and Schoenberg SO:
Post-therapeutic positron emission tomography/computed tomography
for early detection of non-small cell lung cancer recurrence.
Transl Lung Cancer Res. 2:295–303. 2013.PubMed/NCBI
|
|
51
|
Hagberg GE, Mamedov I, Power A, Beyerlein
M, Merkle H, Kiselev VG, Dhingra K, Kubìček V, Angelovski G and
Logothetis NK: Diffusion properties of conventional and
calcium-sensitive MRI contrast agents in the rat cerebral cortex.
Contrast Media Mol Imaging. 9:71–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Turetschek K, Preda A, Novikov V, Brasch
RC, Weinmann HJ, Wunderbaldinger P and Roberts TP: Tumor
microvascular changes in antiangiogenic treatment: Assessment by
magnetic resonance contrast media of different molecular weights. J
Magn Reson Imaging. 20:138–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Samei E, Saunders RS, Badea CT, Ghaghada
KB, Hedlund LW, Qi Y, Yuan H, Bentley RC and Mukundan S Jr:
Micro-CT imaging of breast tumors in rodents using a liposomal,
nanoparticle contrast agent. Int J Nanomedicine. 4:277–282. 2009.
View Article : Google Scholar : PubMed/NCBI
|