Introduction
Corin is a type II transmembrane serine protease
that is highly expressed in cardiomyocytes (1–4). The
human corin gene is mapped to chromosome 4p12-13 (1) and encodes a polypeptide of 1,042 amino
acids (5). In SDS-polyacrylamide gel
electrophoresis and western blot analysis, human corin protein has
an apparent molecular mass of ~190 kDa (6,7). The
full corin protein is usually inactive and must be cleaved by
proprotein convertase subtilisin/kexin type 6 (PCSK6) at
Arg801-Ile802 in order to be activated, which is why a ~40 kDa band
may be detected in cells expressing wild-type corin. A previous
study showed that three different soluble fragments derived from
corin molecules, with molecular masses of ~180, ~160 and ~100 kDa,
could be detected in the culture medium of corin-expressing human
embryonic kidney (HEK) 293 cells by western blotting, and that
these different sized fragments were produced by either
ADAM10-mediated shedding or corin autocleavage (8).
Like other serine proteases, corin also has a
catalytic domain, namely a trypsin-like protease domain, which
plays a prominent role in its biological function. The corin
proteolytic domain cleaves pro-atrial natriuretic peptide (pro-ANP)
to form mature atrial natriuretic peptide (ANP) in the heart
(6,9–11). Once
activated, ANP is released into the circulation. In target organs,
such as the peripheral blood vessels and kidneys, ANP binds to and
activates its receptor, natriuretic peptide receptor-A (NPR-A).
NPR-A increases intracellular cyclic guanosine monophosphate (cGMP)
production to promote vasodilation, natriuresis and diuresis,
consequently maintaining blood pressure and volume balance
(12–14).
Corin plays a vital role in the control of blood
pressure and volume homeostasis. In corin gene knockout mice,
systolic pressure, diastolic pressure and mean arterial pressure
were significantly higher than in wild-type mice (13,15), and
the hypertension was exacerbated by dietary salt loading (16,17).
Furthermore, in African Americans, polymorphisms (T555I/Q568P) in
the corin gene have been shown to be associated with high blood
pressure and cardiac hypertrophy (18).
As well as cardiomyocytes, corin has also been
reported to be expressed in other tissues, including kidney,
pregnant uterus, bone, skin and brain (12,19–21). In
the kidney, corin was detected in epithelial cells, with segmental
expression in the proximal tubule, thick ascending limb, connecting
tubule, and throughout the collecting duct (12,22).
Fang et al reported that the levels of urinary corin were
markedly reduced in chronic kidney diseases, and that the reduction
correlated with disease severity (23). This suggested that corin may be shed
from the cell surface of renal tubules and that urinary corin
levels could be associated with the severity of kidney
diseases.
Proteinuric kidney diseases are diseases associated
with inflammation and immunity. Numerous studies have reported that
inflammatory cytokines are increased in kidney diseases (24,25). In
order to determine the influence of cytokines on corin expression
and the effect of this on proteinuric kidney diseases, we assessed
the levels of urinary corin and plasma cytokines and the
correlations between corin and cytokine levels in vivo and
in vitro.
Materials and methods
Patient samples
Plasma and urine samples were collected at the First
Affiliated Hospital of Soochow University from 31 patients with
proteinuric kidney diseases [15 cases of chronic glomerulonephritis
(CGN) and 16 cases of nephrotic syndrome (NS)] treated in the
Nephrology Department, and from 21 healthy individuals who
underwent a routine health check-up and reported no history of
proteinuria. The present study was approved by the Ethics Committee
of Soochow University (Soochow, China) and conducted in accordance
with the approved guidelines. Written informed consent was obtained
from all individuals recruited to the present study. At the time of
sample collection, we excluded patients with infection, malignant
cancer or diabetic nephropathy, and those with systemic diseases of
the immune system. Characteristics of normal controls and patients
are shown in Table I. Blood samples
were anticoagulated with EDTA, while urine samples were collected
without any anticoagulation. Blood and urine samples were
centrifuged at 1,000 × g for 10 min, then supernatant was collected
and stored at −80°C.
 | Table I.Characteristics of normal controls and
patients. |
Table I.
Characteristics of normal controls and
patients.
| Characteristics | Control (n=21) | Patients (n=31) |
|---|
| Age (years) | 43.00±2.02 | 49.81±2.53 |
| Sex (n) |
|
|
| Male | 12 | 18 |
|
Female | 9 | 13 |
| eGFR (ml/min/1.73
m2) | 98.12±3.69 | 97.10±3.18 |
| CRP (mg/l) | N/D | 1.96±0.47 |
| Proteinuria (g/24
h) | N/D | 1.90±0.41 |
Enzyme-linked immunosorbent assay
(ELISA)
Corin levels in plasma and urine samples were
detected using an ELISA kit (R&D Systems, Minneapolis, MN,
USA). The assay was performed in 96-well plates, with the capture
antibody added the night prior to the assay. During detection,
plasma samples were diluted 1:2 with 1% bovine serum albumin
(BSA).
Plasma cytokines [cytokines interleukin-1β (IL-1β)
and tumor necrosis factor-α (TNF-α)] were also detected with ELISA
kits (Bio Legend System, San Diego, CA). For these assays, plasma
was diluted 1:2.
Urinary albumin comes from the glomeruli, and
increased levels are indicative of glomerular injury. Urinary
β2-microglobulin (β2-MG) mostly originates from the
renal tubules. Albumin and β2-MG levels in the urinary samples were
assessed at the Renal Medicine Laboratory of the First Affiliated
Hospital of Soochow University.
C-reactive protein (CRP) and estimated
glomerular filtration rate (eGFR)
CRP in plasma was detected as part of the routine
biochemical analysis at the First Affiliated Hospital of Soochow
University. eGFR was calculated by the CKD-EPI equation, which
takes into account the creatinine value and patient age. The
creatinine value was also obtained by biochemical analysis.
Cell culture studies
293 cells (26) were
obtained from ATCC. 293-corin cells were stably transfected with
pcDNA3.1-h corin (plasmid containing full-length human corin;
Invitrogen, Carlsbad, USA) (27),
which was constructed by the Cyrus Tang Medical Institute of
Soochow University (Soochow, China) (9,22,28). In
the present study, 293 corin cells were cultured in Dulbecco's
modified Eagle's medium with 500 ng/ml G418, at 37°C with 95% air
and 5% CO2. The pcDNA3.1-h corin plasmid (Invitrogen)
also incorporated a C-terminal V5 tag, allowing the corin protein
to be detected by western blotting using an anti-V5 antibody
(Invitrogen).
In order to study the correlations between cytokine
and corin levels, various concentrations (0, 0.1, 1 and 10 ng/ml)
of the cytokines IL-1β, TNF-α and IL-6 were added to the culture
medium. The group (0 ng/ml) was added 10 ul medium as buffer
control. Subsequently, the conditioned medium was collected and the
cells were lysed with lysis buffer for western blotting.
Western blot analysis
Protein samples collected from conditioned medium or
cell lysates were mixed with loading buffer and heated at 98°C for
5 min. After separation by SDS-polyacrylamide gel electrophoresis,
the proteins were transferred to nitrocellulose membranes. After
incubation with 5% milk, the membranes were probed with the
aforementioned anti-V5-HRP antibody in order to label the V5 tag of
recombinant corin proteins. Prior to use, the anti-V5-HRP antibody
was diluted 1:5,000 in 0.05% Tween Tris-HCl-buffered saline
solution (TBST).
In the western blot analysis, corin protein in the
culture medium was shown as three bands (~180, ~160 and ~100 kDa)
on the films, which were measured by ImageJ. To normalize the
values, the levels of corin in the medium were expressed as corin
in medium/corin in lysate, in order to evaluate corin shedding.
Corin values in the lysate were expressed as corin in lysate/GAPDH
level, in order to evaluate potential differences in the rate of
corin synthesis.
Statistical analysis
The statistical analysis of ELISA measurements was
performed using SPSS 19 software, while western blot analysis
results were analyzed using ImageJ and Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA) software. All data were presented as the
means ± SE. A Pearson's correlation analysis was used to test the
correlations between the levels of two proteins. For statistical
comparison, a Student's t-test or one-way ANOVA were used.
P<0.05 was considered to indicate statistical significance,
while P<0.01 was considered to show marked significance.
Results
Results in vivo
Corin levels in plasma and urine
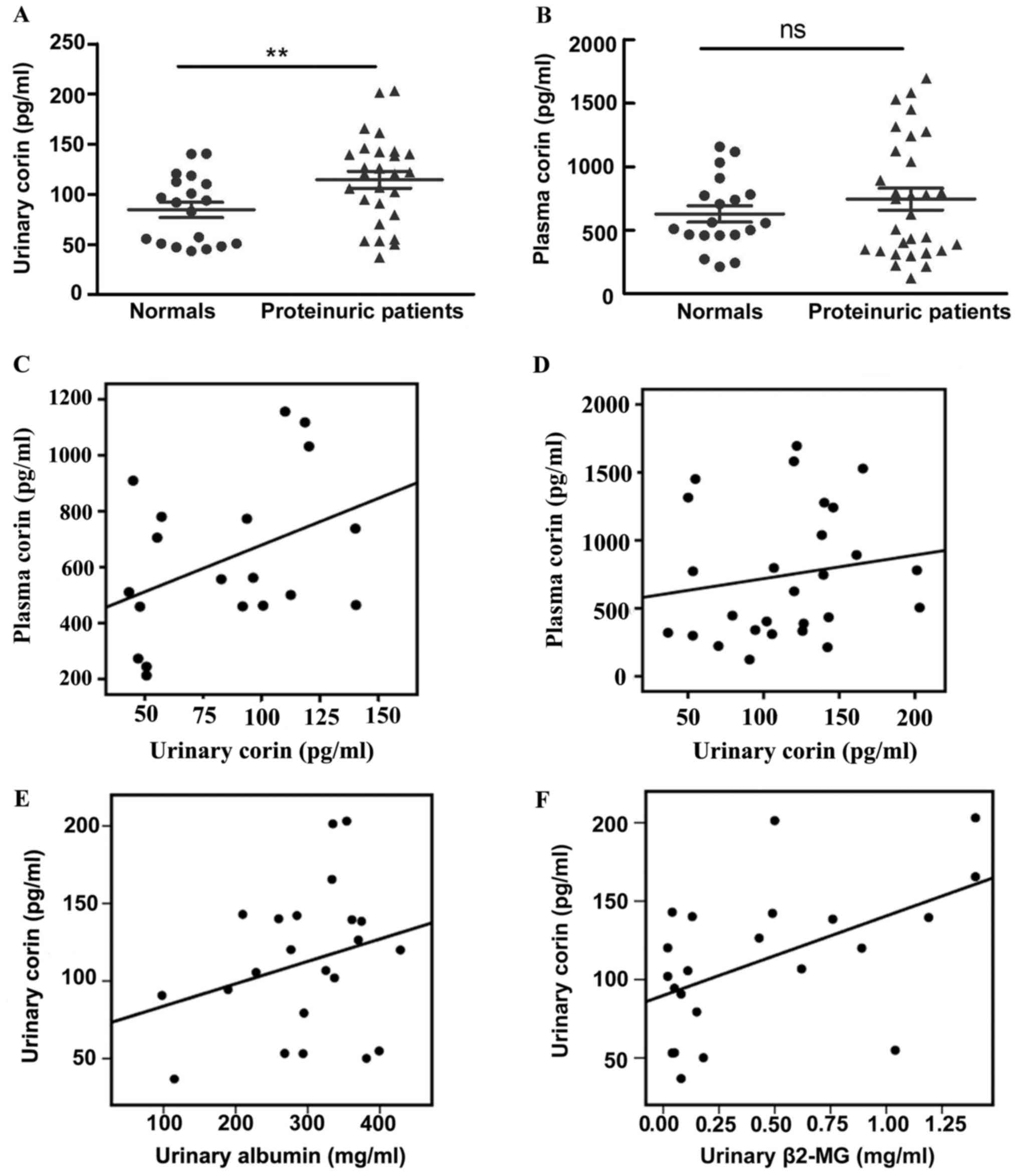
We detected the plasmatic and urinary corin levels
in patients with CGN or NS and in healthy individuals. Corin could
be detected in the plasma and urine (Fig. 1A and B); however, no significant
correlation was found between plasma and urinary corin levels,
whether in healthy individuals (r=0.40, P>0.05) (Fig. 1C) or in patients with proteinuric
kidney disease (r=0.16, P>0.05) (Fig.
1D). To study the origin of urinary corin, we analyzed the
association between corin level and urinary albumin (which mostly
derives from glomeruli) and β2-MG (which mostly originates from the
renal tubules). The level of urinary corin was positively
correlated with urinary β2-MG (r=0.52, P=0.01) (Fig. 1F), but no correlation was found
between urinary corin and albumin levels (Fig. 1E) (r=0.05, P>0.05).
Compared with the healthy group, corin abundance was
increased in the urine of patients with proteinuric kidney disease
(84.45±7.75 vs. 114.50±8.50 pg/ml, respectively; P=0.02) (Fig. 1A). However, no difference in the
level of plasma corin was found between the two groups
[744.90±85.29 (patients group) vs. 627.10±64.41 pg/ml (healthy
group); P>0.05) (Fig. 1B).
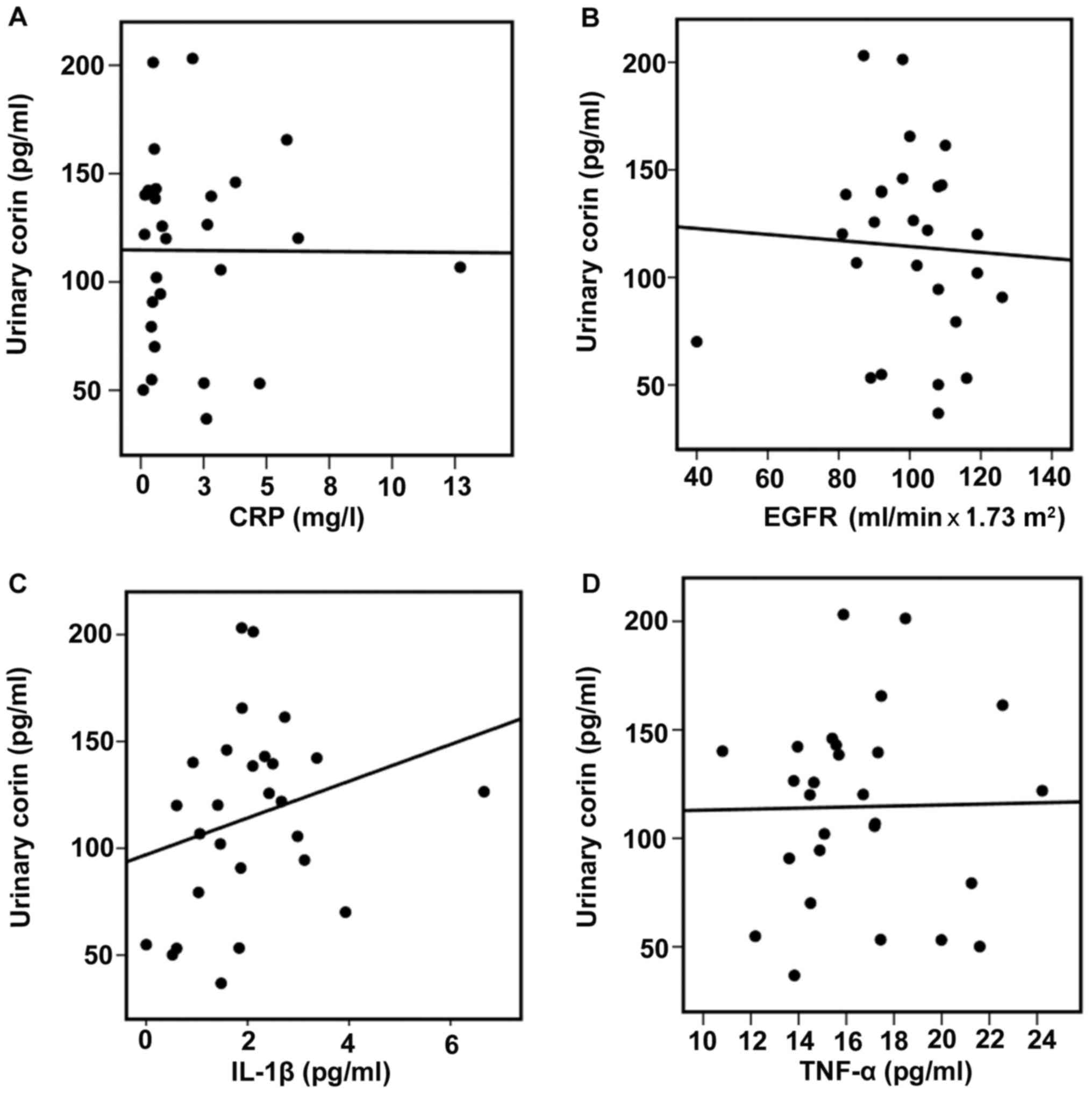
Soluble corin and cytokine levels
To further study a possible explanation for the
increased corin levels in the urine of patients with proteinuric
kidney diseases compared with those of healthy controls, urinary
corin levels were analyzed with respect to plasmatic CRP, eGFR, and
plasma IL-1β and TNF-α levels. There were no correlations between
urinary corin and CRP (r=0.10, P>0.05) or eGFR (r=0.15,
P>0.05) (Fig. 2A and B). No
significant differences in IL-1β or TNF-α levels were found between
healthy controls and patients [IL-1β, 1.84±0.25 (healthy group) vs.
1.87±0.27 pg/ml (patients group), P>0.05; TNF-α, 17.61±0.96
(healthy group) vs. 16.19±0.65 pg/ml (patients group), P>0.05].
However there was a positive correlation between urinary corin and
plasma IL-1β levels (r=0.40; P=0.03) (Fig. 2C). No correlation was found between
urinary corin and plasma TNF-α (Fig.
2D).
Results in vitro
Corin and IL-1β
To further study the correlation between cytokines
and corin, 293-corin cells were cultured. Various concentrations
(0, 0.1, 1 and 10 ng/ml) of IL-1β were added to the culture medium.
After 24 h, conditioned medium was collected and cells were lysed,
and western blot analysis was performed. As the concentration of
IL-1β increased, the amount of corin in the conditioned medium
increased significantly [expression of corin in medium/corin gross
level: 0 ng/ml, 0.20±0.02 (P<0.01 vs. 0.1, 1 and 10 ng/ml); 0.1
ng/ml, 0.38±0.02 (P>0.05 vs. 1 ng/ml; P<0.01 vs. 10 ng/ml); 1
ng/ml, 0.37±0.03 (P<0.01 vs. 10 ng/ml); 10 ng/ml, 0.50±0.05]
(Fig. 3A), whereas the levels of
corin in the lysate were not significantly different between cells
treated with 0, 0.1, 1 or 10 ng/ml of IL-1β (expression of corin in
lysate/GAPDH: 1.48±0.09 vs. 1.42±0.17 vs. 1.74±0.20 vs. 1.57±0.15,
respectively; all P>0.05) (Fig.
3B). Subsequently, we added 1 ng/ml IL-1β to the cell culture
medium for 12, 24, 36 or 48 h, and corin levels in the medium were
shown to increase time-dependently (0.20±0.02 vs. 1.44±0.12 vs.
2.91±0.30 vs. 5.65±0.38, respectively; P<0.01) (Fig. 3C); however, the levels of corin in
the lysate of these groups did not alter significantly
(P>0.05).
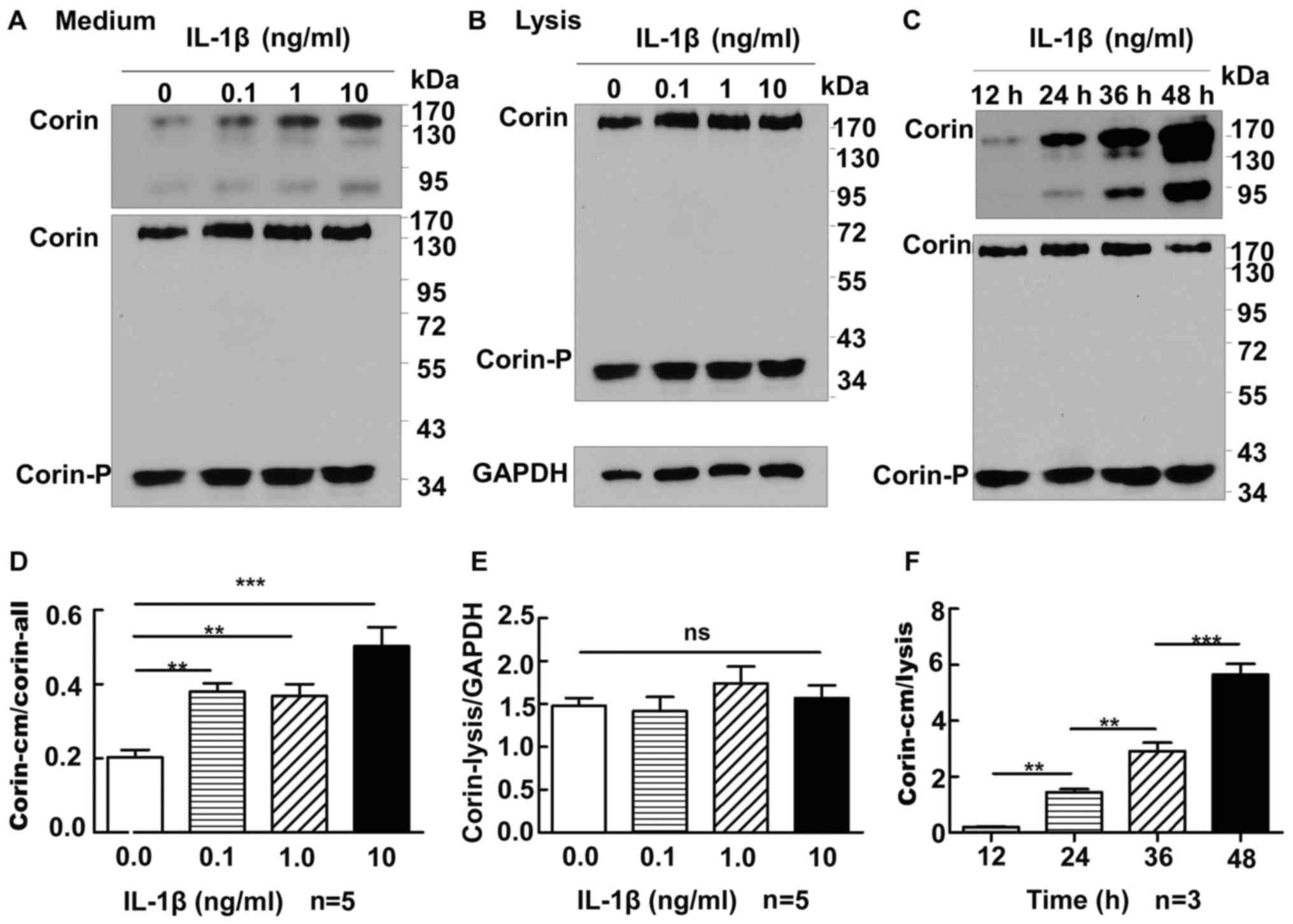 | Figure 3.Correlation between IL-1β and corin in
HEK 293 cells expressing corin. (A) In the cell culture medium,
corin molecules of ~180, ~160 and ~100 kDa could be detected. In
the cell lysate, two bands, ~190 kDa full length corin and a
~40-kDa active form, were detected. The levels of corin in the
culture medium were expressed as corin in medium/corin in lysate.
As IL-1β increased, the level of corin in the cell culture medium
also increased (P<0.05). (B) The amount of corin in the cell
lysate did not alter significantly with different concentrations of
IL-1β (P>0.05). (C) Corin abundance in the culture medium
increased with the duration of IL-1β treatment (P<0.01). (D-F)
Results of statistical analyses of (A), (B) and (C), respectively.
**P<0.01, and ***P<0.0001. One-way ANOVA was used for the
statistical comparison among four groups. IL, interleukin; HEK,
human embryonic kidney. |
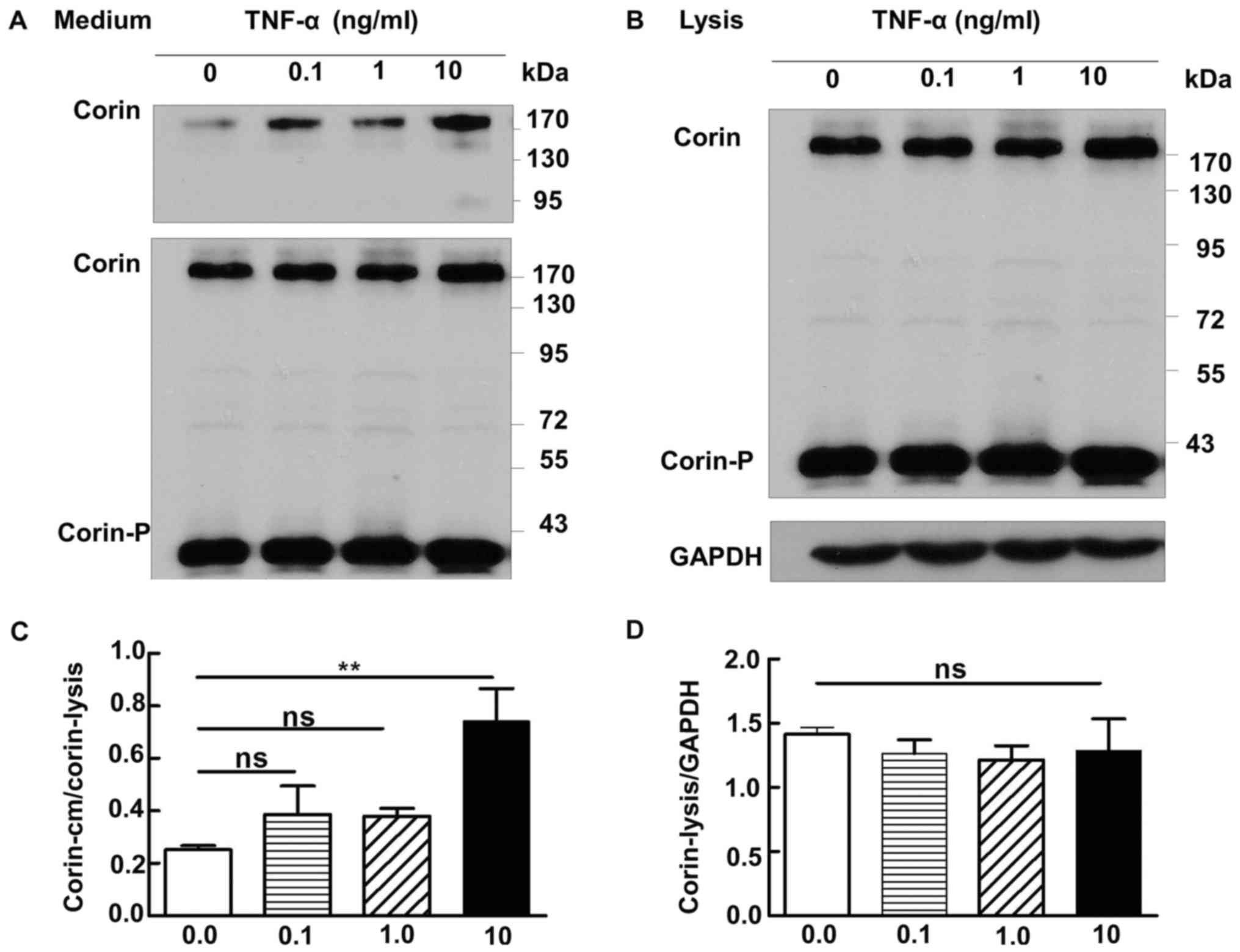
Corin and TNF-α
We cultured 293-corin cells in medium containing
various concentrations of TNF-α (0, 0.1, 1 or 10 ng/ml), then
collected culture medium and cell lysate for analysis by western
blotting. Only when TNF-α reached a concentration of 10 ng/ml was
the level of corin in the medium increased significantly compared
with the control group (0 ng/ml) (P=0.02); no significant increases
were found in cells treated with 0.1 or 1 ng/ml TNF-α (P>0.05)
(Fig. 4A). The levels of corin in
the lysate did not differ significantly between any of the
treatment groups (1.42±0.05 vs. 1.27±0.16 vs. 1.22±0.11 vs.
1.29±0.25, respectively; P>0.05) (Fig. 4B).
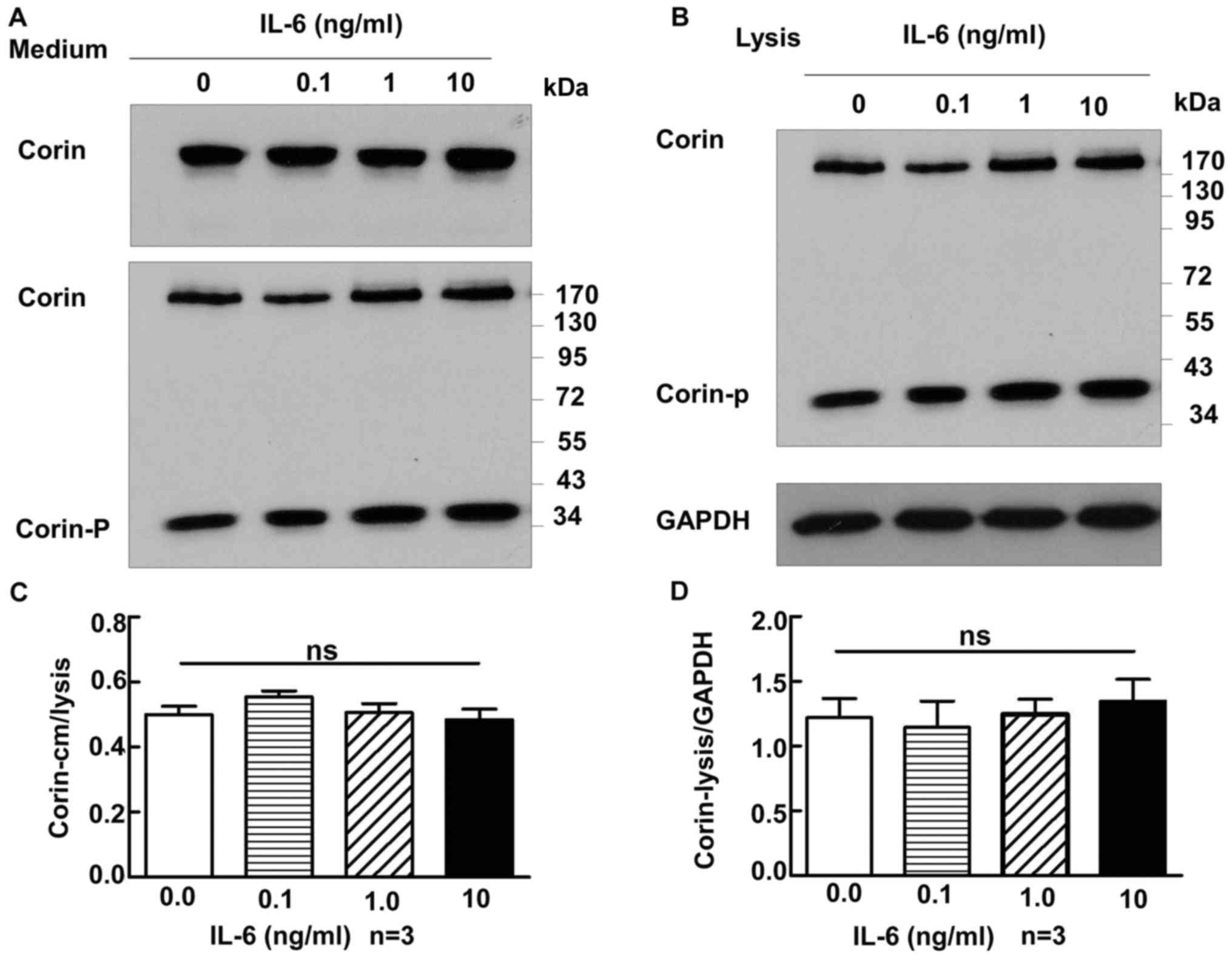
Corin and IL-6
A similar experiment was performed to study the
correlation between IL-6 and corin levels. The concentrations of
IL-6 used were 0, 0.1, 1 and 10 ng/ml. The amount of corin did not
change markedly with increases in IL-6, in either the culture
medium or the cell lysate (medium, 0.50±0.03 vs. 0.56±0.02 vs.
0.51±0.03 vs. 0.48±0.03, respectively, P>0.05; lysate, 1.22±0.15
vs. 1.15±0.20 vs. 1.25±0.12 vs. 1.35±0.17, respectively, P>0.05)
(Fig. 5A and B).
Discussion
Corin is a serine protease that regulates blood
pressure and volume balance (13,14).
Besides cardiomyocytes, corin has also been detected in the kidney.
Corin is distributed in epithelial cells, including those of the
proximal tubule, thick ascending limb, connecting tubule, and
collecting duct, but not the glomerulus (12,22). The
specific distribution of corin in the kidney suggests that corin
may play a specific role in regulating kidney physiology.
Previously, we found that urinary corin in chronic kidney disease
(CKD) was reduced, and that this reduction correlated with disease
severity (23). However, the factors
that can influence corin expression in primary proteinuric kidney
diseases remain largely undetermined. Therefore, in the present
study, we analyzed the relationship between corin and CRP, eGFR and
three of the most common cytokines in order to investigate a
possible corin expression pattern in proteinuric kidney
diseases.
Although corin is a transmembrane protein (1), soluble corin is detectable by ELISA in
plasma and urine samples. This suggests that corin may be shed from
cell membranes into the circulation or urine. The primary source of
corin in the urine is corin shedding from tubule epithelial cells;
however, this is not the case for corin in the plasma, as we found
that there was no correlation between urinary corin and plasmatic
corin levels. As urinary albumin mostly comes from glomeruli and
β2-MG mostly originates from renal tubules, we studied their
correlation with urinary corin levels to confirm the origin of the
urinary corin. We found that the level of urinary corin was
correlated with urinary β2-MG but not urinary albumin, which
suggested that urinary corin may originate from the renal tubules.
Moreover, urinary corin levels in proteinuric patients were
significantly higher than those in healthy controls, indicating
that the increased level of urinary corin may be related to tubular
injury in proteinuric diseases.
Proteinuric kidney diseases are usually associated
with inflammation and immunity, and microinflammation can
accelerate the progression of CKD (24,25). To
further study which factors could influence the level of corin in
the urine, we analyzed the correlation between urinary corin and
plasmatic CRP, eGFR and plasmatic cytokine levels (IL-1β, TNF-α and
IL-6). Although there were no differences in the plasmatic IL-1β
and TNF-α levels between the proteinuric kidney disease group and
the healthy control group, a positive correlation was identified
between urinary corin and plasma IL-1β levels in proteinuric
patients, which suggested that corin levels in the urine were
sensitive to changes in IL-1β levels in the plasma. This may
indicate that, in kidneys with a low-level inflammatory state, the
levels of corin in the urine increase significantly. Compared with
IL-1β, only when TNF-α reached to 10 ng/ml, the level of corin in
the medium would augmented significantly. But the concentration of
TNF-α (10 ng/ml) is higher than the plasma level. To confirm the
relationship between corin and the cytokines IL-1β, TNF-α and IL-6,
we investigated the impact of these three cytokines on corin levels
in vitro. The results revealed that IL-β and TNF-α may
accelerate corin shedding from the cell membrane, without having a
significant effect on corin synthesis; this was demonstrated in
293-corin cells experiments, which showed that stimulation with
increasing concentrations of IL-1β or TNF-α resulted in increased
levels of corin in the cell culture medium, whereas corin abundance
in the cell lysate samples remained unchanged. Corin responded to
IL-1β in a time- and dose-dependent manner. However, IL-6 did not
appear to have an effect on the shedding or synthesis of corin.
In summary, the levels of urinary corin in patients
with proteinuric kidney diseases were higher than those in normal
controls, and increased urinary corin levels were positively
correlated with levels of the cytokines IL-1β. The results
indicated that certain cytokines could accelerate corin shedding
from the cell membrane, but had no significant effect on corin
synthesis. Further studies on corin mRNA levels and downstream
signaling pathway protein (ANP, phosphodiesterase type 5, etc)
stimulated by cytokine must be done in the future. In addition,
IL-1β and TNF-α often act synergistically with other cytokines,
such as IFN-γ in immunoinflammatory responses, whether IFN-γ plays
an important role in corin metabolism still need to study. Both
IL-1β and TNF-α play a role in corin shedding, the binding sites on
the promoter of corin are still unknown. IL-10 is an
anti-inflammatory cytokine, we will go on study whether IL-10 can
revert IL-1β and TNF-α induced corin production in vitro or
not.
Acknowledgements
The present study was supported by a grant from the
Project of Healthy in Jiangsu Province (no. H201415).
References
|
1
|
Hooper JD, Scarman AL, Clarke BE, Normyle
JF and Antalis TM: Localization of the mosaic transmembrane serine
protease corin to heart myocytes. Eur J Biochem. 267:6931–6937.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Q: The serine protease corin in
cardiovascular biology and disease. Front Biosci. 12:4179–4190.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gladysheva IP, Robinson BR, Houng AK,
Kováts T and King SM: Corin is co-expressed with pro-ANP and
localized on the cardiomyocyte surface in both zymogen and
catalytically active forms. J Mol Cell Cardiol. 44:131–142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Sen S, Young D, Wang W, Moravec CS
and Wu Q: Protease corin expression and activity in failing hearts.
Am J Physiol Heart Circ Physiol. 299:H1687–H1692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan W, Sheng N, Seto M, Morser J and Wu Q:
Corin, a mosaic transmembrane serine protease encoded by a novel
cDNA from human heart. J Biol Chem. 274:14926–14935. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan W, Wu F, Morser J and Wu Q: Corin, a
transmembrane cardiac serine protease, acts as a pro-atrial
natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA.
97:pp. 8525–8529. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Cao P, Dong N, Peng J, Zhang C,
Wang H, Zhou T, Yang J, Zhang Y, Martelli EE, et al: PCSK6-mediated
corin activation is essential for normal blood pressure. Nat Med.
21:1048–1053. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang J, Wu S, Wang W, Chen S, Peng J,
Zhang X and Wu Q: Ectodomain shedding and autocleavage of the
cardiac membrane protease corin. J Biol Chem. 286:10066–10072.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu F and Wu Q: Corin-mediated processing
of pro-atrial natriuretic peptide in human small cell lung cancer
cells. Cancer Res. 63:8318–8322. 2003.PubMed/NCBI
|
|
10
|
Chan JC, Knudson O, Wu F, Morser J, Dole
WP and Wu Q: Hypertension in mice lacking the proatrial natriuretic
peptide convertase corin. Proc Natl Acad Sci USA. 102:pp. 785–790.
2005; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu F, Yan W, Pan J, Morser J and Wu Q:
Processing of pro-atrial natriuretic peptide by corin in cardiac
myocytes. J Biol Chem. 277:16900–16905. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polzin D, Kaminski HJ, Kastner C, Wang W,
Krämer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, et
al: Decreased renal corin expression contributes to sodium
retention in proteinuric kidney diseases. Kidney Int. 78:650–659.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armaly Z, Assady S and Abassi Z: Corin: A
new player in the regulation of salt-water balance and blood
pressure. Curr Opin Nephrol Hypertens. 22:713–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klein JD: Corin: An ANP protease that may
regulate sodium reabsorption in nephrotic syndrome. Kidney Int.
78:635–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nigrovic PA, Gray DH, Jones T, Hallgren J,
Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C and Lee DM:
Genetic inversion in mast cell-deficient (Wsh) mice interrupts
corin and manifests as hematopoietic and cardiac aberrancy. Am J
Pathol. 173:1693–1701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Xu-Cai YO, Chen S and Wang W: Corin:
New insights into the natriuretic peptide system. Kidney Int.
75:142–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Shen J, Cui Y, Jiang J, Chen S,
Peng J and Wu Q: Impaired sodium excretion and salt-sensitive
hypertension in corin-deficient mice. Kidney Int. 82:26–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dries DL, Victor RG, Rame JE, Cooper RS,
Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W and Drazner MH: Corin
gene minor allele defined by 2 missense mutations is common in
blacks and associated with high blood pressure and hypertension.
Circulation. 112:2403–2410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ichiki T, Huntley BK, Heublein DM,
Sandberg SM, McKie PM, Martin FL, Jougasaki M and Burnett JC Jr:
Corin is present in the normal human heart, kidney, and blood, with
pro-B-type natriuretic peptide processing in the circulation. Clin
Chem. 57:40–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Charoenpanich A, Wall ME, Tucker CJ,
Andrews DM, Lalush DS and Loboa EG: Microarray analysis of human
adipose-derived stem cells in three-dimensional collagen culture:
Osteogenesis inhibits bone morphogenic protein and Wnt signaling
pathways, and cyclic tensile strain causes upregulation of
proinflammatory cytokine regulators and angiogenic factors. Tissue
Eng Part A. 17:2615–2627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enshell-Seijffers D, Lindon C and Morgan
BA: The serine protease Corin is a novel modifier of the Agouti
pathway. Development. 135:217–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong L, Wang H, Dong N, Zhang C, Xue B and
Wu Q: Localization of corin and atrial natriuretic peptide
expression in human renal segments. Clin Sci (Lond). 130:1655–1664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang C, Shen L, Dong L, Liu M, Shi S, Dong
N and Wu Q: Reduced urinary corin levels in patients with chronic
kidney disease. Clin Sci (Lond). 124:709–717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalavrizioti D, Gerolymos M, Rodi M,
Kalliakmani P, Provatopoulou S, Eleftheriadis T, Mouzaki A and
Goumenos DS: T helper (Th)-cytokines in the urine of patients with
primary glomerulonephritis treated with immunosuppressive drugs:
Can they predict outcome? Cytokine. 76:260–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conley SM, Abais JM, Boini KM and Li PL:
Inflammasome activation in chronic glomerular diseases. Curr Drug
Targets. 18:1019–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stepanenko AA and Dmitrenko VV: HEK293 in
cell biology and cancer research: Phenotype, karyotype,
tumorigenicity, and stress-induced genome-phenotype evolution.
Gene. 569:182–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong N, Zhou T, Zhang Y, Liu M, Li H,
Huang X, Liu Z, Wu Y, Fukuda K, Qin J and Wu Q: Corin mutations
K317E and S472G from preeclamptic patients alter zymogen activation
and cell surface targeting. J Biol Chem. 289:17909–17916. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knappe S, Wu F, Masikat MR, Morser J and
Wu Q: Functional analysis of the transmembrane domain and
activation cleavage of human corin: Design and characterization of
a soluble corin. J Biol Chem. 278:52363–52370. 2003. View Article : Google Scholar : PubMed/NCBI
|