Introduction
Osteosarcoma is a malignant primary tumor of the
bone, which can produce a bone-like tumor comprised of bone
connective tissues, including collagen, elastic fibers and ground
substances (1,2). It is the most common primary tumor of
malignant bone tumors (1).
Osteosarcoma may occur at any age, though has been most frequently
observed in adolescents <25 years old, and is considered to
occur more often in males than females (3). Due to its prevalence, it is important
to enhance the clinical efficacy of treatment for osteosarcoma
(4). Upon diagnosis of osteosarcoma,
in addition to surgery, chemotherapy is crucial for more effective
treatment (5,6).
Previous studies have reported that cell necrosis
was associated with chemotherapy, rather than occurring as a
spontaneous event, in osteosarcoma patients upon receipt of
preoperative chemotherapy (7,8). The
histological response to preoperative chemotherapy, particularly
tissue necrosis, has been implicated as an important clinical
predictor of the outcome of operative treatment for osteosarcoma
(9,10); in patients with a necrosis rate
>70%, a significantly higher rate of disease-free survival (DFS)
was observed when compared with those exhibiting a necrosis rate
<70% (9). However, Li et
al identified no survival advantage for a tumor necrosis rate
of 90% (11). Nevertheless, it has
been suggested that further studies with more patients will aid to
elucidate the predictive value of the necrosis rate at a cutoff of
70% (11). More recently, Kato et
al (12) identified that
anti-tumor necrosis factor (TNF) therapy inhibited metastasis to
the lungs in an osteosarcoma cell line (143B), and thus concluded
that TNF-α inhibition may be a preventive therapeutic strategy for
the pulmonary metastasis of osteosarcoma. Therefore, it is
necessary to clarify the association between necrosis and the
progression and/or metastasis of osteosarcoma.
In osteosarcoma therapy, metastasis and
chemoresistance of malignant cells contribute to treatment failure
(9,13). Previous studies have reported that
high-mobility group box 1 protein (HMGB1) was a critical factor in
the development of chemoresistance (14–16), and
notably, that it promoted drug resistance in osteosarcoma, thus
suggesting a novel target for improving osteosarcoma therapy
(15,17–19).
Recently, Meng et al (20)
observed that the tumorigenesis, invasion and metastasis of
osteosarcoma was associated with overexpression of HMGB1, which may
thus be a potential target for treatment (20). However, to the best of our knowledge,
it has not been clarified whether HMGB1 is involved in cell
necrosis during the development and progression of osteosarcomas.
Therefore, the present study investigated the potential molecular
mechanisms underlying cell necrosis and the levels of HMGB1 in
osteosarcoma, with the aim of providing novel insights to improve
the treatment of human osteosarcomas.
Materials and methods
Cell lines and agents
The human osteosarcoma cell lines MG63, Saos-2 and
U2OS were purchased from the American Type Culture Collection
(Manassas, VA, USA). Human skeletal muscle cells (HSkMCs), as a
normal control cell line, were purchased from Tongpai Biotechnology
Co., Ltd. (Shanghai, China). Cells were cultured in Dulbecco's
modified Eagle's medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) containing 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA) and penicillin-streptomycin under 5% CO2 at 37°C
for 48 h. Doxorubicin (DXR; cat. no. D1515) and MTT (cat. no.
M2003) were obtained from Sigma-Aldrich (Merck KGaA).
Fluorescence-activated cell sorting
(FACS) assay
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) dual staining was used for a cell
apoptosis assay according to the manufacturer's protocols (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Briefly, MG63 cells were treated with or without 0.5 µg/ml of DXR
for 48 h. The untreated MG63 were used as negative control cells.
The human osteosarcoma cancer cells (2×106 cells/well)
were washed twice in cold PBS and fixed in 4% paraformaldehyde for
30 min at room temperature. The cells were then washed twice and
RNase A (Sigma-Aldrich; Merck KGaA) was added to a final
concentration of 100 ng/ml. Annexin V-FITC (0.1 µg/µl) and PI (0.05
µg/µl) were added to the cells and the cells were incubated for 15
min on ice. Subsequently, the cells were analyzed by FCS Express 6
Flow Cytometry (FACScan; BD Biosciences, Franklin Lakes, NJ,
USA).
MTT assay
An MTT assay was used to assess cell viability and
proliferation. Briefly, MG63 and U2OS cells (1×104
cells/well) were plated in a 96-well plate. The cells were treated
with different concentrations of DXR (0, 0.1, 0.5 or 1.0 µg/ml) for
24, 48 or 72 h. The control group (0 µg/ml DXR) was treated with
0.1% dimethyl sulfoxide (DMSO). At each time point, 20 µl MTT (5
mg/ml) was added and the cells were incubated for 4 h at 37°C prior
to removal of the supernatant. DMSO (100 µl; Sigma-Aldrich; Merck
KGaA) was added to dissolve the formazan crystals and a
spectrophotometer (DU-7400; Beckman Coulter, Inc., Brea, CA, USA)
was used to measure the optical density (OD) values at a wavelength
of 490 nm. The inhibitory rate following DXR treatment was
calculated with the following formula: Inhibitory rate (%)=[1-(OD
of the experimental samples/OD of the control)]x100. Maximal
inhibitory concentration refers to the drug concentration that
achieves the highest inhibition, indicating how much drug is needed
to highly inhibit a biological process in pharmacological research
(21).
Western blot analysis
Western blot assay was used to determine the
relationship between DXR and HMGB1 in osteosarcoma cells. In one
experiment, MG63 cells were treated with 0.1, 0.5 and 1.0 µg/ml of
DXR for 24 h. In the other, MG63 cells were treated with 0.5 µg/ml
of DXR for 24, 48 and 72 h. Untreated cells (0 µg/ml of DXR) were
used as negative controls. Whole cell extracts were prepared and
SDS-PAGE was used to separate them as previously described
(22). The primary antibodies used
were mouse monoclonal anti-HMGB1 (cat. no. ab77302; Abcam,
Cambridge, UK), mouse monoclonal anti-β-actin (C-2, cat. no.
sc-8432; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), while
the secondary antibody was goat anti-mouse immunoglobulin
G-horseradish peroxidase (cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.,). The protein bands were visualized with an
enhanced chemiluminescence detection kit (Amersham; GE Healthcare,
Chicago, IL, USA).
ELISA
A human HMGB1 ELISA kit (E-EL-H1554c; Wuhan
Elabscience Biotechnology Co., Ltd., Wuhan, China) was used to
determine the concentration of HMGB1 in the supernatants (following
centrifugation at 1,500 × g for 10 min at room temperature) of
DXR-treated MG63 and U2OS cells after 48 h. In the experiment,
different concentrations of DXR were used; 1/2 half maximal
inhibitory concentration (IC50) and IC50 of each cell line. Here,
the untreated MG63 cells or U2OS cells were used as negative
controls. The experiment was performed according to the kit
protocol using a Benchmark Microplate Reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The samples were assayed in duplicate.
Standard curves were used to calculate the concentrations of HMGB1
in the samples.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Multiple comparisons were
performed by one-way analysis of variance followed by a Tukey's
test. Pairwise comparisons were performed by two sets of
independent samples t-tests. The data were depicted as the mean ±
standard deviation, and P<0.05 was considered to indicate
statistical significance.
Results
HMGB1 levels are increased in human
osteosarcoma cell lines
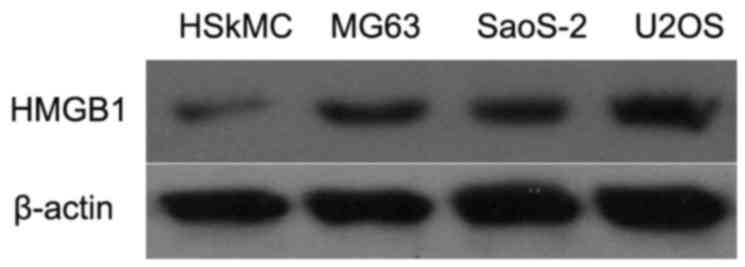
To evaluate the role of HMGB1 in human osteosarcoma
cells, western blot analysis was firstly used to determine the
levels of HMGB1 in MG63, Saos-2 and U2OS cells, and in control
HSkMCs. As depicted in Fig. 1, the
levels of HMGB1 were markedly increased in the MG63, SaoS-2 and
U2OS cells when compared with that in the HSkMCs. These data
suggested that HMGB1 may act as an oncogene in the development of
human osteosarcoma.
Treatment with DXR increases the
necrotic rate of MG63 cells
It has been reported that large-scale cell necrosis
may be induced by cancer thermotherapy (23). In the present study, the necrosis
inducer DXR was administered to human osteosarcoma cells, and the
role of HMGB1 during the process of cell necrosis was investigated.
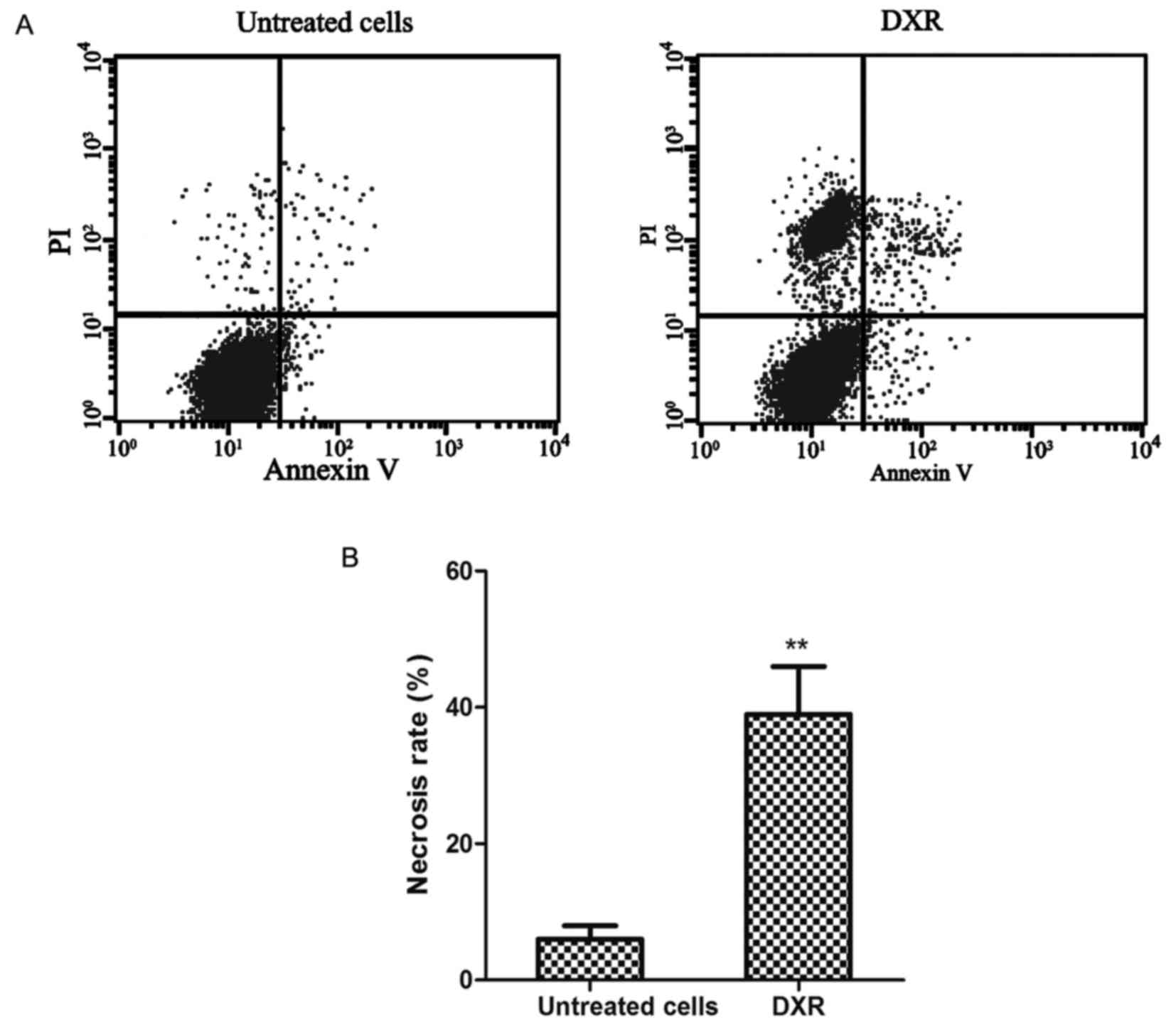
The human osteosarcoma cancer MG63 cells were incubated with or
without 0.5 µg/ml DXR for 48 h, and the rates of cell apoptosis and
necrosis were assessed by Annexin V-FITC/PI staining of the
DXR-treated and untreated groups (Fig.
2A). It was observed that DXR induced cell death in the
osteosarcoma cells, which was identified to be mainly of the cell
necrosis type by the markedly higher proportion of PI-positive
(necrotic) cells than Annexin V-positive/PI-negative (apoptotic)
cells. Furthermore, as depicted in Fig.
2B, the rate of necrosis was significantly increased in the
DXR-treated group when compared with that in the untreated group
(P<0.01, 39.2 vs. 6.5%). This result demonstrated that DXR
induced cell necrosis in human osteosarcoma cancer cells.
DXR reduces the viability of human
osteosarcoma cells
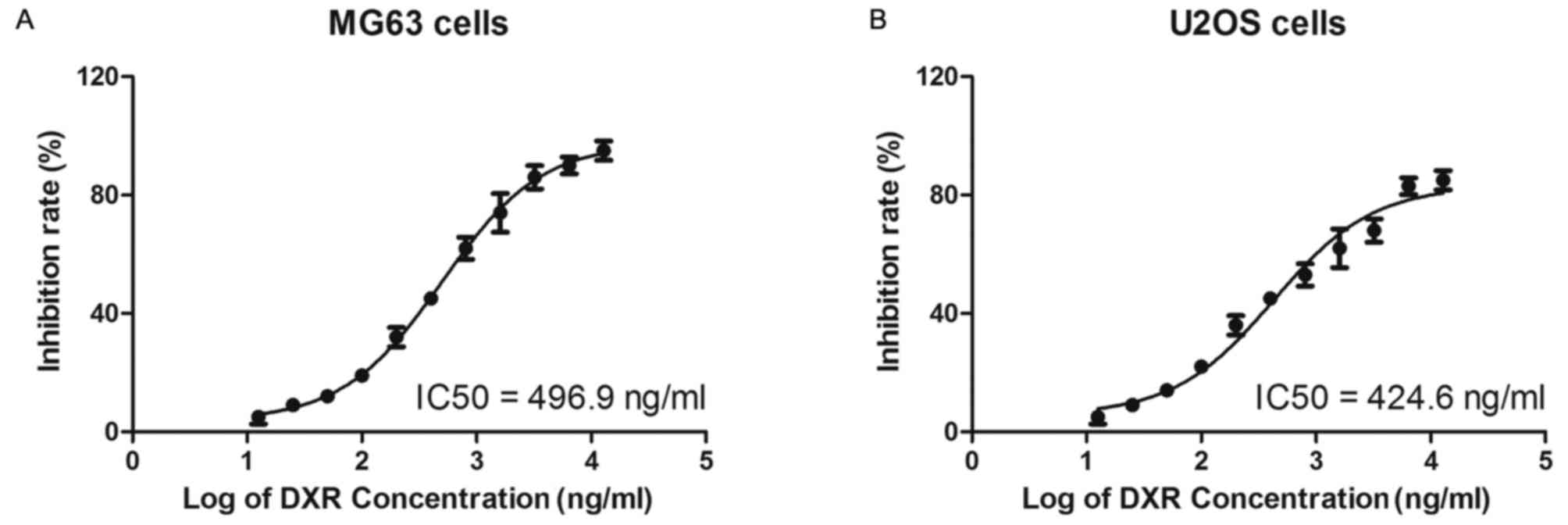
The MG63 and U2OS cells were treated with increasing
concentrations of DXR (0, 12.5, 25, 50, 100, 200, 400, 800, 1,600,
3,200, 6,400 and 12,800 ng/ml) for 24 h and cell viability was
determined by MTT assay. As depicted in Fig. 3, the half IC50 values of DXR in the
MG63 and U2OS cells were 496.9 and 424.6 ng/ml, respectively.
DXR reduces the viability of
osteosarcoma cells in apparent dose- and time-dependent
manners
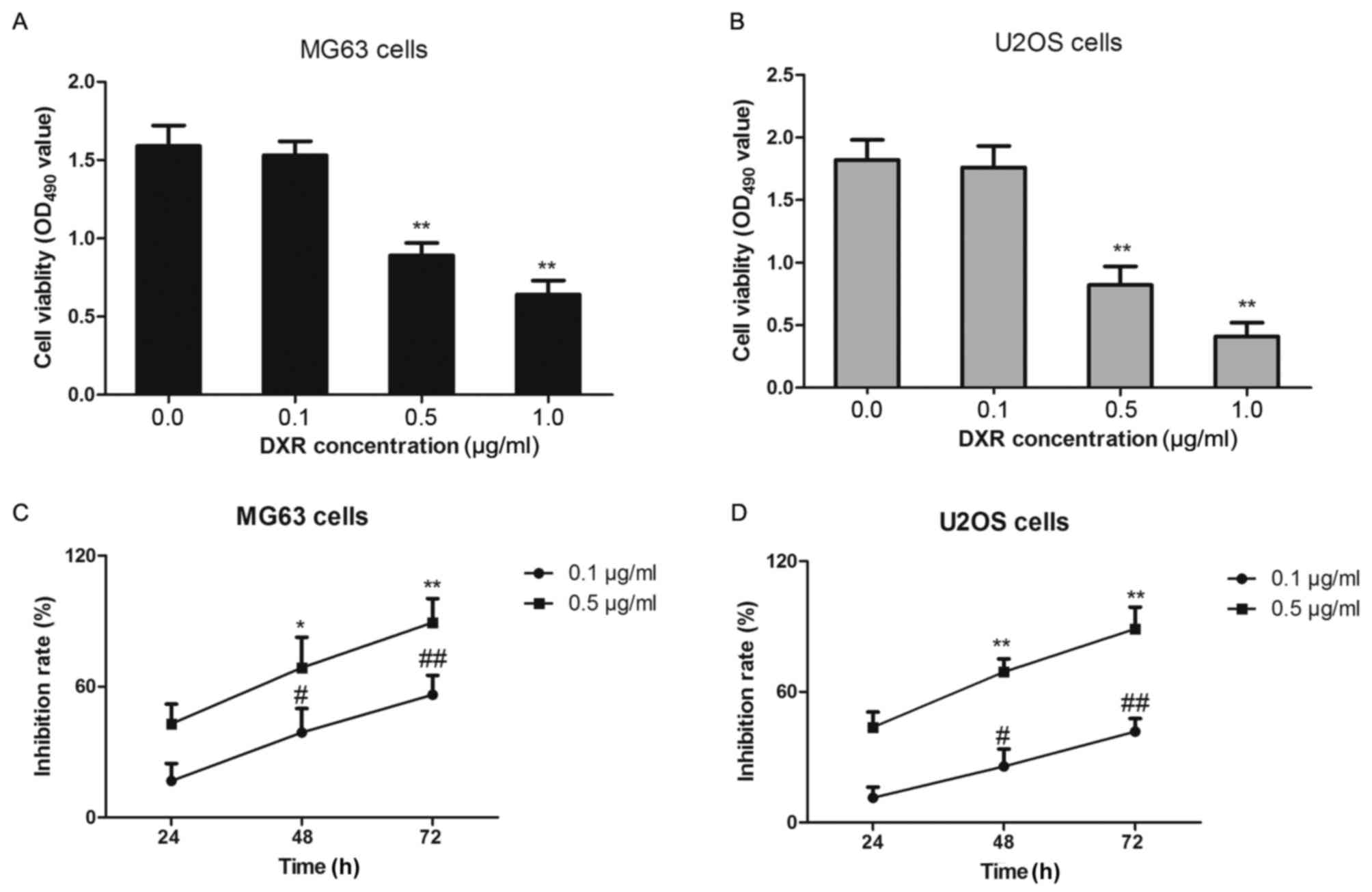
MG63 and U2OS cells were treated with increasing
concentrations of DXR (0, 0.1, 0.5 or 1.0 µg/ml) for 24 h and cell
viability was determined by MTT assay. MG63 cells and U2OS cells
grew well and the number of passages was <8, and so they were
selected for this experiment. As depicted in Fig. 4A and B, the viability of each cell
line was significantly decreased between 0.5 and 1.0 µg/ml,
relative to the untreated cells in each group (P<0.01), in an
apparent dose-dependent manner. Subsequently, the MG63 and U2OS
cells were treated with 0.1 or 0.5 µg/ml DXR for 24, 48 and 72 h,
respectively. The data from this time-course assay indicated that
DXR inhibited cell proliferation in a time-dependent manner
(Fig. 4C and D). Notably, for each
cell line, the inhibition rates were significantly higher at 48 and
72 h compared with that at 24 h (P<0.05) following treatment
with DXR (0.1 and 0.5 µg/ml).
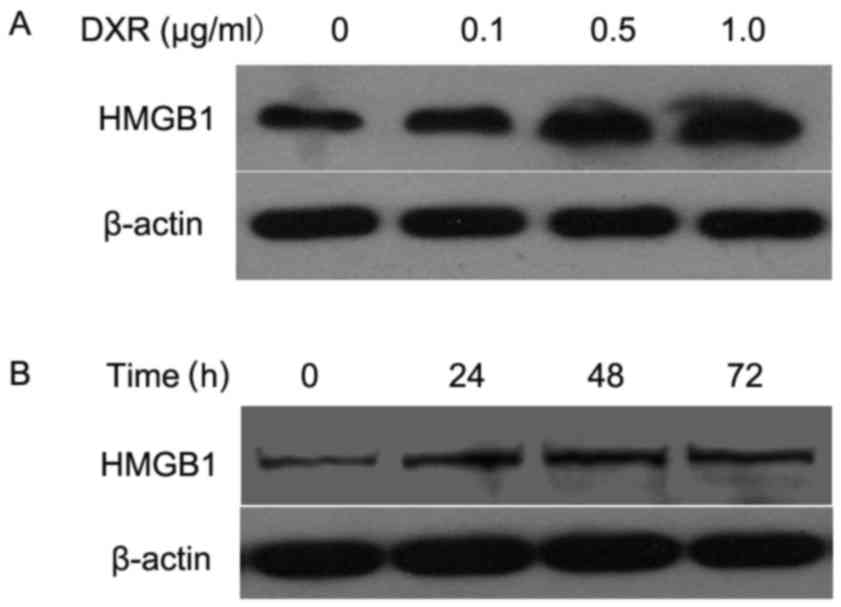
DXR treatment increases the levels of
HMGB1 in osteosarcoma cells
To determine whether DXR treatment affected the
production of DXR in osteosarcoma cells, MG63 cells were treated
with 0.1, 0.5 and 1.0 µg/ml of DXR for 24 h, and the expression of
HMGB1 was assessed by western blot analysis. As depicted in
Fig. 5A, the level of HMGB1 was
gradually upregulated as the concentration of DXR increased.
Subsequently, 0.5 µg/ml of DXR was used to treat MG63 cells for 24,
48 and 72 h, and western blotting was repeated. The results
indicated that HMGB1 expression was increased at 24 h and reached a
maximum by 48 h when compared with that at 0 h (Fig. 5B). These data suggested that the
level of HMGB1 was gradually increased by DXR treatment in dose-
and time-dependent manners in the human osteosarcoma cells.
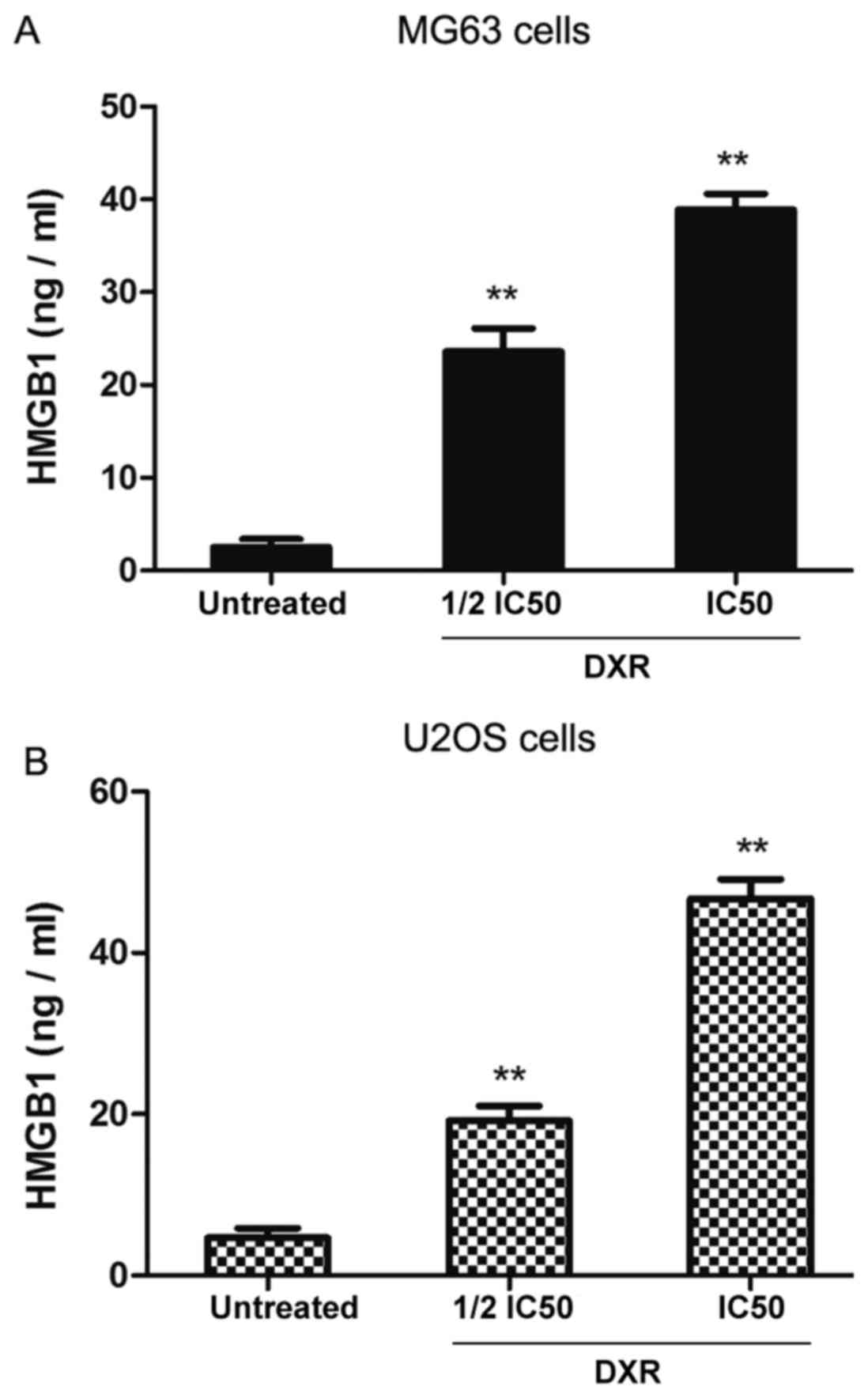
DXR treatment increases the secretion
of HMGB1 from MG63 and U2OS cells
Furthermore, the presence of HMGB1 was determined in
the culture media of MG63 and U2OS cells treated with two different
concentrations of DXR (½ IC50 and IC50 of each cell line) by ELISA.
As depicted in Fig. 6, DXR increased
the levels of HMGB1 in the culture media of MG63 and U2OS cells in
an apparent dose-dependent manner when compared with untreated
cells in each group (P<0.01). Therefore, the results
demonstrated that the necrosis inducer DXR significantly increased
the expression and secretion of HMGB1 in the human osteosarcoma
cells.
Discussion
Chemotherapy is a critical treatment for patients
with osteosarcoma (24–26). However, conventional chemotherapeutic
agents not only elicit cell apoptosis, but also induce cell
necrosis (27). Song et al
(10) reported that following
chemotherapy, the rate of tumor cell necrosis in control group
tumors was increased by approximately 50%, regardless of tumor
volume and location. However, Li et al (11) investigated whether tumor necrosis was
associated with DFS and overall survival rates of patients with
osteosarcoma and observed no survival advantage at a tumor necrosis
rate of 90%. Thus, in the present study, the underlying molecular
mechanism of cell necrosis and its potential effects on the
proliferation and/or progression of osteosarcoma cells were
investigated.
The levels of HMGB1 in human osteosarcoma cells were
firstly detected, and the results demonstrated that HMGB1
expression was markedly increased in the MG63, SaoS-2 and U2OS
osteosarcoma cell lines when compared with control HSkMCs.
Subsequently, to simulate the cell/tissue necrosis associated with
chemotherapy in osteosarcoma, the necrosis inducer DXR was
administered to the MG63 and U2OS cells. The osteosarcoma cells
were treated with increasing concentrations of DXR, and the results
demonstrated that DXR effectively induced cell death in the
osteosarcoma cell lines. Notably, the DXR-induced cell death was
identified to be mainly of the cell necrosis type, which was
determined by FACS analysis. The osteosarcoma MG63 cells were
further treated with different concentrations of DXR for 24–72 h.
It was observed that the levels of HMGB1 were gradually increased
with increasing concentrations of DXR, and that the levels of HMGB1
were upregulated in an apparent time-dependent manner.
IC50 is typically used to measure the efficacy of a
drug (28). In the present study,
the IC50 values of DXR in the MG63 and U2OS cells were 496.9 and
424.6 ng/ml, respectively. This was consistent with Rajkumar
(29), who reported that the
osteosarcoma cell line 143B had an IC50 of 0.4 µmol/l, while in a
doxorubicin drug-resistant osteosarcoma cell line (143B-DR-DOX),
the IC50 was 75 µmoll/l. Drug resistance remains a challenge for
clinical therapy in human cancers, including osteosarcoma (30,31).
Future research should investigate the effects of combined therapy
with necrosis inducer or apoptosis inducer on the proliferation of
osteosarcoma cell lines.
In conclusion, the present study addressed the
hypothesis that HMGB1 contributes to the development of
chemoresistance in osteosarcoma. From the current findings, it may
be speculated that necrotic osteosarcoma cancer cells produce and
secrete HMGB1, which has previously been indicated to serve an
important role as a tumor inducer that promotes the survival and
proliferation of osteosarcoma cells. The present results may
provide novel insights for improving the clinical treatment of
human osteosarcomas.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272015) and the
Heilongjiang Provincial Department of Education Fund (grant no.
12541394).
References
|
1
|
Nthumba PM: Osteosarcoma of the jaws: A
review of literature and a case report on synchronous multicentric
osteosarcomas. World J Surg Oncol. 10:2402012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertucci F, Araujo J and Giovannini M:
Pancreatic metastasis from osteosarcoma and Ewing sarcoma:
Literature review. Scand J Gastroenterol. 48:4–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fayda M, Kebudi R, Dizdar Y, Gorgun O, Gun
F, Aksu G and Ayan I: Spontaneous pneumothorax in children with
osteosarcoma: Report of three cases and review of the literature.
Acta Chir Belg. 112:378–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014.PubMed/NCBI
|
|
5
|
Haddox CL, Han G, Anijar L, Binitie O,
Letson GD, Bui MM and Reed DR: Osteosarcoma in pediatric patients
and young adults: A single institution retrospective review of
presentation, therapy, and outcome. Sarcoma. 2014:4025092014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
PosthumaDeBoer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Springfield DS, Schakel ME Jr and Spanier
SS: Spontaneous necrosis in osteosarcoma. Clin Orthop Relat Res.
1–237. 1991.
|
|
8
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sami SH, Rafati AH and Hodjat P: Tissue
necrosis after chemotherapy in osteosarcoma as the important
prognostic factor. Saudi Med J. 29:1124–1129. 2008.PubMed/NCBI
|
|
10
|
Song WS, Jeon DG, Cho WH, Kong CB, Cho SH
and Lee SY and Lee SY: Spontaneous necrosis and additional tumor
necrosis induced by preoperative chemotherapy for osteosarcoma: A
case-control study. J Orthop Sci. 20:174–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Ashana AO, Moretti VM and Lackman
RD: The relation of tumour necrosis and survival in patients with
osteosarcoma. Int Orthop. 35:1847–1853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato H, Wakabayashi H, Naito Y, Kato S,
Nakagawa T, Matsumine A and Sudo A: Anti-tumor necrosis factor
therapy inhibits lung metastasis in an osteosarcoma cell line.
Oncol. 88:139–146. 2015. View Article : Google Scholar
|
|
13
|
Gorlick R and Meyers PA: Osteosarcoma
necrosis following chemotherapy: Innate biology versus
treatment-specific. J Pediatr Hematol Oncol. 25:840–841. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinotti S, Patrone M, Manfredi M,
Gosetti F, Pedrazzi M, Marengo E and Ranzato E: HMGB1
osteo-modulatory action on osteosarcoma SaOS-2 cell line: An
integrated study from biochemical and -Omics approaches. J Cell
Biochem. 117:2559–2569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu T, Zhang W, Yang G, Li H, Chen Q, Song
R and Zhao L: HMGB1 overexpression as a prognostic factor for
survival in cancer: A meta-analysis and systematic review.
Oncotarget. 7:50417–50427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Wang S, Chen Y, Liu G and Yang X:
miR-22 targets the 3′UTR of HMGB1 and inhibits the HMGB1-associated
autophagy in osteosarcoma cells during chemotherapy. Tumour Biol.
35:6021–6028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Wang X, Li J, Yang C, Xing Z, Chen
R and Xu F: HMGB1 knockdown effectively inhibits the progression of
rectal cancer by suppressing HMGB1 expression and promoting
apoptosis of rectal cancer cells. Mol Med Rep. 14:1026–1032. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia Q, Xu J, Chen H, Gao Y, Gong F, Hu L
and Yang L: Association between an elevated level of HMGB1 and
non-small-cell lung cancer: A meta-analysis and literature review.
OncoTargets Ther. 9:3917–3923. 2016. View Article : Google Scholar
|
|
20
|
Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu
X and Zheng M: HMGB1 promotes cellular proliferation and invasion,
suppresses cellular apoptosis in osteosarcoma. Tumour Biol.
35:12265–12274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aykul S and Martinez-Hackert E:
Determination of half-maximal inhibitory concentration using
biosensor-based protein interaction analysis. Anal Biochem.
508:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia J, Yu X, Song X, Li G, Mao X and Zhang
Y: Inhibiting the cytoplasmic location of HMGB1 reverses cisplatin
resistance in human cervical cancer cells. Mol Med Rep. 15:488–494.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rem AI, Oosterhuis JA, Korver JG and van
den Berg TJ: Transscleral laser thermotherapy of hamster Greene
melanoma: Inducing tumour necrosis without scleral damage. Melanoma
Res. 11:503–509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uhl M, Saueressig U, van Buiren M, Kontny
U, Niemeyer C, Köhler G, Ilyasov K and Langer M: Osteosarcoma:
Preliminary results of in vivo assessment of tumor necrosis after
chemotherapy with diffusion- and perfusion-weighted magnetic
resonance imaging. Invest Radiol. 41:618–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samimi MA, Mirkheshti N and Pazouki A:
Assessing the percent of necrosis after neoadjuvant chemotherapy
with 24 h infusional cisplatin/3 days Doxorubicin intermittent with
Ifosfamide-Doxorubicin for osteosarcoma. Int J Hematol Oncol Stem
Cell Res. 8:5–8. 2014.PubMed/NCBI
|
|
26
|
Cui Q, Li D, Liu C, Guo J, Liu S, Liu Y,
Wang X and Zeng Y: The significance of MGMT protein detection in
evaluation of osteosarcoma necrosis rate after cisplatin
chemotherapy. Bos J Basic Med Sci. 11:80–83. 2011. View Article : Google Scholar
|
|
27
|
Bajpai J, Gamnagatti S, Kumar R, Sreenivas
V, Sharma MC, Khan SA, Rastogi S, Malhotra A, Safaya R and Bakhshi
S: Role of MRI in osteosarcoma for evaluation and prediction of
chemotherapy response: Correlation with histological necrosis. Ped
Radiol. 41:441–450. 2011. View Article : Google Scholar
|
|
28
|
Blanchard N, Richert L, Coassolo P and
Lave T: Qualitative and quantitative assessment of drug-drug
interaction potential in man, based on Ki, IC50 and inhibitor
concentration. Curr Drug Metab. 5:147–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rajkumar T and Yamuna M: Multiple pathways
are involved in drug resistance to doxorubicin in an osteosarcoma
cell line. Anticancer Drugs. 19:257–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan Y, Xu L, Zhuo N, Ge J, Chen G and Lu
XB: The clinical significance of neoadjuvant chemotherapy in
improving the drug resistance of osteosarcoma. Minerva Med.
108:479–481. 2017.PubMed/NCBI
|
|
31
|
Wang Y and Teng JS: Increased multi-drug
resistance and reduced apoptosis in osteosarcoma side population
cells are crucial factors for tumor recurrence. Exp Ther Med.
12:81–86. 2016. View Article : Google Scholar : PubMed/NCBI
|