Introduction
Salidroside (SAL) is a phenylpropanoid glycoside
extracted from Rhodiola rosea L., which is used in
traditional Tibetan medicine and exhibits a wide range of
pharmacological effects including anti-oxidative, anti-apoptotic,
anti-inflammatory, anti-depressive, anti-aging and anti-fatigue
effects (1–4). It has been demonstrated that SAL is
neuroprotective against ischemic cerebral injury, a leading cause
of disability and mortality worldwide and is therefore considered
to be a major public health problem (5). It has been demonstrated that SAL
reduces the infarct volume and improves neurological deficit scores
in a rat model of transient focal cerebral ischemia and reperfusion
(6). In addition, SAL improves
learning and spatial memory during hypoxia treatment, indicating
that SAL has a neuroprotective effect during hypoxia (7). Another study determined that SAL
prevents the cerebral ischemic injury caused by cerebral artery
occlusion and reperfusion in vivo, and neurotoxicity induced
by hydrogen peroxide in vitro (8). Pretreatment with SAL also reduces the
cellular damage that occurs following global cerebral
ischemia/reperfusion (I/R) injury in rats (9). Although the protective effects of SAL
on cerebral I/R injury have been identified, its underlying
neuroprotective mechanisms remain unclear. Therefore, the present
study used hypoxia/reperfusion (H/R)induced human brain vascular
smooth muscle cells (HBVSMCs) as an in vitro cell model of
cerebral I/R injury (10) to
investigate the mechanisms underlying SAL-mediated neuroprotective
activity.
Sirtuin 1 (SIRT1) is an oxidized nicotinamide
adenine dinucleotide-dependent deacylase and is expressed in
various tissues of the body including the heart, liver, muscle,
kidney, endothelium and adipose, and its expression is particularly
high in the brain (11). SIRT1
serves an essential role in a number of different processes
including inflammation, cellular senescence, apoptosis, aging and
stress resistance in the central nervous system (CNS). SIRT1 also
serves a neuroprotective role in diseases of the CNS (12). SIRT1 has been specifically identified
as a mediator of cerebral ischemia injury and may therefore be
exploited as a potential target for treatments of this disease
(13). A number of studies have
demonstrated that SIRT1 undergoes profound changes in expression
and activity, which is associated with the changes in mitochondrial
function that occur following hypoxic-ischemia and reoxygenation
injury (14,15). Previous studies have demonstrated
that the SIRT1-mediated deacetylation and phosphorylation of
downstream targets including forkhead box protein O3α (FOXO3α),
which is a ubiquitously expressed mammalian forkhead transcription
factor and highly expressed in the adult brain, promotes cell
survival, mitochondrial function, apoptosis and inflammation in
response to severe stress (16,17). A
number of studies have indicated that FOXO3α serves important roles
in neuronal survival in normal and disease conditions and the
regulation of FOXO3α mediated by SIRT1 contributes to
neuroprotection in vitro and in vivo (18–20).
Further studies have determined that SIRT1-activated multiple
signaling pathways, such as FOXO3α, mediate a variety of
neuroprotective agents including leptin, two tetrahydroxystilbene
and icariin and may attenuate ischemic injury following stroke
(21) and cerebral ischemia
(22,23). However, it remains unknown whether
SIRT1-mediated signaling pathways serve a similar role in the
neuroprotection exhibited by SAL against cerebral ischemia
injury.
The present study therefore investigated whether SAL
attenuates H/R injury, resulting in a neuroprotective effect, via
regulation of the SIRT1/FOXO3α pathway. To the best of our
knowledge, the current study is the first to investigate whether
the SIRT1/FOXO3α signaling pathway contributes to the SAL-mediated
prevention of H/R injury in vitro and may be a potential
therapeutic target for the treatment of cerebral ischemic
injury.
Materials and methods
Reagents
SAL, Sirtinol and
3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
reagent were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). Hoechst 33342 solution, the BCA protein assay kit,
radioimmunoprecipitation (RIPA) lysis buffer and BeyoECL Plus were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). The caspase-3 enzyme-linked immunosorbent assay (ELISA) kit
was purchased from the Nanjing Jiancheng Bioengineering Research
Institute (cat no. H076; Nanjing, China). The Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) staining kit was
obtained from Sigma-Aldrich; Merck KGaA. Antibodies against SIRT1
(cat no. 9475), phosphorylated (p)-FOXO3α (cat no. 9466), B-cell
lymphoma 2 (Bcl-2; cat no. 3498) and Bcl-2 associated X protein
(Bax; cat no. 2772) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). GAPDH (cat no. 10494-1-AP) and secondary
antibodies were obtained from ProteinTech Group, Inc. (Chicago, IL,
USA).
Cell culture
Human brain vascular smooth muscle cells (HBVSMC)
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and maintained in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C in a humidified atmosphere consisting of 5% CO2.
The medium was replenished three times a week.
H/R model and drug treatment
To establish an in vitro model of I/R injury,
HBVSMCs were incubated at 37°C for 4, 6 or 8 h in a hypoxic (H)
chamber (94% N2, 5% CO2 and 1% O2; Biospherixhypoxia chamber) to
induce oxygen-deficiency and then reoxygenated (R) by culture at
37°C in a standard incubator (5% CO2 and 20% O2) for 16 h. HBVSMCs
were divided into four groups: Normal group (HBVSMCs were cultured
in normal medium and standard incubator); H (4 h)/R (16 h) group; H
(6 h)/R (16 h) group; H (8 h)/R (16 h) group. To determine the
protective effects of SAL on H/R injury, cells were pretreated with
SAL (100, 200 or 400 µM) for 30 min and then underwent hypoxia for
8 h and reoxygenation for 16 h. HBVSMCs were divided into four
groups: Normal group; H (8 h)/R (16 h) group; SAL+H (8 h)/R (16 h)
group; SAL (400 µM) group. To identify the role of the SIRT1/FOXO3α
pathway in the SAL-induced beneficial effects against H/R injury,
cells were pretreated with sirtinol (10 µM) for 30 min. Cell were
then incubated with SAL (400 µM) for 30 min prior to exposure to
hypoxia for 8 h followed by reoxygenation for 16 h. HBVSMCs were
divided into four groups: Normal group; H (8 h)/R (16 h) group;
SAL+H (8 h)/R (16 h) group; Sirtinol (10 µM) + SAL+H (8 h)/R (16 h)
group. All treatments were performed in triplicate.
Cell viability assay
The viability of HBVSMCs underlying different
processing conditions as described was examined using an MTT assay
following the manufacturer's protocol. Briefly, cells were seeded
into 96-well plates at a density of 1×104 cells/well overnight at
37°C. MTT reagent (10 µl) was added to each well and cells were
incubated for a further 4 h at 37°C. Formazan was subsequently
dissolved in dimethyl sulfoxide. A microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was then used to measure
absorbance at 490 nm. Cell viability (%) was calculated for all
groups, as a proportion of the control. Each experiment was
independently performed in triplicate.
Measurement of caspase-3 activity
The activity of caspase-3 in HBVSMCs was evaluated
using a commercial caspase-3 ELISA kit following the manufacturer's
protocol. Briefly, cells were trypsinized in RIPA lysis buffer and
centrifuged at 12,000 × g at 4°C for 10 min. The protein
concentration of each group was determined using a BCA protein
assay kit and equal amounts of protein were incubated with 5 µl
Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide, 0.2 mM) at 4°C
for 4 h in the dark. Absorbance was measured at a wavelength of 405
nm using a microplate reader and caspase-3 activity was calculated
as follows: Optical density (OD) (experimental group)/OD (control
group). Each experiment was independently performed in
triplicate.
Hoechst 33342 staining
HBVSMCs were seeded in 24-well culture plates at a
density of 1×105 cells/well. After cells reached ~70% confluence,
cells were treated as previously described, washed with cold
phosphate-buffered saline (PBS) three times and fixed with 4%
paraformaldehyde at 4°C for 10 min in the dark. Cells were then
washed with PBS and incubated with Hoechst 33342 (1 µg/ml) for 10
min at room temperature in the dark. Following three washes with
PBS, cells were observed using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). To calculate the average rate of
apoptosis, 5 different, random sections of each group were
assessed. The apoptosis rate (%) was calculated for all groups and
compared with the control group. Each experiment was performed in
triplicate.
Annexin V-FITC/PI staining
The apoptosis rate of the HBVSMCs was determined
using an Annexin V-FITC/PI staining kit, following the
manufacturer's instructions. In brief, HBVSMCs were digested with
0.25% trypsin and washed with PBS. Subsequently, cells were
harvested and resuspended in the binding buffer contained in the
staining kit (106 cells/ml), prior to mixing with Annexin V-FITC (5
µl) and PI (10 µl). Following incubation for 15 min in the dark at
room temperature, the apoptosis ratio was determined using flow
cytometry. The experiments were repeated three times
independently.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HBVSMCs in the logarithmic phase were seeded onto
6-well plates at a density of 1×104 cells/well. Total RNA was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
quantity and purity of RNA was detected using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.). First strand
cDNA was reverse-transcribed from equal amount of RNA using the
Prime Script RT reagent kit (Takara Bio Inc., Otsu, Japan). For
RT-qPCR, the primer sequences of SIRT1 were as follows: Forward,
5′-TCATTCCTGTGAAAGTGATGACGA-3′ and reverse,
5′-CTGCCCTAGTGTCATATCATCCAA-3′. The primer sequences of the GAPDH
internal control were as follows: Forward,
5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse,
5′-ATGGTGGTGAAGACGCCAGTA-3′. The PCR process was performed as
follows: 50°C for 2 min, 95°C for 2 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 2 min. mRNA expression was calculated
and normalized using the 2-∆∆Cq method (24) and expressed as a fold of the control.
The assay was performed in triplicate for each sample.
Western blot analysis
H/R-injured HBVSMC in the presence and absence of
SAL or sirtinol were harvested and lysed in RIPA buffer containing
protease inhibitors for 30 min at 4°C. Total proteins were
collected following centrifugation at 12,000 × g for 10 min at 4°C
and quantified using the BCA Protein assay kit. Equal amounts of
proteins (30 µg) were separated by 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). Non-specific protein binding was blocked with 5% non-fat
milk for 2 h at room temperature. Subsequently, the membranes were
incubated with primary antibodies against SIRT1 (1:1,000), Bax
(1:1,000), Bcl-2 (1:1,000), FOXO3α (1:1,000) and GAPDH (1:2,000)
overnight at 4°C. Following three washes with Tris-buffered saline
containing 0.05% Tween-20, the membrane was incubated with
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat
no. LS-C146625; LifeSpan BioSciences, Seattle, WA, USA) for 2 h at
room temperature. Protein bands were analyzed using the BeyoECL
Plus kit, an enhanced chemiluminescence detection system. The
detected protein was quantified using Image J2 software (National
Institutes of Health, Bethesda, MD, USA) and expressed as a
percentage compared with GAPDH expression. Each experiment was
independently performed in triplicate.
Statistical analysis
Data are expressed as mean ± standard error of the
mean of three independent experiments. The statistical differences
among different groups were assessed using one-way analysis of
variance followed by a least significant difference test. P<0.05
was considered to represent a statistically significant
difference.
Results
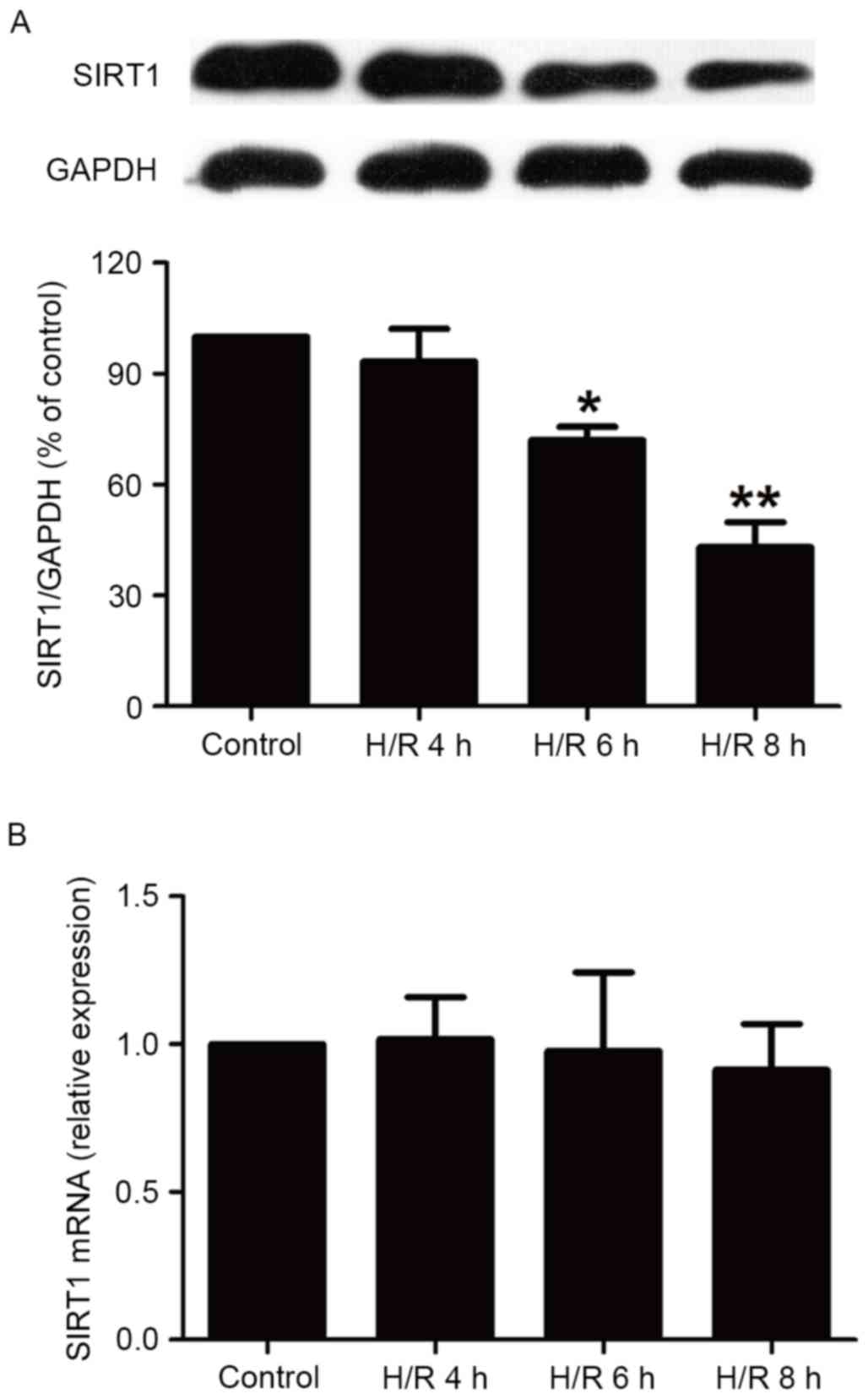
H/R treatment reduces levels of SIRT1
protein in HBVSMCs
It has been demonstrated that SIRT1 modulates
pathways involved in oxidative stress, apoptosis and inflammation
to protect against ischemia/hypoxia (13). Therefore, the current study assessed
the impact of H/R on the expression of SIRT1 in HBVSMCs. The
present study demonstrated that the expression of SIRT1 in HBVSMCs
exposed to hypoxia for 6 and 8 h followed by reoxygenation for 16 h
was significantly reduced compared with the control group
(P<0.05; Fig. 1A), while a
shortage of oxygen (4 h) followed by reoxygenation for 16 h did not
significantly affect SIRT1 expression. In addition, levels of SIRT1
mRNA were measured using RT-qPCR and it was demonstrated that H/R
treatment did not significantly affect SIRT1 mRNA levels in HBVSMCs
(Fig. 1B). These results indicate
that the upregulation of SIRT1 protein expression may affect
cerebral H/R injury. Notably, 8 h hypoxia induced the largest
decline in the expression of SIRT1 protein (P<0.01), therefore
the duration of hypoxia HBVSMCs were subjected to in subsequent
experiments was 8 h.
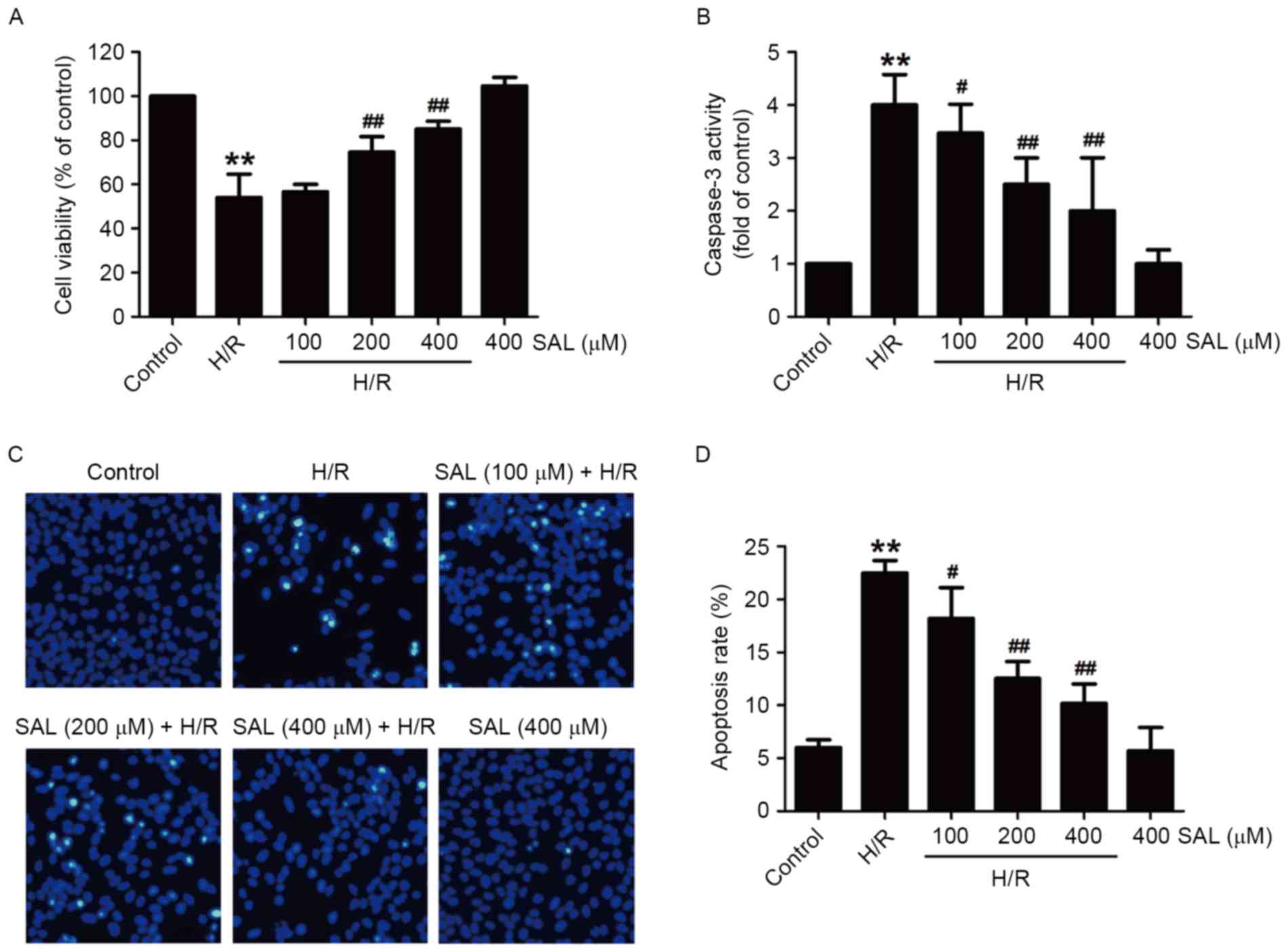
SAL attenuates H/R-induced
cytotoxicity and apoptosis in HBVSMCs
To investigate the protective effects of SAL on
cerebral H/R injury, the viability of cells and apoptosis under
hypoxia (8 h)/reoxygenation (16 h) treatment in the presence or
absence of SAL was measured in HBVSMCs. Compared with the control
group, the viability of HBVSMCs in the H/R group was significantly
reduced (P<0.01). However, the downregulation of cell viability
induced by H/R was significantly reversed following pretreatment
with 200 or 400 µM SAL in HBVSMCs (P<0.01; Fig. 2A). In addition, pretreatment of
HBVSMCs with SAL significantly reduced the H/R-induced increase in
caspase-3 activity, a key regulatory factor in the process of
apoptosis, in a concentration-dependent manner (P<0.05; Fig. 2B). Furthermore, following H/R
treatment, HBVSMCs exhibit the phenomenon of chromatin condensation
and fragmentation. Chromatin was stained brightly following H/R,
however this was reversed following SAL treatment (Fig. 2C). Pretreatment with SAL also
significantly reversed the upregulation of the apoptosis rate
induced by H/R in a dose-dependent manner (P<0.05; Fig. 2D). Notably, the viability, caspase-3
activity and apoptosis rate of HBVSMCs treated with 400 µM SAL that
did not undergo H/R were not significantly affected, suggesting
that SAL alone has no effect on apoptosis. These results indicate
that SAL treatment alleviates H/R-induced injuries following
cerebral H/R injury.
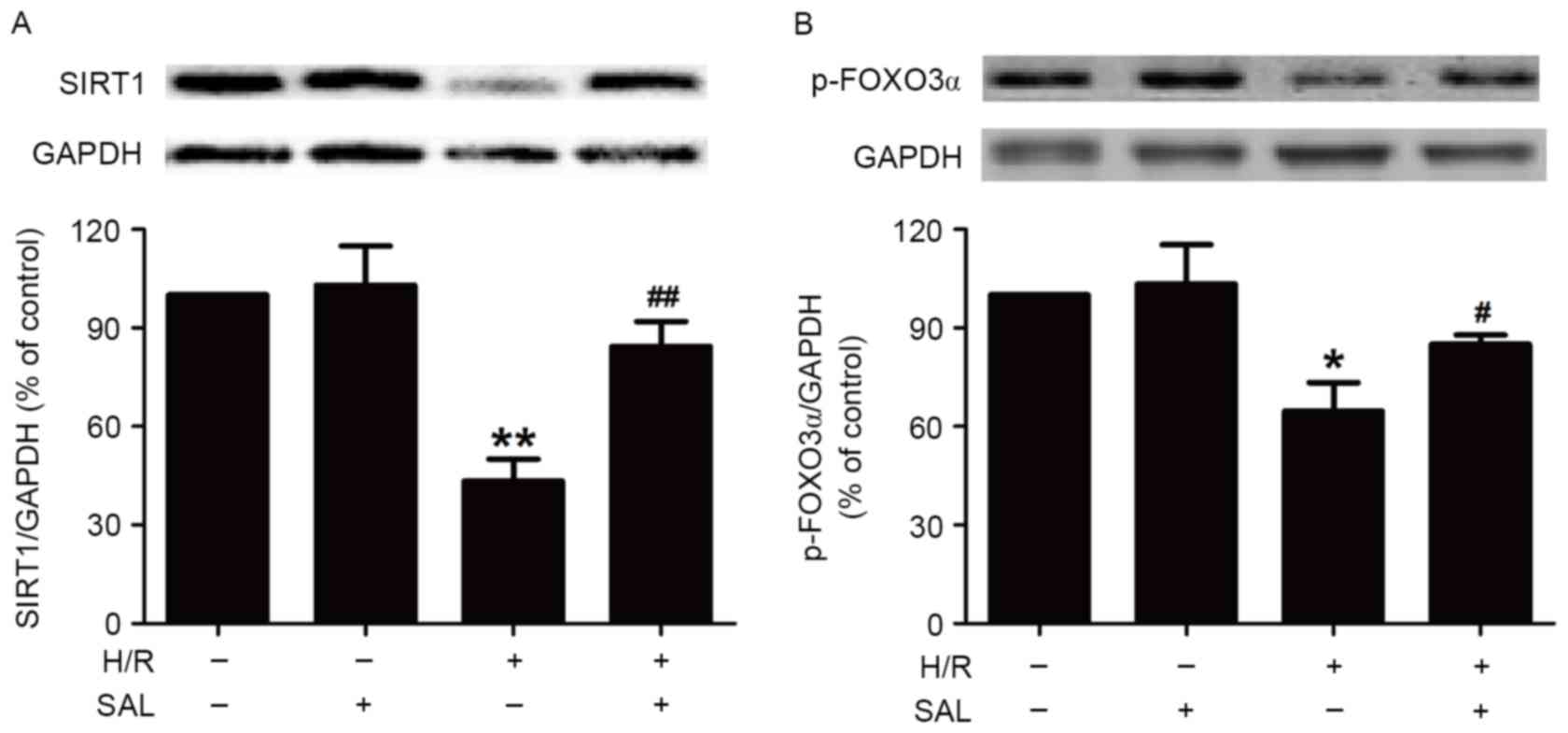
SAL prevents the H/R-induced
disturbance of SIRT1 signaling pathway in HBVSMCs
To investigate whether SAL protects HBVSMCs against
H/R injury by modulating the SIRT1 signaling pathway, the present
study measured the effect of SAL on the expression of SIRT1 protein
in the presence or absence of H/R treatment. Pretreatment with SAL
(400 µM) during H/R significantly increased the expression of SIRT1
protein in HBVSMCs compared with the H/R group (P<0.01; Fig. 3A), whereas SAL had no effect on the
expression of SIRT1 in HBVSMCs that did not undergo H/R, indicating
that the upregulation of SIRT1 may be involved in the protective
role of SAL in H/R injury. In addition, the present study
demonstrated that the downregulation of p-FOXO3α induced by H/R
treatment (P<0.05) was reversed by pretreatment with SAL (400
µM) in HBVSMCs (P<0.05; Fig. 3B).
SAL treatment alone had no influence on the expression of p-FOXO3α.
These results suggest that the promotion of the SIRT/FOXO3α pathway
may contribute to the inhibition of SAL on H/R injury.
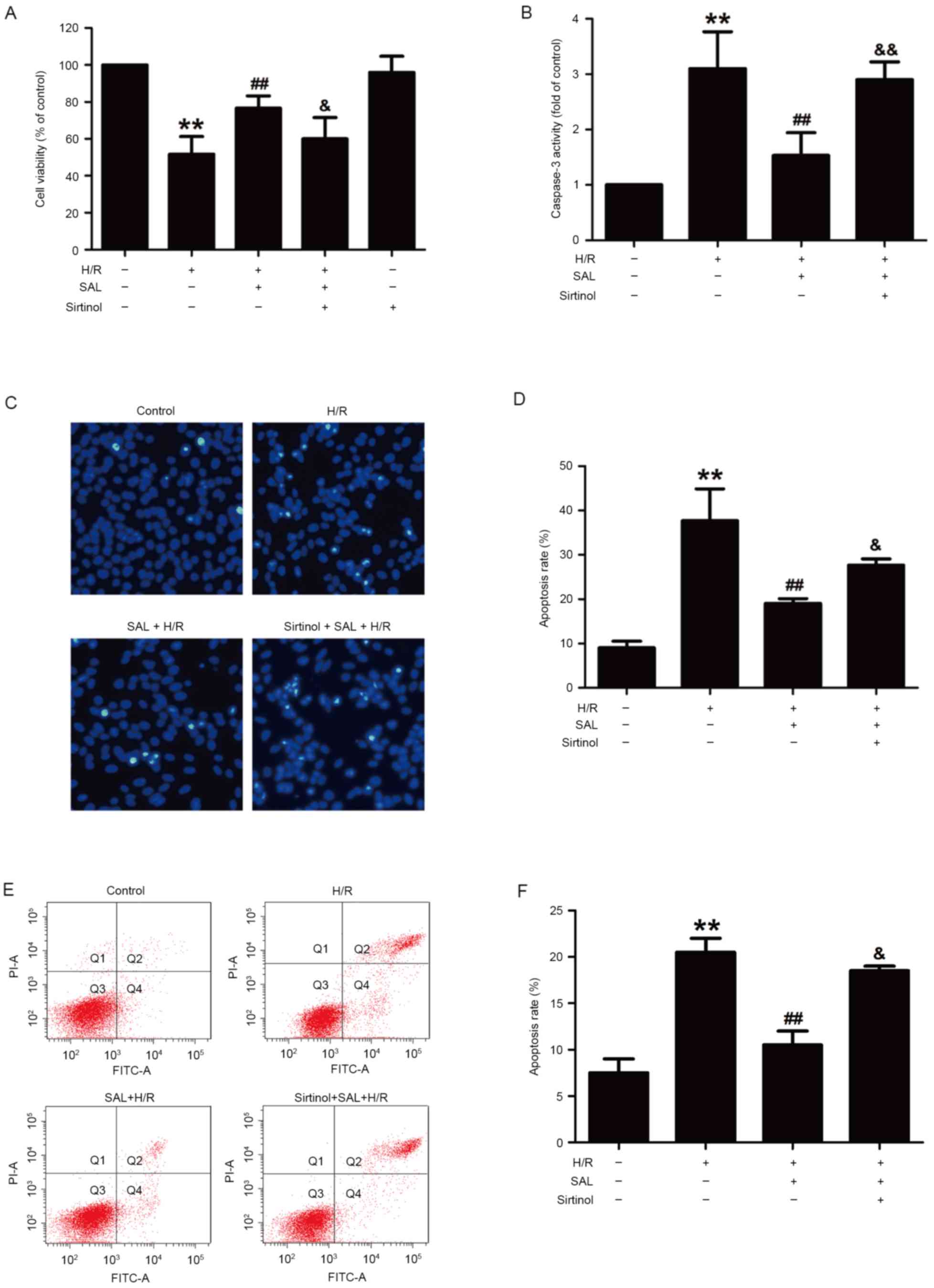
Blocking the SIRT1/FOXO3α pathway
attenuates the protective effect of SAL against H/R-induced
cytotoxicity and apoptosis in HBVSMC
To confirm the contribution of the SIRT1/FOXO3α
pathway to the SAL-induced reversal of H/R injury, sirtinol, a
SIRT1-specific inhibitor, was used in subsequent experiments. The
results demonstrated that pretreatment with sirtinol (10 µM) for 30
min significantly reduced the viability of HBVSMCs compared with
that of the SAL and H/R co-treatment group (P<0.05), whereas
sirtinol itself did not affect the viability of HBVSMCs (Fig. 4A). This indicates that SAL acted via
the SIRT1/FOXO3α pathway to reverse the decrease in cell viability
induced by H/R treatment. The impact of sirtinol on apoptosis was
also investigated and it was demonstrated that sirtinol reversed
the inhibitory effects of SAL (400 µM) on caspase-3 activity
(P<0.01; Fig. 4B). This increased
activity was also evident from the morphological changes induced by
H/R treatment that occurred in apoptotic cells, characterized by
nuclear condensation, fragmentation and bright blue fluorescence
(Fig. 4C). The apoptosis rate was
significantly increased following pretreatment with sirtinol
compared with the H/R and SAL group (P<0.05; Fig. 4D). In addition, Annexin V-FITC/PI
staining determined that sirtinol significantly inhibited the
reversal effect of SAL (400 µM) on the H/R induced upregulation of
the apoptosis ratio in HBVSMCs (P<0.05; Fig. 4E and F). These results indicate that
the SIRT1/FOXO3α pathway mediated the SAL-induced protection
against H/R-induced injury.
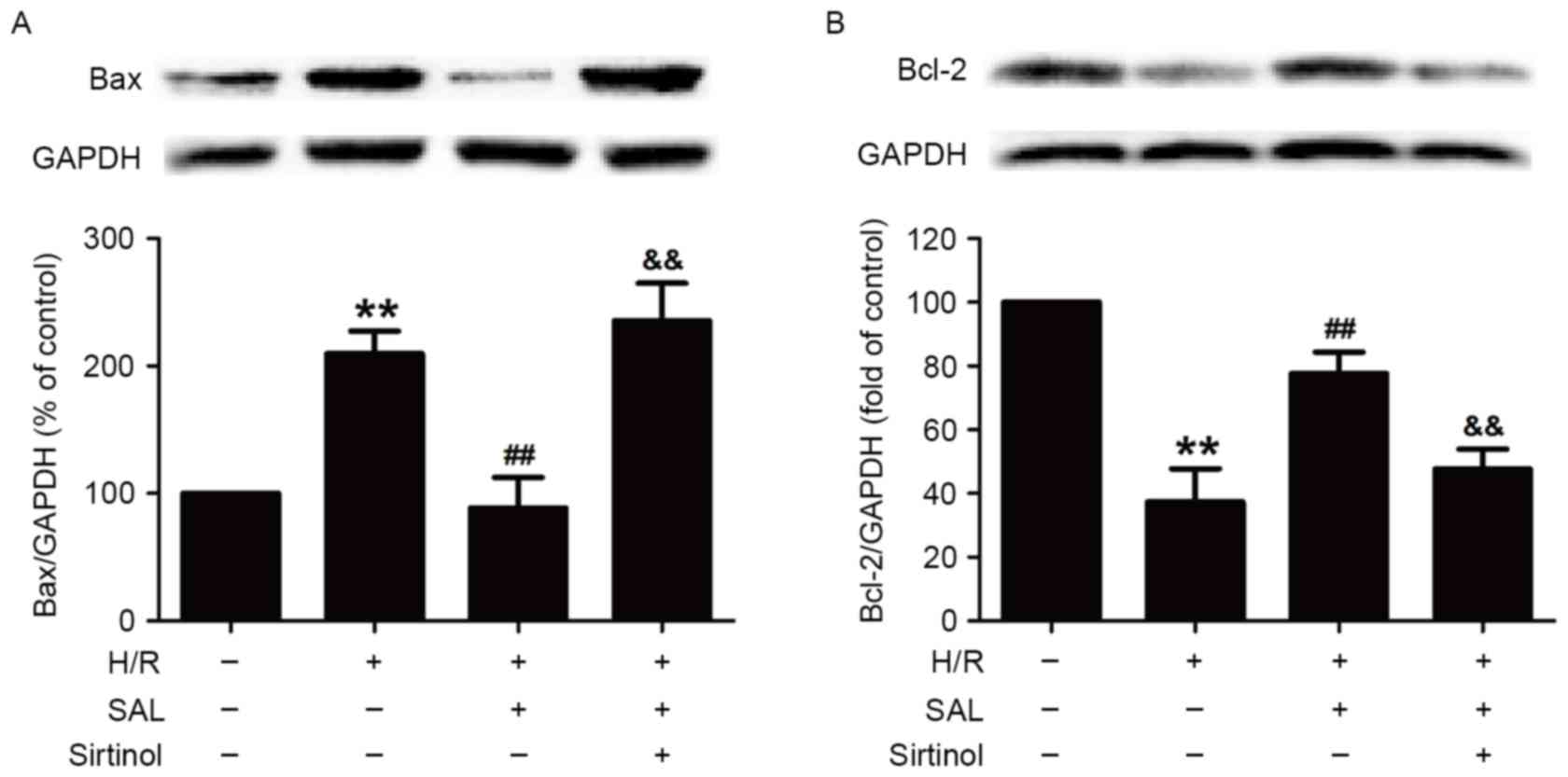
Inhibition of the SIRT1/FOXO3α pathway
prevents the reversal effects of SAL on the H/R-induced
alternations of Bax and Bcl-2 protein levels in HBVSMCs
Finally, it was investigated whether the
SIRT1/FOXO3α pathway was involved in the protective effect of SAL
against the H/R-induced change in the expression of
apoptosis-related proteins in HBVSMCs. Compared with the control
group, H/R significantly increased the expression of the
pro-apoptotic protein Bax (P<0.01) and this increase was
reversed by pretreatment with SAL (400 µM; P<0.01; Fig. 5A). Notably, pretreatment with
sirtinol (10 µM) significantly attenuated the SAL-induced
downregulation of Bax expression in HBVSMCs compared with SAL and
the H/R co-incubation group (P<0.01; Fig. 5A). Additionally, the significant
decrease in the expression of the anti-apoptotic protein Bcl-2
induced by H/R was also significantly attenuated by SAL
pre-treatment (400 µM; P<0.01; Fig.
5B). However, sirtinol reversed the SAL-induced increase in
Bcl-2 expression (P<0.01; Fig.
5B). These results demonstrate that the SIRT1/FOXO3α pathway
mediated the inhibitory role of SAL in H/R injury and may be
involved in promoting the expression of proteins in the Bcl-2
family.
Discussion
A number of studies have demonstrated that SAL
exhibits neuroprotective effects following cerebral ischemic injury
(6,25,26).
SIRT1 regulates a variety of signaling pathways and is therefore
considered to be a key mediator of cerebral ischemia (13,27).
However, to the best of our knowledge, the role of the SIRT1
signaling pathway in the protection of SAL against H/R injury
remains unknown. In the current study, HBVSMCs were used to
establish an in vitro model of H/R injury to investigate the
protective effects of SAL on H/R injury and the underlying
protective mechanism of SAL during H/R. The current study indicated
that the SIRT1/FOXO3 pathway is a mediator of SAL and exhibits
beneficial roles in H/R-treated HBVSMCs. This suggests that SAL may
be used clinically to treat patients with I/R injury and that the
SIRT1/FOXO3 pathway may be a potential therapeutic target for
treatments of cerebral ischemia.
Cerebral I/R-induced cytotoxicity and apoptosis
serve a pivotal role in brain injury caused by I/R, in which
apoptosis greatly contributes to the cell death that occurs
following cerebral I/R injury (28).
Caspase-3 has been identified as a pivotal mediator of apoptosis
and previous studies have determined that the activity and
expression of caspase-3 are upregulated in in vivo and in
vitro models of ischemic stroke (29–31).
Consistent with the above studies, the present study demonstrated
that H/R-treated HBVSMCs, an appropriate and reproducible in
vitro cell model of cerebral I/R injury, exhibited improved
mimicking of the pathological conditions of I/R injury, including a
decrease in cell viability and increases in caspase-3 activity and
the cellular apoptosis rate. It has previously been demonstrated
that SAL markedly inhibits cellular apoptosis and therefore has a
neuroprotective effect (32) and the
neuroprotective properties of SAL have been demonstrated in mice
subjected to I/R injury, as well as in cultured nerve cells
(25,26). Therefore, the present study
investigated the effect of SAL on H/R injury in HBVSMCs and
identified that pretreatment with SAL, which had no significant
influence on cell viability and apoptosis under normal conditions,
reversed H/R-induced cytotoxicity and apoptosis and increased
caspase-3 activity in HBVSMCs, confirming the results of a previous
study by Lai et al (33). The
results of the present study clearly indicate that SAL pretreatment
exhibits neuroprotective effects against H/R injury, which prompted
further investigation of the underlying mechanisms.
Over the last decade, a number of studies have
identified that SIRT1 serves a neuroprotective role in cerebral
ischemia (15,34,35).
Accumulating evidence indicates that SIRT1 is involved in a number
of important physiological processes, including apoptosis,
oxidative stress and the cell cycle, and therefore serves an
important biological function in transcriptional regulation and
signal transduction (13,36). Furthermore, intervention of the SIRT1
signaling pathway improves a variety of age-related diseases
including coronary heart disease, type II diabetes and cerebral
ischemia/reperfusion (37–39). Therefore, it was hypothesized that
the protective functions of SAL are due to the regulation of the
SIRT1 pathway that occurs following H/R injury.
In present study, it was demonstrated that H/R
treatment significantly reduces the expression of SIRT1 protein in
HBVSMCs, although the level of SIRT1 mRNA was unaffected by H/R.
Therefore, inducing the upregulation of SIRT1 protein may
contribute to H/R injury and this is consistent with the results of
a previous study by Lv et al (40). The current study also assessed the
effects of pretreating HBVSMCs with SAL and demonstrated that SAL
markedly reversed the downregulation of SIRT1 protein expression,
suggesting that the upregulation of SIRT1 may be involved in the
protective effect of SAL against H/R injury. FOXO3α, which is
highly expressed in the brain and heart, is involved in the
regulation of apoptosis, cell survival, the cell cycle and
oxidative stress (41).
Transcriptional activity is regulated by SIRT1, which induces the
phosphorylation of FOXO3α, regulates the activity of FOXO3α and
inhibits apoptosis (42,43). Fukunaga and Shioda (44) confirmed that the expression of FOXO3α
protein was inhibited in chronic cerebral ischemia. The current
study demonstrated that H/R treatment decreased the expression of
FOXO3α protein, while this inhibitory action was eradicated by SAL
pretreatment in HBVSMCs. These results indicate that the
SIRT1/FOXO3α pathway may mediate the neuroprotective effect induced
by SAL. Notably, H/R downregulated the levels of p-FOXO3α more than
the levels of SIRT1, which may be due to the presence of other
regulatory signals contributing to the changes in FOXO3α levels
following H/R treatment, such as SIRT6 (45) and the phosphatidylinositol
3-kinase/Akt signaling pathway (46), leading to a smaller decrease in
levels of p-FOXO3α compared with SIRT1. In addition, the current
study indicated that the inhibition of SIRT1 by sirtinol eradicated
the reversal effect of SAL against the H/R-induced downregulation
of cell viability and upregulation of apoptosis in HBVSMCs. This is
similar to the results of a previous study, which demonstrated that
sirtinol blocks the resveratrol-induced neuroprotection following
cerebral ischemic damage (47).
Notably, sirtinol did not affect cell viability and apoptosis in
HBVSMCs that did not undergo H/R treatment, which is consistent
with the results of previous studies (48,49).
These results indicate that the activation of the SIRT1/FOXO3α
pathway contributes to the neuroprotection induced by SAL in cells
that have undergone H/R injury.
Bcl-2 family proteins are a critical checkpoint in
apoptotic signal transduction cascades, which irreversibly damages
cellular constituents (50). The
ratio of Bcl-2/Bax protein is a determining factor in the
modulation of apoptotic cell death, which is associated with the
mitochondrial apoptotic pathway (51). Additionally, cerebral ischemia injury
may activate the mitochondrial apoptotic pathway, as demonstrated
by changes in caspase-like enzyme activation, cytochrome c release
and the expression of Bcl-2 family proteins (52). In the present study, inhibition of
the SIRT1/FOXO3α pathway prevented the reversal effect of SAL on
the H/R-induced alternation of Bax and Bcl-2 in HBVSMCs, indicating
that the SAL-activated SIRT1/FOXO3α pathway may improve
mitochondrial function, thus protecting against I/R injury.
In conclusion, the current study demonstrated that
pretreatment of HBVSMCs with SAL protects against H/R-induced
cytotoxicity and apoptosis and that this neuroprotection depends on
the activation of the SIRT1/FOXO3α pathway. These results may aid
the development of novel strategies against cerebral ischemia and
provide a foundation for a novel pharmacological approach to treat
neuroprotective effects in patients with cerebral I/R injury or
associated diseases by increasing activation of the SIRT1/FOXO3α
pathway.
Acknowledgements
This study was supported by a grant from the First
Affiliated Hospital of Zhengzhou University.
References
|
1
|
Gao J, He H, Jiang W, Chang X, Zhu L, Luo
F, Zhou R, Ma C and Yan T: Salidroside ameliorates cognitive
impairment in a d-galactose-induced rat model of Alzheimer's
disease. Behav Brain Res. 293:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang SJ, Yu HY, Kang DY, Ma ZQ, Qu R, Fu Q
and Ma SP: Antidepressant-like effects of salidroside on olfactory
bulbectomy-induced pro-inflammatory cytokine production and
hyperactivity of HPA axis in rats. Pharmacol Biochem Behav.
124:451–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao L, Li H, Zhang J, Yang F, Huang A,
Deng J, Liang M, Ma F, Hu M and Huang Z: Salidroside protects
Caenorhabditis elegans neurons from polyglutamine-mediated toxicity
by reducing oxidative stress. Molecules. 19:7757–7769. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xian H, Zhao J, Zheng Y, Wang M, Huang J,
Wu B, Sun C and Yang Y: MADP, a salidroside analog, protects
hippocampal neurons from glutamate induced apoptosis. Life Sci.
103:34–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipp LL: Brain perfusion and oxygenation.
Crit Care Nurs Clin North Am. 26:389–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han J, Xiao Q, Lin YH, Zheng ZZ, He ZD, Hu
J and Chen LD: Neuroprotective effects of salidroside on focal
cerebral ischemia/reperfusion injury involve the nuclear erythroid
2-related factor 2 pathway. Neural Regen Res. 10:1989–1996. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barhwal K, Das SK, Kumar A, Hota SK and
Srivastava RB: Insulin receptor A and Sirtuin 1 synergistically
improve learning and spatial memory following chronic salidroside
treatment during hypoxia. J Neurochem. 135:332–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi TY, Feng SF, Xing JH, Wu YM, Li XQ,
Zhang N, Tian Z, Liu SB and Zhao MG: Neuroprotective effects of
Salidroside and its analogue tyrosol galactoside against focal
cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro.
Neurotox Res. 21:358–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou YQ, Cai ZY, Mao YF, Li JB and Deng XM:
Effects of salidroside-pretreatment on neuroethology of rats after
global cerebral ischemia-reperfusion. Zhong Xi Yi Jie He Xue Bao.
7:130–134. 2009.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kiseleva TN and Chudin AV: Experimental
model of ocular ischemic diseases. Vestn Ross Akad Med Nauk.
97–103. 2014.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dali-Youcef N, Lagouge M, Froelich S,
Koehl C, Schoonjans K and Auwerx J: Sirtuins: The ‘magnificent
seven’, function, metabolism and longevity. Ann Med. 39:335–345.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pallàs M, Casadesús G, Smith MA,
Coto-Montes A, Pelegri C, Vilaplana J and Camins A: Resveratrol and
neurodegenerative diseases: Activation of SIRT1 as the potential
pathway towards neuroprotection. Curr Neurovasc Res. 6:70–81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng X, Tan J, Li M, Song S, Miao Y and
Zhang Q: Sirt1: Role Under the Condition of Ischemia/Hypoxia. Cell
Mol Neurobiol. 37:17–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poulose N and Raju R: Sirtuin regulation
in aging and injury. Biochim Biophys Acta. 1852:2442–2455. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Duan W, Li Y, Yan J, Yi W, Liang
Z, Wang N, Yi D and Jin Z: New role of silent information regulator
1 in cerebral ischemia. Neurobiol Aging. 34:2879–2888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH,
Ho TJ, Kuo WW, Chang CH, Huang CY and Lin WT: Resveratrol enhanced
FOXO3 phosphorylation via synergetic activation of SIRT1 and
PI3K/Akt signaling to improve the effects of exercise in elderly
rat hearts. Age (Dordr). 36:97052014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin W, Zhao W, Ho L, Wang J, Walsh K,
Gandy S and Pasinetti GM: Regulation of forkhead transcription
factor FoxO3a contributes to calorie restriction-induced prevention
of Alzheimer's disease-type amyloid neuropathology and spatial
memory deterioration. Ann N Y Acad Sci. 1147:335–347. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng K, Li Y, Long L, Li D, Jia Q, Wang Y,
Shen Q, Tang Y, Wen L, Kung HF and Peng Y: Knockdown of FoxO3a
induces increased neuronal apoptosis during embryonic development
in zebrafish. Neurosci Lett. 484:98–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mojsilovic-Petrovic J, Nedelsky N,
Boccitto M, Mano I, Georgiades SN, Zhou W, Liu Y, Neve RL, Taylor
JP, Driscoll M, et al: FOXO3a is broadly neuroprotective in vitro
and in vivo against insults implicated in motor neuron diseases. J
Neurosci. 29:8236–8247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kops GJ, Dansen TB, Polderman PE, Saarloos
I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH and Burgering
BM: Forkhead transcription factor FOXO3a protects quiescent cells
from oxidative stress. Nature. 419:316–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avraham Y, Davidi N, Porat M, Chernoguz D,
Magen I, Vorobeiv L, Berry EM and Leker RR: Leptin reduces infarct
size in association with enhanced expression of CB2, TRPV1, SIRT-1
and leptin receptor. Curr Neurovasc Res. 7:136–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG and
Xu Y: Icariin protects against brain injury by enhancing
SIRT1-dependent PGC-1alpha expression in experimental stroke.
Neuropharmacology. 59:70–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang
YJ, Wu WN, Dong LD and Chen JG: Protection by tetrahydroxystilbene
glucoside against cerebral ischemia: Involvement of JNK, SIRT1, and
NF-kappaB pathways and inhibition of intracellular ROS/RNS
generation. Free Radic Biol Med. 47:229–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Deng A, Zhou T and Ding F:
Pretreatment with
2-(4-methoxyphenyl)ethyl-2-acetamido-2-deoxy-β-D-pyranoside
attenuates cerebral ischemia/reperfusion-induced injury in vitro
and in vivo. PLoS One. 1:e1001262014. View Article : Google Scholar
|
|
26
|
Han T: Effects of salidroside pretreatment
on expression of tumor necrosis factor-alpha and permeability of
blood brain barrier in rat model of focal
cerebralischemia-reperfusion injury. Asian Pac J Trop Med.
6:156–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koronowski KB and Perez-Pinzon MA: Sirt1
in cerebral ischemia. Brain Circ. 1:69–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakka VP, Gusain A, Mehta SL and Raghubir
R: Molecular mechanisms of apoptosis in cerebral ischemia: Multiple
neuroprotective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao JJ, Song JQ, Pan SY and Wang K:
Treatment with isorhamnetin protects the brain against ischemic
injury in mice. Neurochem Res. 41:1939–1948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn SM, Kim HN, Kim YR, Choi YW, Kim CM,
Shin HK and Choi BT: Emodin from Polygonum multiflorum ameliorates
oxidative toxicity in HT22 cells and deficits in photothrombotic
ischemia. J Ethnopharmacol. 188:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Zhao Y, Zheng C, Meng Y and Yang Y:
Synthesis, biological activity of salidroside and its analogues.
Chem Pharm Bull (Tokyo). 58:1627–1629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai W, Zheng Z, Zhang X, Wei Y, Chu K,
Brown J, Hong G and Chen L: Salidroside-mediated neuroprotection is
associated with induction of early growth response genes (Egrs)
across a wide therapeutic window. Neurotox Res. 28:108–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi N, Zhu C and Li L: Rehabilitation
training and resveratrol improve the recovery of neurological and
motor function in rats after cerebral ischemic injury through the
Sirt1 signaling pathway. Biomed Res Int. 2016:17321632016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu H and Wang B: SIRT1 exerts
neuroprotective effects by attenuating cerebral
ischemia/reperfusion-induced injury via targeting p53/microRNA-22.
Int J Mol Med. 39:208–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mellini P, Valente S and Mai A: Sirtuin
modulators: An updated patent review (2012–2014). Expert Opin Ther
Pat. 25:5–15. 2015.PubMed/NCBI
|
|
37
|
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ,
Li T, Fan J, Peng ZW and Yan WJ: Nrf2/antioxidant defense pathway
is involved in the neuroprotective effects of Sirt1 against focal
cerebral ischemia in rats after hyperbaric oxygen preconditioning.
Behav Brain Res. 309:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Silent information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Kreutzenberg SV, Ceolotto G, Papparella
I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP
and Avogaro A: Downregulation of the longevity-associated protein
sirtuin 1 in insulin resistance and metabolic syndrome: Potential
biochemical mechanisms. Diabetes. 59:1006–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv H, Wang L, Shen J, Hao S, Ming A, Wang
X, Su F and Zhang Z: Salvianolic acid B attenuates apoptosis and
inflammation via SIRT1 activation in experimental stroke rats.
Brain Res Bull. 115:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lam EW, Francis RE and Petkovic M: FOXO
transcription factors: Key regulators of cell fate. Biochem Soc
Trans. 34:722–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gomes AR, Yong JS, Kiew KC, Aydin E,
Khongkow M, Laohasinnarong S and Lam EW: Sirtuin1 (SIRT1) in the
acetylation of downstream target proteins. Methods Mol Biol.
1436:169–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zakhary SM, Ayubcha D, Dileo JN, Jose R,
Leheste JR, Horowitz JM and Torres G: Distribution analysis of
deacetylase SIRT1 in rodent and human nervous systems. Anat Rec
(Hoboken). 293:1024–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fukunaga K and Shioda N:
Pathophysiological relevance of forkhead transcription factors in
brain ischemia. Adv Exp Med Biol. 665:130–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang XX, Wang XL, Tong MM, Gan L, Chen H,
Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, et al: SIRT6 protects
cardiomyocytes against ischemia/reperfusion injury by augmenting
FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol.
111:132016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li
L, Wu W and Hann S: p38α MAPK-mediated induction and interaction of
FOXOa and p contribute to the inhibited-growth and
induced-apoptosis of human lung adenocarcinoma cells by berberine.
J Exp Clin Cancer Res. 33:362014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Della-Morte D, Dave KR, DeFazio RA, Bao
YC, Raval AP and Perez-Pinzon MA: Resveratrol pretreatment protects
rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling
protein 2 pathway. Neuroscience. 159:993–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee JH, Moon JH, Nazim UM, Lee YJ, Seol
JW, Eo SK, Lee JH and Park SY: Melatonin protects skin keratinocyte
from hydrogen peroxide-mediated cell death via the SIRT1 pathway.
Oncotarget. 7:12075–12088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W,
Zhao G, Zhai M, Liu L, Yi D, et al: Berberine attenuates myocardial
ischemia/reperfusion injury by reducing oxidative stress and
inflammation response: Role of silent information regulator 1. Oxid
Med Cell Longev. 2016:16896022016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng JH, Viacava Follis A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857:1243–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|