Introduction
Mesenchymal stem cells (MSCs) are a key type of
multipotent stem cells in clinical applications (1). MSCs are ubiquitous and can be isolated
from bone marrow, skin, heart, adipose tissue, brain, deciduous
teeth, umbilical cord (UC) and peripheral blood (2). In specific in vivo
microenvironments or when cultured in specific differentiation
medium, MSCs can be induced to differentiate into many cell types
including adipocytes, tenocytes, osteoblasts and visceral mesoderm
(3). MSCs also reduce immune
responses by suppressing the activation and proliferation of immune
cells such as T cells (4), B cells
(5), natural killer cells (6) and antigen-presenting cells, and the
complement system (7). Regarding
surface marker expression, MSCs are negative for co-stimulatory
molecules CD80, CD86 and human leukocyte antigen-II and positive
for CD29, CD90 and CD59, which makes MSCs unable to stimulate allo-
or xenogeneic lymphocytes due to a lack of immunogenicity (8). MSCs also secrete multiple trophic
molecules that can benefit remote tissues and serve a key function
in tissue repair (9).
Based on cellular characteristics, MSCs are now the
best candidate in many cell-based therapies. Their
immunosuppressive capability serves a key function in the induction
of transplantation tolerance and protects solid organ grafts from
rejection (10). In autoimmune
disease treatment, engraftment of MSCs improves the levels of
serological markers and stabilizes renal function by preventing the
appearance of serious adverse events (11). MSCs have also been widely used in
many treatment trials, including stem cell-based therapies for
liver cirrhosis (12), cerebral
palsy (13), type I diabetes
(14), multiple sclerosis (15) and graft-vs.-host disease (16). However, the effect of MSCs on lung
cancer cells remains unclear. In the present study, UC-derived MSCs
(UCMSCs) were co-cultured with H1299 lung cancer cells in order to
evaluate how MSCs influence the biological functions of H1299
cells.
Materials and methods
Cells and co-culture system
H1299 cells (State key Laboratory of Biological
Treatment, Sichuan University; Chengdu, China) were maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) and in a sterile humidified incubator with 5%
CO2 at 37°C. UCMSCs were obtained from the Sichuan
Umbilical Cord Blood Stem Cell Bank (Chengdu, China). After
dissociation in a 37°C water bath, UCMSCs were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) at 1×105 cells/well in 6-well plates,
and all cells were grown in a sterile humidified incubator with 5%
CO2 at 37°C. The medium was changed every 2 days, and
adherent cells were harvested after 2 weeks by 0.25% trypsin
(Gibco; Thermo Fisher Scientific, Inc.) treatment. Only UCMSCs from
passages 6 or lower were used for co-culture. UCMSCs were
co-cultured with H1299 cells in DMEM with 10% FBS and in a sterile
humidified incubator with 5% CO2 at 37°C for 24, 48, 72
and 96 h. The co-culture ratio of UCMSCs to H1299 cells was 2:1.
H1299 cells were considered the control group and underwent the
same culturing time (24, 48, 72 and 96 h).
Transwell-based invasion assay
An invasion assay was conducted using 24-well (8-µm
pore size) Transwell plates (Corning, Inc., Corning, NY, USA).
H1299 cells were plated in the upper chambers in DMEM at
3×104 cells/well, which were pre-coated with 20%
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), while UCMSCs
were maintained in the bottom chamber in DMEM with 1% FBS. After 24
or 48 h, invading H1299 cells were detected by crystal violet
staining at room temperature for 30 min (which was synchronous with
the culturing time) and all the results were observed using light
microscopy (magnification, ×100).
Assays for proliferation, apoptosis
and cell cycle analysis
CCK-8 detection (Cell Counting Kit-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was performed to
assess H1299 cell proliferation at 24, 48 and 72 h in co-culture.
At 24, 48 and 72 h in co-culture, apoptosis was evaluated by
staining of H1299 cell cultures with 3 µl Annexin V (fluorescein
isothiocyanate) at room temperature for 20 min, followed by
counterstaining with 5 µl propidium iodide at room temperature for
5 min and detection by flow cytometry. Cell cycle progression among
H1299 cells was also investigated by flow cytometry.
Western blot analysis
Co-cultured H1299 cells were collected by two washes
with cold PBS, and proteins were extracted using
radioimmunoprecipitation assay protein lysis reagent (Pierce;
Thermo Fisher Scientific, Inc.) containing 1X protease inhibitors
(Roche Diagnostics, Indianapolis, IN, USA). The protein
concentration was measured using a Micro BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Sodium dodecyl
sulfate-polyacrylamide gel (12%) electrophoresis was used to
separate proteins (30 µg per lane), which were then transferred
onto nitrocellulose membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). After blocking with 5% fat-free milk at room
temperature for 1 h, membranes were incubated with primary
antibodies at 4°C overnight and then horseradish
peroxidase-conjugated secondary antibody at room temperature for 2
h (1:5,000; cat. no. ab6789&ab6721; Abcam, Cambridge, UK).
Antigen-antibody complexes were visualized using an enhanced
chemiluminescence reagent (GE Healthcare, Chicago, IL, USA).
Primary antibodies were as follows: AKT (1:800; cat. no. AF6261),
p-AKT (1:800; cat. no. AF0016), phosphoinositide 3-kinase (PI3K;
1:800; cat. no. AF6242), p-PI3K (1:800; cat. no. AF3241), signal
transducer and activator of transcription 3 (STAT3; 1:1,000; cat.
no. AF6294), p-STAT3 (1:1,000; cat. no. AF3294), extracellular
signal-regulated kinase 1/2 (ERK1/2; 1:1,000; cat. no. AF0155),
p-ERK1/2 (1:1,000; cat. no. AF1015), mechanistic target of
rapamycin (mTOR; 1:800; cat. no. AF6308), p-mTOR (1:800; cat. no.
AF3310) (all Affinity Biosciences, Zhenjiang, China) and GAPDH
(1:2,000; cat. no. 200608; Zen BioScience Co., Ltd., Chengdu,
China).
Data analysis
Image Lab software 5.1 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and ModFit LT version 4.1 (Verity Software
House, Topsham, ME, USA) were used for analysis of western blotting
and cell cycle data, respectively. All analyzed data are expressed
as the mean ± standard error, as calculated using SPSS 19.0 (IBM
Corp, Armonk, NY, USA). T-tests were performed to evaluate
inter-group differences. P<0.05 was considered to indicate a
statistically significant difference. All figures were generated
using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
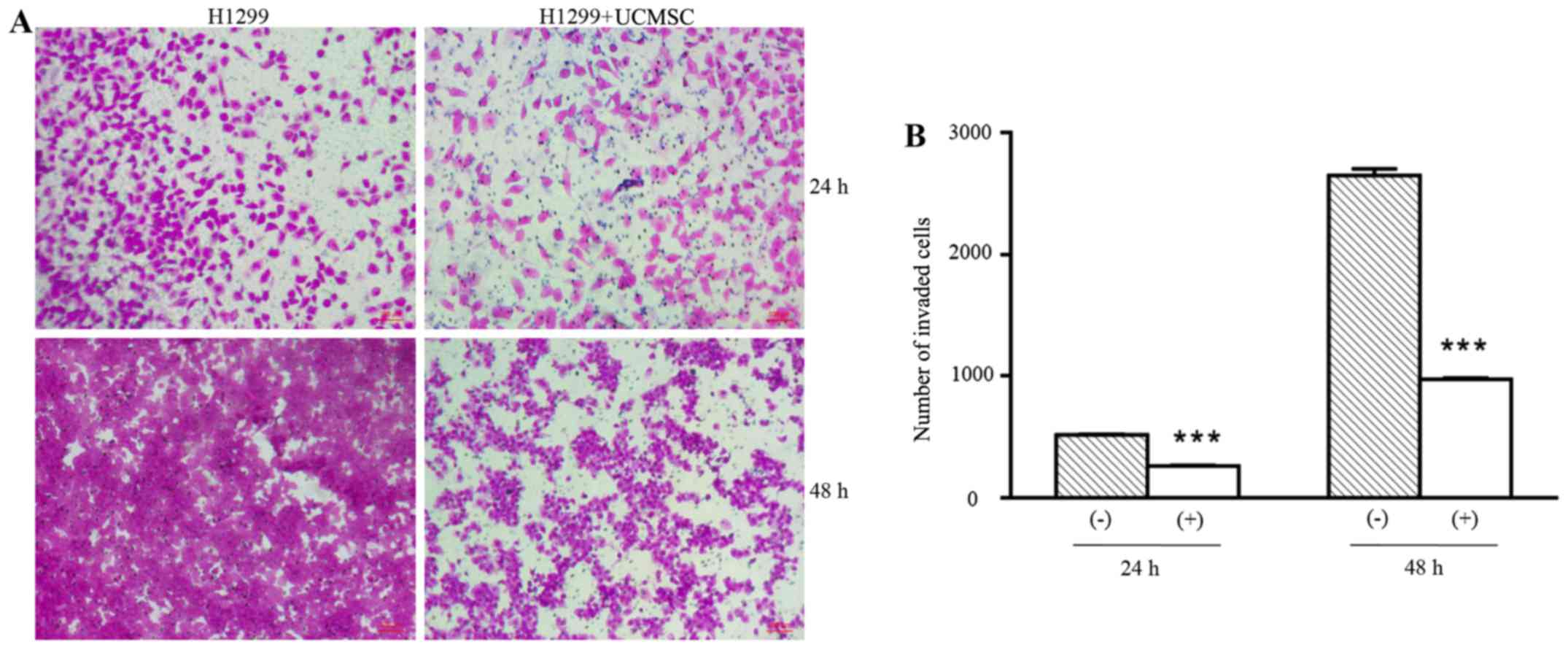
UCMSCs significantly suppressed
invasion of H1299 cells
The invasion ability of H1299 cells in the presence
of UCMSCs was detected in Transwell chambers coated with Matrigel.
As shown in Fig. 1, the number of
invading H1299 cells was significantly reduced in the co-culture
group compared with the control (H1299 cells only) group following
24 and 48 h in culture (P<0.001). This indicated that UCMSCs
could inhibit the invasion of H1299 cells.
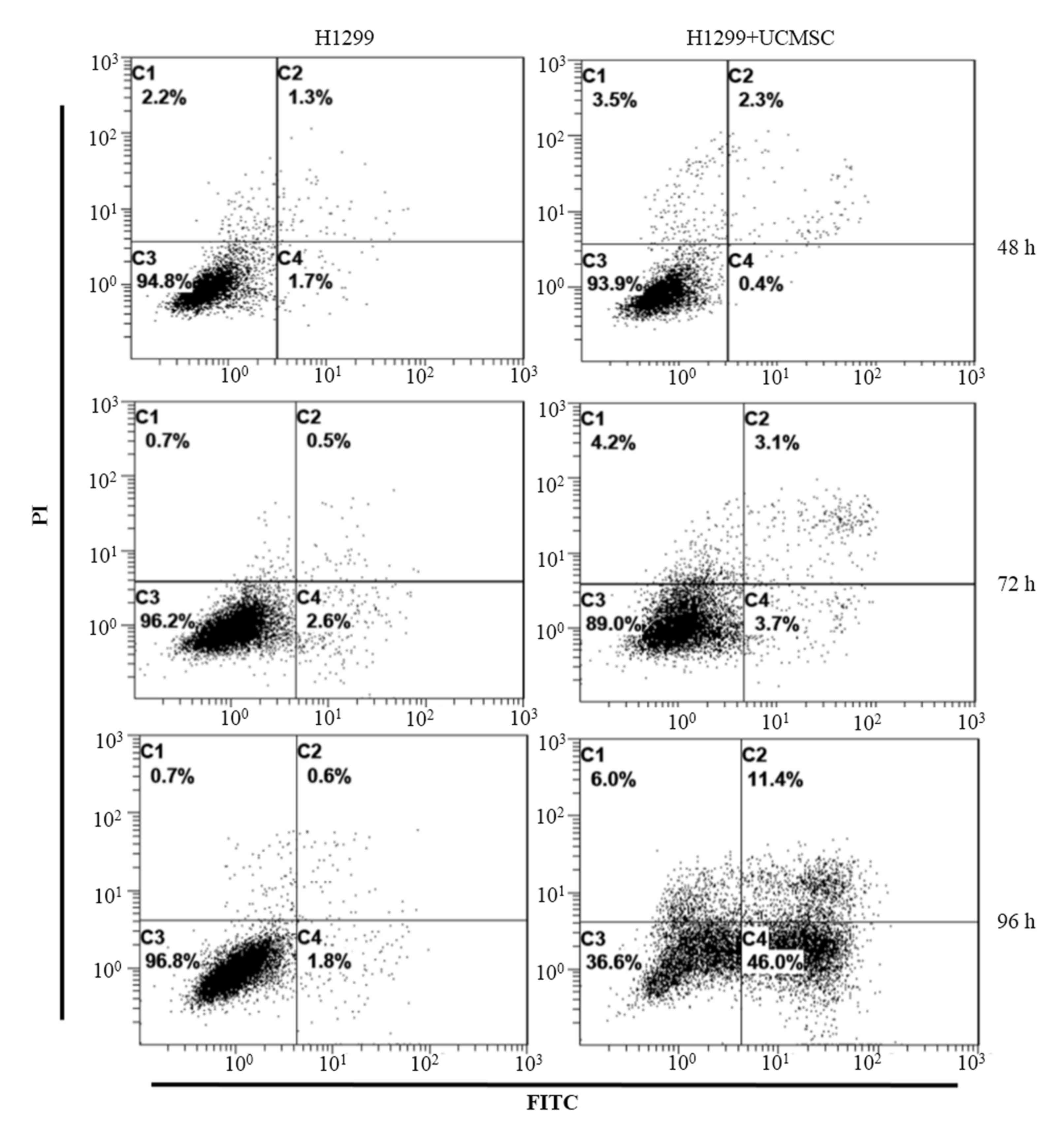
UCMSCs induced apoptosis of H1299
cells
Flow cytometry was conducted to identify changes in
the apoptosis rate of H1299 cells in co-culture with UCMSCs
according to Annexin V staining. The results indicated that the
apoptosis rates of H1299 cells did not differ markedly between the
control and co-culture groups at either 48 h (3.0 vs. 2.7%) or 72 h
(3.1 vs. 6.8%; Fig. 2). However,
after 96 h in co-culture, the apoptosis rate of H1299 cells in the
co-culture group (57.4%) was greater than that among H1299 cells
cultured alone (2.4%).
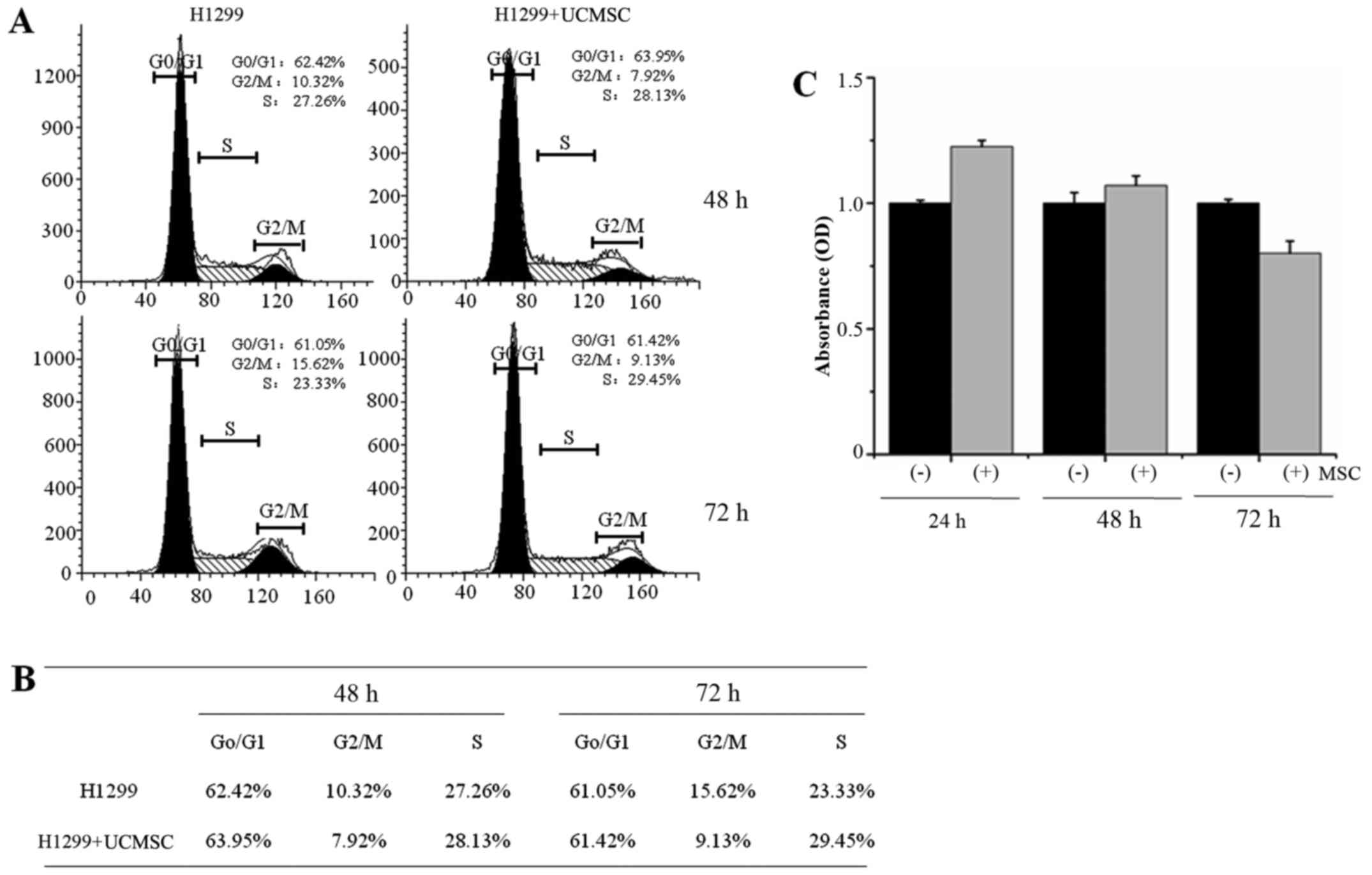
UCMSCs inhibited H1299 cell
proliferation but exhibited no effect on cell cycle
progression
The cell cycle distribution of H1299 cells was
detected by flow cytometry. The results indicated no differences
between H1299 cells cultured alone and with UCMSCs for either 48 h
(control cells: G0/G1 62.42%, G2/M 10.32%, S 27.26%; co-cultured
cells: G0/G1 63.95%, G2/M 7.92%, S 28.13%) or 72 h (control cells:
G0/G1 61.05%, G2/M 15.62%, S 23.33%; co-cultured cells: G0/G1
61.42%, G2/M 9.13%, S 29.45%; Fig. 3A
and B). The proliferation of H1299 cells was then evaluated by
CCK8 assay after 24, 48 or 72 h in co-culture with UCMSCs. The
results indicated that H1299 cell proliferation was not
significantly influenced by UCMSCs in co-culture, although a slight
increase after 24, 48 h and a slight decrease after 72 h were
observed.
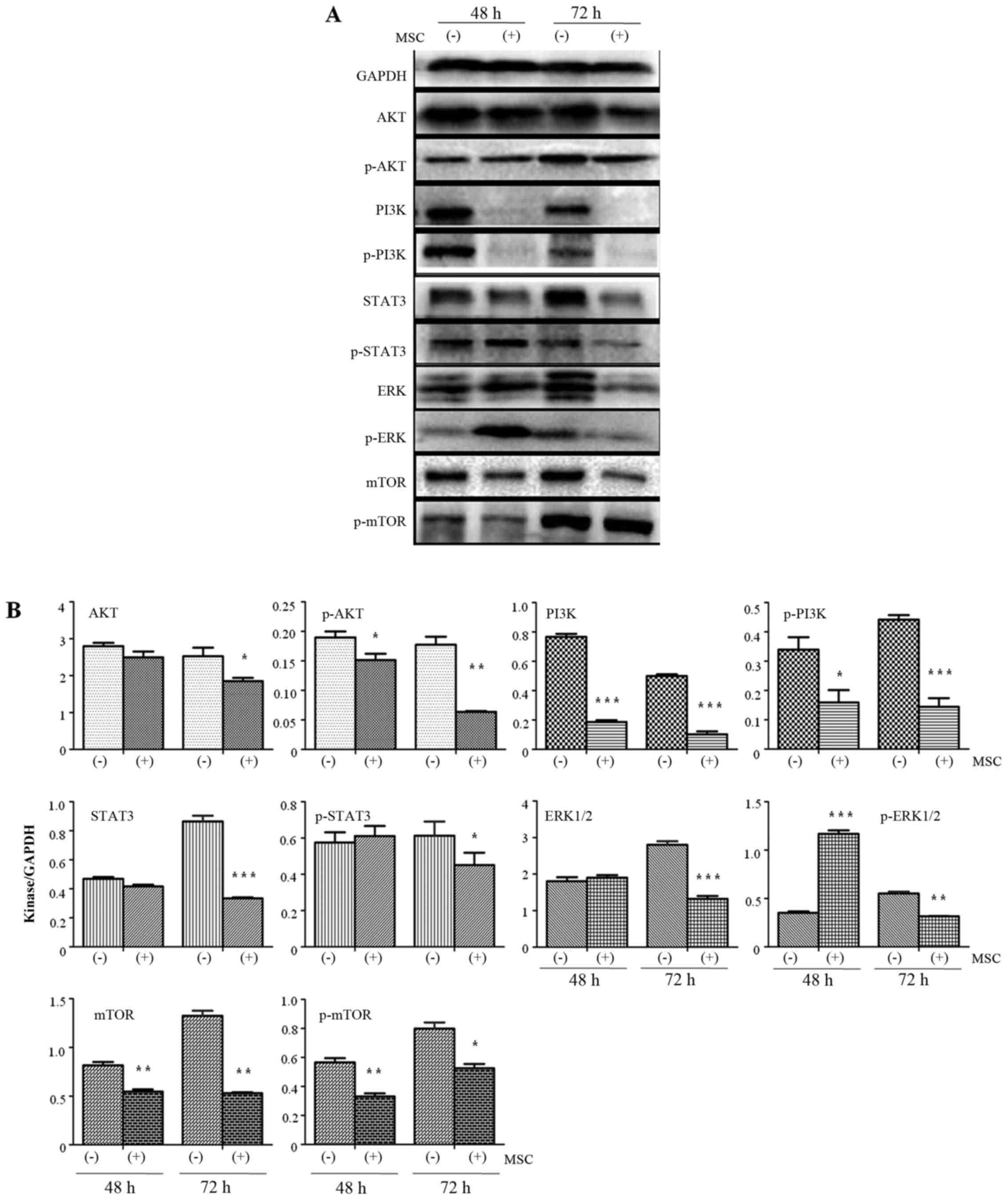
UCMSCs inhibited PI3K/AKT kinase
expression in H1299 cells
Previous research has demonstrated that the
PI3K/AKT/STAT3 pathway serves a key function in mediating the
occurrence of tumor metastasis (17). In the present study, the expression
of these kinases was detected by western blotting in order to
explore the mechanisms by which UCMSCs inhibit the invasive ability
of H1299 cells (Fig. 4). Expression
of AKT in H1299 cells exhibited no change after 48 h in co-culture
with UCMSCs compared with the control cells. However, the
expression of AKT was significantly inhibited at 72 h (P<0.05).
p-AKT expression was significantly inhibited in co-cultured H1299
cells compared with control cells at 48 (P<0.05) and 72 h
(P<0.01). PI3K and p-PI3K expression levels were also
significantly reduced after 48 (PI3K, P<0.001; p-PI3K,
P<0.05) and 72 h (P<0.001) in co-culture compared with
control cells. Co-culture with UCMSCs resulted in inhibited
expression of STAT3 (P<0.001) and p-STAT3 (P<0.05) in H1299
cells only after 72 h in co-culture. ERK1/2 expression was
significantly decreased after 72 h (P<0.001). p-ERK expression
was significantly increased after 48 h (P<0.001) and then
significantly inhibited after 72 h (P<0.01) in co-culture
compared with control cells. Finally, after 48 and 72 h in
co-culture with UCMSCs, expression of mTOR (P<0.01) and p-mTOR
(48 h, P<0.01; 72 h, P<0.05) was significantly inhibited.
These observations indicated that the AKT/PI3K/STAT3/mTOR pathways
are involved in the effects of USMSCs on the biological functions
of H1299 cells.
Discussion
To the best of our knowledge, the effect of UCMSCs
on the biological functions of lung cancer cells has not previously
been reported. In the present study, H1299 cells were co-cultured
with UCMSCs in order to investigate how UCMSCs influenced the
biological functions of H1299 cells. This was analyzed using
numerous detection methods, including CCK8 assay (proliferation),
flow cytometry (apoptosis and cell cycle), Transwell Matrigel assay
(invasion) and western blot analysis (expression of kinases). The
results indicated that UCMSCs inhibited invasion and induced
apoptosis of H1299 cells, but exerted little influence on H1299
cell cycle distribution or proliferation. Further analyses
suggested that expression of multiple kinases, including AKT, PI3K,
ERK, STAT3 and mTOR, in either phosphorylated or non-phosphorylated
states, was significantly suppressed in H1299 cells co-cultured
with UCMSCs. Thus, the present study indicated that UCMSCs could
inhibit the biological functions of H1299 cells by suppressing
activation of AKT/PI3K/STAT3/mTOR signaling.
Experimental evidence on the effects of MSCs on
tumor cells remains contradictory. Consistent with the present
results, Wu et al (18)
demonstrated that microvesicles from human UCMSCs could inhibit the
functions of T24 bladder cancer cells. The study also demonstrated
that this proliferation suppression was mediated by cell cycle
arrest and the induction of apoptosis was mediated by increased
expression of caspase 3. In a study of the effects of MSCs on
cholangiocarcinoma, Liu et al (19) established xenograft models by
injection of HCCC-9810 cells and identified that conditioned medium
from UCMSCs inhibited proliferation and induced apoptosis in a
dose- and time-dependent manner. In another study, α-smooth muscle
actin-positive MSCs were co-engrafted with pancreatic cancer cells
in severe combined immunodeficiency mice and the results confirmed
that MSCs could promote epithelial-mesenchymal transition (20). Mechanistic analysis indicated that
the ‘stemness’ of pancreatic cells was enhanced by Notch-associated
signaling regulated by MSCs (20).
Zhang et al (21) co-cultured
breast cancer and prostate cancer cells with bone marrow-derived
MSCs and identified that treatment with MSCs significantly enhanced
angiogenesis within the tumor in nude mice. Considering all these
findings together, the effects of MSCs on the biological functions
of cancer cells remain controversial. Possible explanations for
this include heterogeneity of MSCs or variations in
microenvironments.
To the best of our knowledge, the present study was
the first to demonstrate that UCMSCs could suppress lung cancer
cell functions by inhibiting invasion and inducing apoptosis. In
the present mechanistic analysis, it was identified that expression
of multiple key kinases (AKT, PI3K, STAT3 and mTOR) by H1299 cells
was inhibited in the presence of UCMSCs. This may indicate that
AKT/PI3K/STAT3 signaling is important in the UCMSC-mediated
regulation of H1299 cell functions. Further studies are required to
confirm this hypothesis.
References
|
1
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glennie S, Soeiro I, Dyson PJ, Lam EW and
Dazzi F: Bone marrow mesenchymal stem cells induce division arrest
anergy of activated T cell. Blood. 105:2821–2827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren G, Su J, Zhang L, Zhao X, Ling W,
L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, et al: Species
variation in the mechanisms of mesenchymal stem cell-mediated
immunosuppression. Stem Cells. 27:1954–1962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sotiropoulou PA, Perez SA, Gritzapis AD,
Baxevanis CN and Papamichail M: Interactions between human
mesenchymal stem cells and natural killer cells. Stem Cells.
24:74–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y,
Yu XD and Mao N: Human mesenchymal stem cells inhibit
differentiation and function of monocyte-derived dendritic cells.
Blood. 105:4120–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronthos S, Franklin DM, Leddy HA, Robey
PG, Storms RW and Gimble JM: Surface protein characterization of
human adipose tissue derived stromal cells. J Cell Physiol.
189:54–63. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bassi E, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cell. 3:1–8. 2011. View Article : Google Scholar
|
|
10
|
Rasmusson I: Immune modulation by
mesenchymal stem cells. Exp Cell Res. 312:2169–2179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kharaziha P, Hellström PM, Noorinayer B,
Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost
M, Zali MR and Soleimani M: Improvement of liver function in liver
cirrhosis patients after autologous mesenchymal stem cell
injection: A phase I–II clinical trial. Eur J Gastroenterol
Hepatol. 21:1199–1205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J,
Chen L, Lv S, Li Y, Yu S, et al: Human umbilical cord mesenchymal
stem cells improve liver function and ascites in decompensated
liver cirrhosis patients. J Gastroenterol Hepatol. 2 Suppl
27:S112–S120. 2012. View Article : Google Scholar
|
|
14
|
Vojtassák J, Danisovic L, Kubes M, Bakos
D, Jarábek L, Ulicná M and Blasko M: Autologous biograft and
mesenchymal stem cells in treatment of the diabetic foot. Neuro
Endocrinol Lett. 2 Suppl 27:S134–S137. 2006.
|
|
15
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noël D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu S, Ju GQ, Du T, Zhu YJ and Liu GH:
Mcirovesicles derived from human umbilical cord Wharton's jelly
mesenchymal stem cells attenuate bladder tumor cell growth in vitro
and in vivo. PLoS One. 8:e613662013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Han G, Liu H and Qin C: Suppression
of cholangiocarcinoma cell growth by human umbilical cord
mesenchyaml stem cells: A possible role of wnt and Akt signaling.
PLoS One. 8:e628442013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabashima-Niibe A, Higuchi H, Takaishi H,
Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto
Y, Kawachi S, et al: Mesenchymal stem cells regulate
epithelial-mesenchymal transition and tumor progression of
pancreatic cancer cells. Cancer Sci. 104:157–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang T, Lee YW, Rui YF, Cheng TY, Jiang
XH and Li G: Bone marrow-derived mesenchymal stem cells promote
growth and angiogenesis of breast and prostate tumors. Stem Cell
Res Ther. 4:702013. View
Article : Google Scholar : PubMed/NCBI
|