Introduction
Stroke is ranked in the second place among all the
lethal diseases. It seriously affects the quality of life of
patient and also brings huge economic pressure on the family and
society (1–3). Clinically, stroke is divided into
ischemic stroke and hemorrhagic stroke, where the ischemic stroke
rate accountes for ~80% of the incidences of stroke (4–6). The
occurrence of ischemic stroke is due to the lack of cerebral blood
supply and oxygen caused by various reasons, resulting in brain
tissue necrosis. Reperfusion after cerebral ischemia sometimes does
not only restore the normal brain function, but also aggravates
tissue damage and dysfunction, which is called cerebral
ischemia-reperfusion injury. Due to the compensation of human body,
the damage caused by reperfusion only occurs on one side, that is,
focal ischemia-reperfusion injury (7–9).
Evidence showed that inflammation is closely related to the death
of neuronal cells and causes neurological deficits, in which
nuclear transcription factor-κB (NF-κB) is one of the key genes
that cause inflammation (10).
Toll-like receptor (TLR) is a transmembrane receptor and its
subtype which is extensively studied and has clear function is
called TLR4. TLR is mainly involved in the identification of
different pathogens related molecules and leads to increased
secretion in inflammatory cells, which as a result cause systematic
inflammatory response (11). It is
not yet clear whether TLR4/NF-κB signaling pathway is involved in
focal cerebral ischemia-reperfusion injury. In this study, our aim
is to explore the relationship between neurological damage caused
by ischemia-reperfusion and TLR4 and NF-κB signaling pathway in
order to establish the focal cerebral ischemia-reperfusion injury
model in rats and obtain the explicit mechanism of
ischemia-reperfusion injury.
Materials and methods
Instruments and materials
The material used in this study were:
Chrysanthemum ester (NF-κB inhibitor), chloral hydrate (both
from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai,
China), triphenyltetrazole oxide (TTC; Sigma, St. Louis, MO, USA),
ECL luminescent solution (Invitrogen, Carlsbad, CA, USA), DMSO
(Sigma), RIPA lysate, protease inhibitor, phosphatase inhibitor
(both from Servicebio, Wuhan, China). In addition kits and
instruments used in this study were: TRIzol kit (Invitrogen),
reverse transcription kit (Invitrogen), rabbit anti-rat NF-κB,
rabbit anti-rat TLR4, rabbit anti-rat glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), rabbit anti-rat NF-κB p65/p50 polyclonal
antibody (dilution, 1:1,000; cat. nos. 4764, 14358 and 13586; Cell
Signaling Technology, Beverly, MA, USA), goat anti-rabbit IgG
(heavy and lightchain) polyclonal antibody (dilution, 1:1,000; cat.
no. 7074; Cell Signaling Technology, Beverly, MA, USA), terminal
deoxynucleotidyltransferase-mediated dUTP nick end labelling
(TUNEL) kit (Invitrogen), fluorescent inverted microscope (Thermo
Fisher Scientific, Darmstadt Germany), pipette (Eppendorf, Hamburg,
Germany), small animal anesthesia system (RWD Co., Ltd., Shenzhen,
China), ultraviolet spectrophotometer (Beckman Coulter, Inc., Brea,
CA, USA), electronic balance, low temperature centrifuge (both from
Thermo Fisher Scientific). Other related equipments and reagents
are described in the relevant part of this study.
Experimental animals
In this study, male SD rats, 8 weeks old, and
weighing between 220–280 g, were obtained by the Animal
Experimental Center of Jiangxi Province with the experimental
animal certificate number: SCXK (Gan) 2015–0019. Before the
experiment, all rats were adapted to the environment for 1 week and
the diurnal rhythm was strictly followed during the adaptation
period. The environment was kept quiet and the room temperature was
25°C. The animals were allowed to drink and eat freely. The
experiments on animal with the corresponding methods were approved
by the Ethics Committee of Qingdao Central Hospital.
Experimental grouping and
establishment of middle cerebral artery occlusion (MCAO) animal
model
The 36 SD rats were randomly divided into sham
operation group (group S), blank control group (group C) and
Chrysanthemum ester group (group CE). All rats were
anesthetized by intraperitoneal injection of 4% chloral hydrate
before operation and fixed on the mouse plate after anesthesia.
After shaving the neck hair, the neck skin from the center of the
rat neck was cut carefully, the muscle tissue were divided with
tweezers to find the left common carotid artery. The rats in group
S were also subjected to anesthesia and blood vessel separation
whereas the rats in group CE were given 5 mg/kg of
Chrysanthemum ester intraperitoneally 2 h before the
operation. The left common carotid artery from rats in groupC and
CE was isolated, and then the internal carotid artery and external
carotid artery were isolated. The external carotid artery and
common carotid artery was carefully ligated with suture. After
clamping the distal end of carotid artery with the arterial clip,
an incision was carefully opened with a syringe needle at the
proximal end of the internal carotid artery and a thread with a
diameter of 0.3 mm was inserted with the depth between 18.0±2.0 mm
so that it could reach the beginning of the middle cerebral artery
and completely block the arterial blood supply. At the same time,
the internal carotid artery was ligated, and the thread was left
outside (~1 cm), and the skin was sutured. After the operation, the
rats were placed on a warm blanket until they were awake. After 2 h
of ischemia, the thread was gently pulled out and the rats were
under reperfusion for 24 h.
Detection of physiological
indicators
During the operation, blood gas, blood pressure and
body temperature of the animals was closely monitored by
physiological signal recorder (Powerlab, Nuremberg, Germany).
Supine position was taken for the rats during the surgery and left
groin incision was also made. After separating the femoral artery,
the pressure converters treated with heparin was inserted, blood
pressure and arterial blood gas was monitored 20 min before placing
the thread, 20 min after placing the thread and 20 min after
removing the thread. In addition, the rectal temperature of the
rats was monitored during the course of the experiment.
Neurological score
The neurological impairment score was obtained at 2,
8 and 24 h after cerebral ischemia-reperfusion, and the
neurological behavior score was computed according to the Zea-Longa
standard. In this standard, 0 point indicates no symptoms of
neurological deficit that is normal activity, 1 point indicates
that rat contralateral forepaws cannot stretch freely (mild
neurological deficits), 2 points indicate circling to the
contralateral side while walking (moderate neurological deficits),
3 points indicate body dumping to the contralateral side while
walking (severe neurological deficits) and 4 points indicate loss
of consciousness and unable to walk spontaneously. The neurological
scores of all rats was counted and analyzed statistically.
TTC staining
After 24 h of taking reperfusion and after
neurological scores, the rats were sacrificed. The brain was cut
into 3 mm slices by the coronal plane, placed in 2% TTC solution,
incubated in the incubator at 37°C for 5 min in the dark and then
placed in 4% paraformaldehyde to get fixed. Survival tissue in the
TTC solution was dyed bright red, necrotic tissue in the TTC
solution was pale. After fixing for 24 h, all the brain films were
taken, the colorless parts were analyzed by using Image software
and then the percentage of infarct volume was calculated.
Determination of brain cell
apoptosis
Frozen slides of the brain tissue of each group were
taken out from the −80°C refrigerator, slightly dried and washed
with phosphate-buffered saline (PBS) for 5 min, 1% Triton X-100
membrane for 15 min, washed with PBS 3 times, 5 min each. Then
incubated in 100 mM glycine for 20 min, washed with PBS 3 times, 5
min each. The experiment was in strict accordance with the TUNEL
kit instructions: TUNEL reaction solution was prepared, the slide
was added with TUNEL reaction solution and placed in a wet box,
incubated in 37°C incubator in the dark for 1 h, washed with PBS 3
times, 5 min each. Added with DAPI (DAPI:ultrapure water, 1:50) for
10 min, washed with ultrapure water 3 times, 5 min each. The
surrounding area of the tissue was aspired, one drop of
anti-fluorescent quenching mounting medium was added for sealing,
the slide was observed under fluorescence microscope and pictures
were taken, preserved at 4°C in the dark, in which the cells with
yellow-green fluorescence were positive, that is, apoptotic
cells.
Gene and protein expression in
TLR4/NF-κB signaling pathway
Samples of the brain tissue of each group were
taken, ground in liquid nitrogen and transferred to a centrifuge
tube containing 1 ml of chloroform, vortexed for 10 sec for the
tissue samples to completely dissolve in chloroform, after 10 min
ice bath, the sample was centrifuged at 4°C and 3,280 × g for 20
min, the supernatant was transferred to 1.5 ml of RNase-free
centrifuge tube, added with isopropyl alcohol of the same volume,
centrifuged at 4°C and 3,280 × g for 15 min, added with 500 µl of
pre-cooled ethanol to wash the precipitation, then centrifuged at
3,280 × g for 15 min, the supernatant was discarded and the sample
was dried completely at room temperature, the precipitation was
dissolved in 60 µl of DEPC water and placed at −80°C. Two
microliters of RT primer and 10 µl of RNA and 1 µl of dNTP mixture
were mixed and placed at 65°C for 5 min, and cDNA was obtained by
reverse transcription strictly according to the reverse
transcription kit. The expression of TLR4 and NF-κB mRNA was
detected by semi-quantitative PCR with GAPDH as the internal
reference and using the cDNA as template. The PCR reaction
conditions were as follows: 95°C pre-denaturation 5 min, 95°C 30
sec, 64°C 25 sec, 72°C 30 sec, a total of 35 cycles, extension at
72°C for 7 min. Primers were synthesized by Shanghai Sangon
Biotechnology Co., Ltd. The sequences are shown in Table I, and after the reaction,
electrophoresis was performed using 2% agarose gel and the samples
were observed by UV imaging. The low temperature was kept by
placing the sample on ice, lysate was added at 1:10 ratio according
to the weight of the brain tissue, the lysate contained 1% protease
inhibitor and 1% of the phosphatase inhibitor, the tissue was
homogenized using a homogenizer, 5 sec each time, until no visible
tissue samples was observed, the EP tube was placed on ice during
homogenizing, then the sample was centrifuged at 4°C, 3,280 × g for
10 min, the supernant was obtained and the protein was quantitated
using BCA protein quantitation kit. Twelve percent of the separated
gel and 5% of the concentrated gel were prepared. After the gel was
solidified, the same concentration of each protein sample was added
to each well, electrophoresed at 80 V until the band reached the
end of the glass plate, then transferred into a film using wet
transfer method, transferring was conducted at 100 V, for 90 min.
After transfer, the film was closed with 3% BSA-TBST for 1 h, the
target bands were cut, each monoclonal antibody was prepared
according to 1:1,000 with 5% BSA-TBST, using rabbit anti-GAPDH as
internal reference antibody, incubated overnight at 4°C with rabbit
anti-TLR4, rabbit anti-NF-κB and rabbit anti-NF-κB p65/p50
antibody, washed three times with TBST and incubated for 2 h with
the secondary antibody prepared at 1:5,000 ratio with 5% skim milk,
then washed with TBST again, adding ECL luminescent liquid (1:1 mix
of A liquid and B liquid) in the dark, then the fixation time was
determined according to the protein band fluorescence intensity,
fixed after the development, then the bands were scanned and gray
value analysis was performed using ImageJ software.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene | Primer sequences |
|---|
| NF-κB | F:
5′-AACTGTTCCCCCTCATCTTC-3 |
|
| R:
5′-TCCTACAAGCTCGTGGGGGT-3 |
| TLR4 | F:
5′-AGACATCCAAAGGAATACTGCAA-3 |
|
| R:
5′-GCCTTCATGTCTATAGGTGATGC-3 |
| GAPDH | F:
5′-ATGGGGAAGGTGAAGGTCG-3 |
|
| R:
5′-CCATCACGCCACAGTTTCC-3 |
Statistical analysis
The data were processed and analyzed by SPSS 19.0
software (SPSS Inc., Chicago, IL, USA) and expressed as mean value
± standard deviation. Moreover, t-test was used to compare the
results between the groups. In addition, the analysis of variance
(ANOVA) was used to compare the data among multiple groups.
Homogeneity of variance was checked and if the variances were
homogeneous, Bonferroni method was used for comparison. On the
other hand, if the variances were not homogeneous, Welch method and
multiple comparison using Dunnett's T3 method were employed. The
level of significance was set at P<0.05.
Results
Detection of physiological
indexes
In order to rule out the effect of the operation
method on the physiological indexes of rats, these indexes were
monitored. The blood gas and body temperature of the SD rats in
each group were tested during the pretreatment period and during
the operation. The results showed (Table II) that there was no significant
difference in the physiological indicators between the two groups
(P>0.05).
 | Table II.Physiological indexes of the rats. |
Table II.
Physiological indexes of the rats.
| Physiological
indexes | pH | PaCO2
(mmHg) | PaO2
(mmHg) | Rectal temperature
(°C) |
|---|
| Group S | 7.16±0.14 | 43.5±1.9 | 95.1±1.3 | 36.5±0.2 |
| Group C | 7.22±0.11 | 43.1±1.3 | 98.6±3.8 | 36.2±0.1 |
| Group CE | 6.72±0.15 | 41.7±2.2 | 95.1±2.6 | 35.9±0.8 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 |
| t-value | 1.036 | 0.892 | 0.573 | 1.328 |
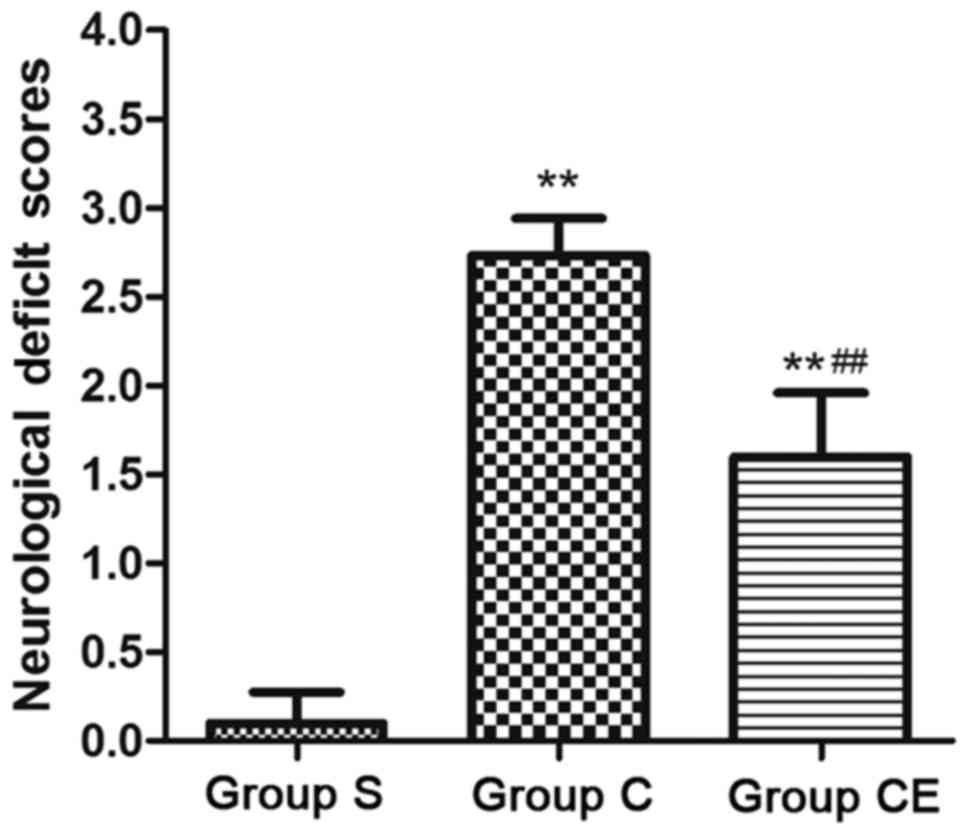
Neurological function score
The neurological function scores were obtained in
each group after focal ischemia-reperfusion and the results are
presented in Fig. 1. The results
showed that the neurological function scores of group C and CE were
significantly higher than those of group S (P<0.01). On the
other hand, the score of neurological function for group CE was
significantly lower than that of group C (P<0.01).
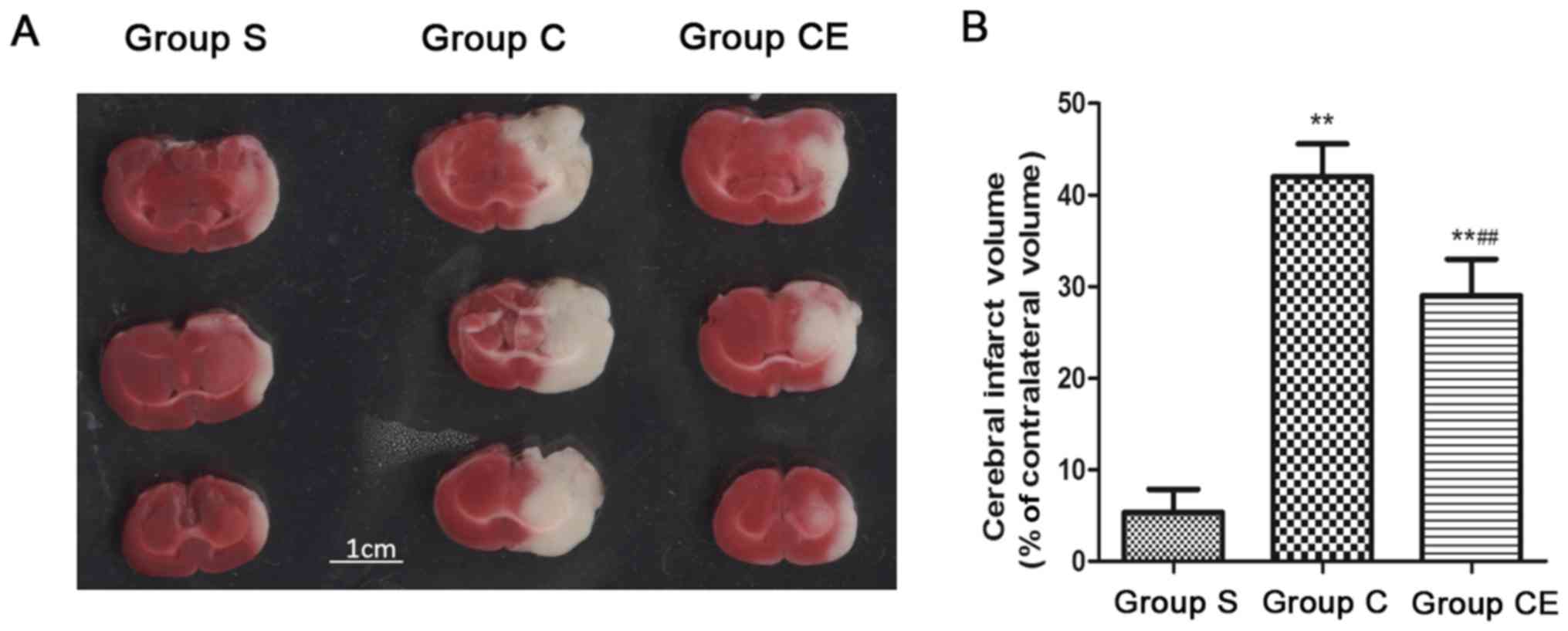
TTC staining
The rat model of focal ischemia-reperfusion was
established. The rats in group C and CE were stained with TTC after
2 h of ischemia and 24 h after reperfusion and at the same time,
rats in group S were also sacrificed and the brain slice was
stained with TTC. The results showed (Fig. 2) that the infarction area of group S
was significantly smaller than that of group C and CE (P<0.01),
the infarction area of group CE was significantly smaller than that
of group C, the differences were statistically significant
(P<0.01).
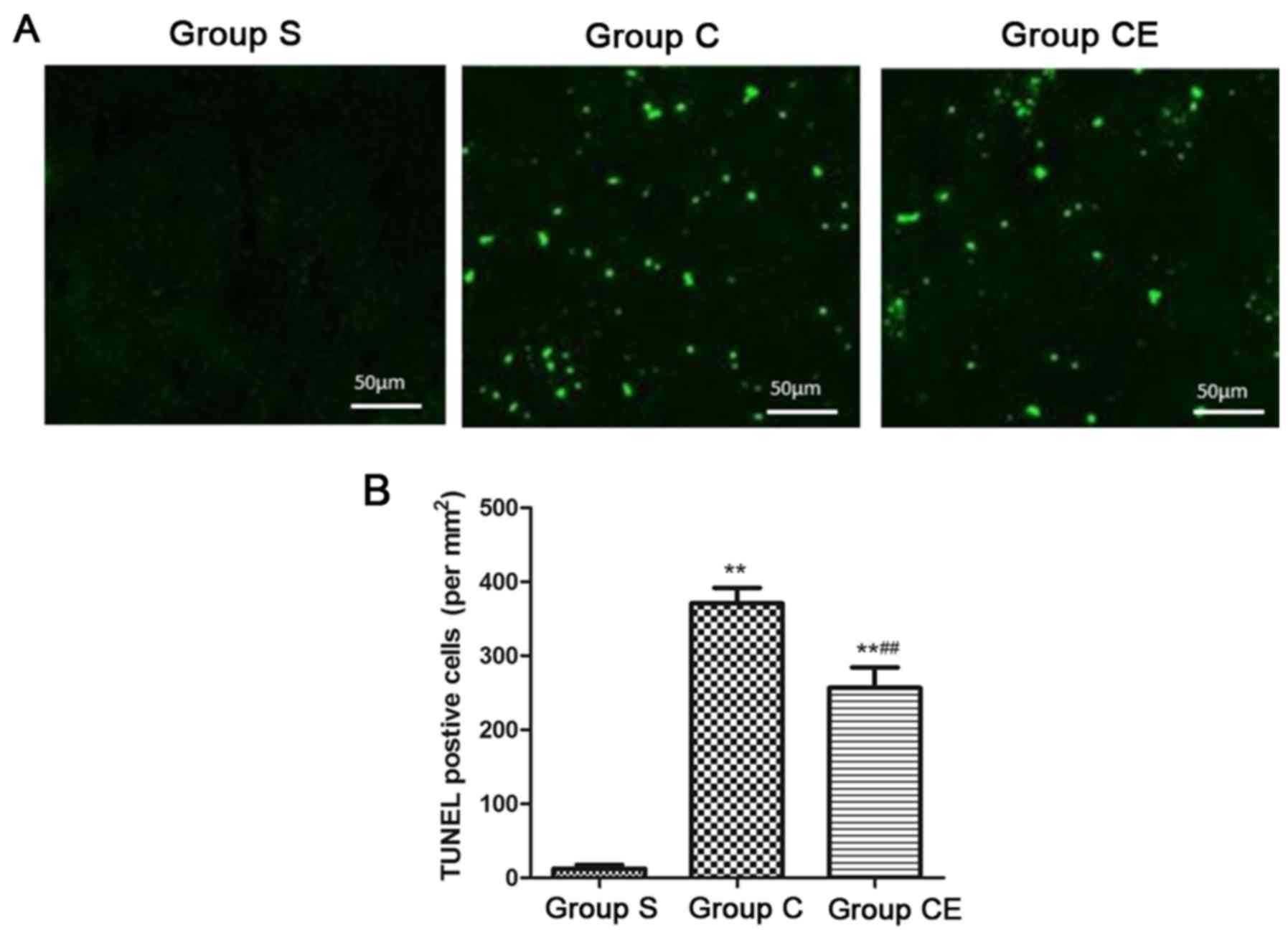
Brain cell apoptosis level
In order to establish rat model of focal
ischemia-reperfusion, rats in group C and CE were stained with
TUNEL after 2 h of ischemia and 24 h after reperfusion and at the
same time, rats in group S were also sacrificed and the brain
tissue was stained with TUNEL. Apoptosis is shown in Fig. 3. The TUNEL positive cells were barely
not observed in the cortex of group S, the number of TUNEL-positive
cells in the cortex of the group C and group CE was significantly
higher than that of group S (P<0.01), and the TUNEL-positive
cells in group CE were significantly lower than in group C
(P<0.01).
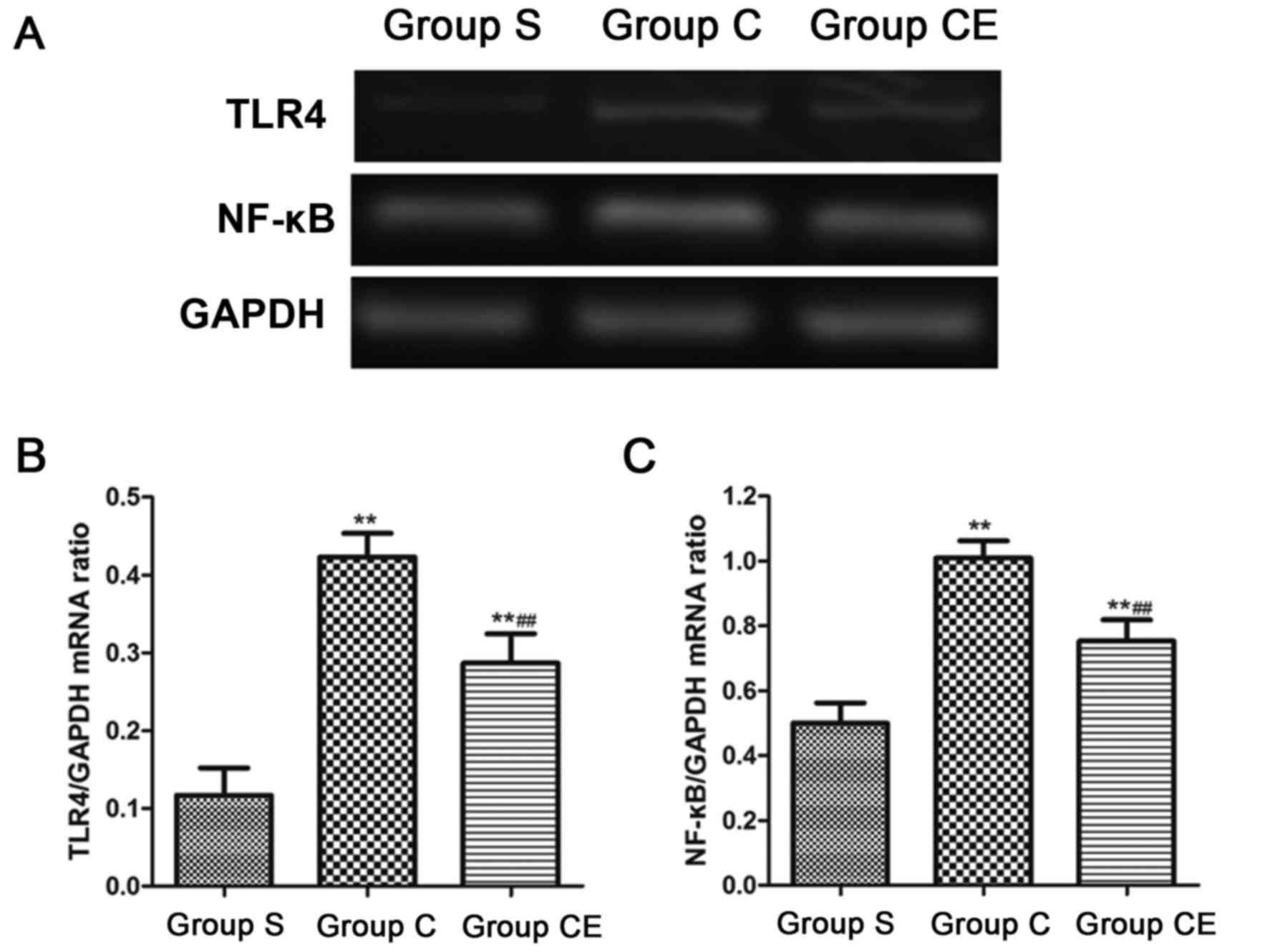
Semi-quantitative PCR detection of
mRNA expression
The expression of NF-κB and TLR4 mRNA in the brain
tissue of each group was detected by semi-quantitative PCR. The
results showed that the expression levels of NF-κB and TLR4 in
group C and CE were significantly higher than those in group S
(P<0.01). The expression of NF-κB and TLR4 in group CE was lower
than that in group C (P<0.01) (Fig.
4).
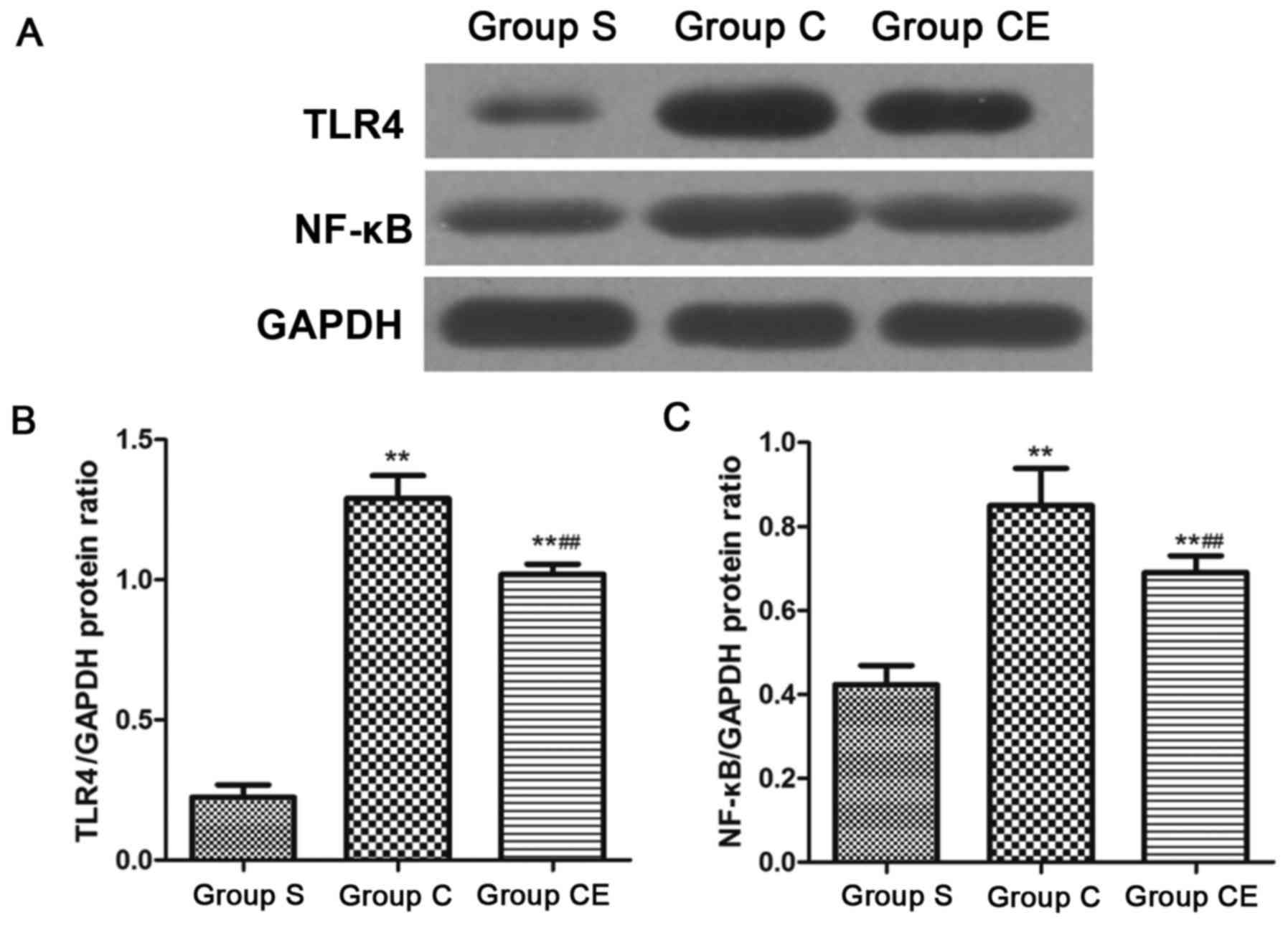
Protein expression detection with
western blot analysis
The expression of NF-κB and TLR4 was detected by
western blot analysis. The results are shown in Fig. 5. The expression of NF-κB and TLR4 in
groups C and CE were significantly higher than that of group S
(P<0.01). Moreover, the expression of NF-κB and TLR4 in group CE
was lower than group C (P<0.01). In addition, the expression of
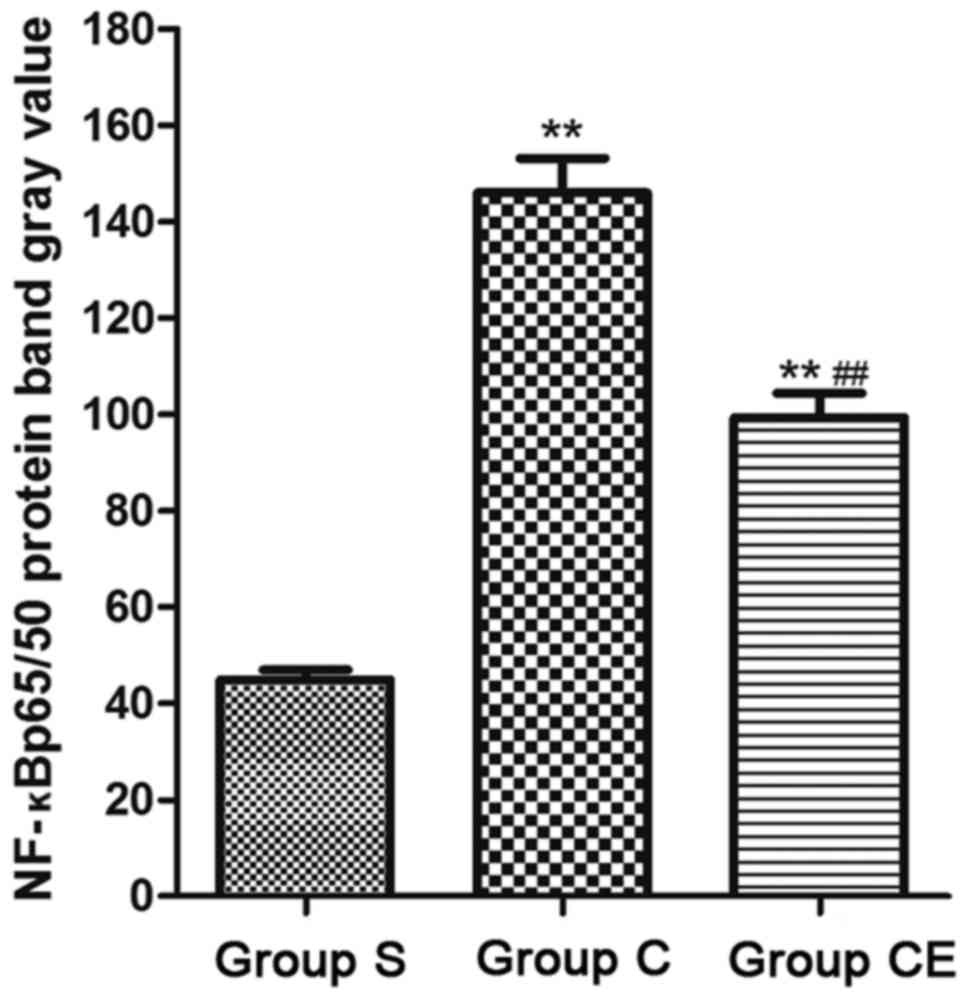
NF-κB p65/p50 was detected (Fig. 6)
and the expression of NF-κB p65/p50 in group CE and group C were
significantly higher than group S (P<0.01).
Discussion
Stroke is one of the most serious diseases that
affect human health. It is considered among the three major lethal
diseases with cardiovascular disease and cancer (12). In Western developed countries,
ischemic cerebrovascular disease accounts for 85% of total strokes.
Ischemic stroke is due to the embolization or thrombosis of the
brain vessels, mainly middle cerebral artery, which leads to brain
blood supply interruption and then the emergence of energy
metabolism disorders, ion steady-state imbalance, free radical
production and excitatory neurotoxicity (13). TLR4 is widely distributed in the
central nervous system. Studies have shown that TLR4 recognizes
endogenous ligands released by ischemia-reperfusion injury, causing
a series of inflammatory responses that seriously affect
neurological function (14). The
activation of NF-κB can lead to the expression of adhesion
molecules and the corresponding receptors which play vital roles in
the inflammatory response (15). The
role of NF-κB/TLR4 signaling pathway in cerebral
ischemia-reperfusion injury is gradually increasing and the
regulation of NF-κB/TLR4 signaling pathway can reduce the damage
caused by ischemia-reperfusion in brain tissue (16).
In this study, we investigated whether TLR4 and
NF-κB are involved in the regulation of nerve injury by studying
focal cerebral ischemia-reperfusion injury in rats. The inhibitor
of NF-κB and Chrysanthemum ester was taken as a control. It
was clarified whether NF-κB is involved in the regulation of
cerebral ischemia-reperfusion injury. Furthermore, the
physiological indexes of rats were monitored during the operation,
which showed that the MCAO model did not affect the physiological
status of the rats. The MCAO animal model could simulate the
ischemia-reperfusion injury of the human body, and it was highly
consistent with the pathological changes of the human body with the
advantage of a short operation time and causing little damage to
the experimental animals (17).
Studies have shown that NF-κB is involved in the regulation of
brain injury because NF-κB activation is closely related to
neurological impairment (18). The
neurological function of rats was scored after the injury and the
results showed that the scores of rats in group C were
significantly increased. This means that ischemia-reperfusion could
cause serious damage to the neurological function of rats.
Moreover, the score in group CE was significantly lower than that
of group C which indicates that inhibiting NF-κB has a protective
effect on the rat's neurological function. In addition, TTC
staining and TUNEL staining were used to evaluate the damage of
brain tissue caused by ischemia-reperfusion from the morphological
and apoptotic levels. The results showed that ischemia-reperfusion
could significantly increase the cerebral infarct size and increase
the level of apoptosis in brain tissue. However,
Chrysanthemum ester can significantly reduce the level of
cerebral infarction and apoptosis, and can fight against
ischemia-reperfusion which in fact plays a protective role on the
brain tissue of rats. The expression of NF-κB signaling pathway and
TLR4 protein was studied by using semi-quantitative PCR and western
blot analysis. The results show that the expression of NF-κB and
TLR4 in group C were significantly higher than that in group S, and
NF-κB p65/p50 was significantly higher than that in group S. This
indicates that ischemia-reperfusion injury can increase the
expression of NF-κB and TLR4, activating NF-κB signaling pathway
which causes brain injury. The endogenous and exogenous substances
in focal cerebral ischemia-reperfusion injury can increase the
expression of NF-κB, activating NF-κB signaling pathway. NF-κB is
transformed from p50 homodimer to p60 heterodimer with
transcriptional activity; activated NF-κB was transferred from the
cytoplasm to the nucleus to play a transcriptional role, which
produces a series of inflammatory factors, promotes the production
of inflammatory response. Moreover, NF-κB p65/p50 was increased
which indicates the activation of NF-κB signaling pathway, which
can further lead to the release of a large number of inflammatory
factors. These factors cause inflammation which leads to increased
levels of apoptosis in brain tissue, and thus causing neurological
function damage. Endogenous substances produced from brain damage
can become TLR4 ligand, thereby increasing the inflammatory
response (19,20). Compared with group C the CE showed
reduced levels of NF-κB and NF-κB p65/p50, which further
demonstrate that NF-κB plays a role in cerebral
ischemia-reperfusion injury.
In conclusion, the model of cerebral
ischemia-reperfusion injury in rats confirmed that
ischemia-reperfusion injury could increase the expression of TLR4
and NF-κB, activate TLR4/NF-κB signaling pathway, induce neuronal
apoptosis and affect neuronal function. By inhibiting NF-κB,
reducing the expression of TLR4, inhibiting TLR4/NF-κB signaling
pathway can play a protective role on focal ischemia-reperfusion
injury.
Acknowledgements
This study was supported by Qingdao Health Science
and Technology Plan Project (no. 2016WJZD046).
References
|
1
|
Park JS, Hwang NK, Oh DH and Chang MY:
Effect of head lift exercise on kinematic motion of the
hyolaryngeal complex and aspiration in patients with dysphagic
stroke. J Oral Rehabil. 17:554–559. 2016.
|
|
2
|
Keppel HJM: A novel selective AMPA
antagonist for stroke, neuropathic pain or epilepsy? Drug
Development Lessons Learned. Drug Dev Res. 95:126–132. 2016.
|
|
3
|
Seto SW, Chang D, Jenkins A, Bensoussan A
and Kiat H: Angiogenesis in ischemic stroke and angiogenic effects
of Chinese herbal medicine. J Clin Med. 5:630–645. 2016. View Article : Google Scholar
|
|
4
|
Vasileva D, Lubenova D, Mihova M,
Grigorova-Petrova K and Dimitrova A: Orthostatic reactivity in
patients with ischemic stroke in the chronic period. Open Access
Maced J Med Sci. 3:851–855. 2015. View Article : Google Scholar
|
|
5
|
Boisserand LS, Kodama T, Papassin J,
Auzely R, Moisan A, Rome C and Detante O: Biomaterial applications
in cell-based therapy in experimental stroke. Stem Cells Int.
2016:68105622016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang Y, Huang J, Tian J, Cao Y, Zhang G,
Wang C, Cao Y and Li J: The prevalence and risk factors of stroke
in patients with chronic schizophrenia. Neuropsychiatr Dis Treat.
12:1131–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crivera C, Nelson WW, Schein JR and Witt
EA: Attitudes toward anticoagulant treatment among nonvalvular
atrial fibrillation patients at high risk of stroke and low risk of
bleed. Patient Prefer Adherence. 10:795–805. 2016.PubMed/NCBI
|
|
8
|
Wang Y and Bajorek B: Clinical pre-test of
a computerised antithrombotic risk assessment tool for stroke
prevention in atrial fibrillation patients: Giving consideration to
NOACs. J Eval Clin Pract. 22:892–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hastrup S, Damgaard D, Johnsen SP and
Andersen G: Prehospital acute stroke severity scale to predict
large artery occlusion: Design and comparison with other scales.
Stroke. 21:487–491. 2016.
|
|
10
|
Zhang H, Li L, Sun Y, Zhang X, Zhang Y, Xu
S, Zhao P and Liu T: Electroacupuncture could regulate the NF-κB
signaling pathway to ameliorate theinflammatory injury in focal
cerebral ischemia/reperfusion model rats. J Neurochem. 137:713–725.
2016.
|
|
11
|
Oshiro AH, Otsuki DA, Hamaji MW, Rosa KT,
Ida KK, Fantoni DT and Auler JO Jr: Differential Roles of TLR2 and
TLR4 in acute focal cerebral ischemia/reperfusion injury in mice.
Brain Res. 70:577–590. 2015.
|
|
12
|
Gauberti M, Obiang P, Guedin P, Balossier
A, Gakuba C, Diependaele AS, Chazalviel L, Vivien D, Young AR, Agin
V, et al: Thrombotic stroke in the anesthetized monkey (Macaca
mulatta): Characterization by MRI - a pilot study. Cerebrovasc Dis.
33:329–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinohara H, Kadoi Y, Takahashi K, Saito S,
Kawauchi C and Mizutani A: Time course of changes in cerebral blood
flow velocity after tourniquet deflation in patients with diabetes
mellitus or previous stroke under sevoflurane anesthesia. J Anesth.
25:409–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying W, Pengfei G and Yuhong Z: TLR2 and
TLR4 in the brain injury caused by cerebral ischemia and
reperfusion. Mediators Inflamm. 9:2–9. 2016.
|
|
15
|
Lu L, Zhang G, Song C, Wang X, Qian W,
Wang Z, Liu Y, Gong S and Zhou S: Protection of ischemic post
conditioning against transient focal ischemia-induced brain damage
is associated with inhibition of neuroinflammation via modulation
of TLR2 and TLR4 pathways. J Neuroinflammation. 16:686–691.
2016.
|
|
16
|
Tomuschat C, O'Donnell AM, Coyle D, Dreher
N, Kelly D and Puri P: Neuroprotective effect of kaempferol
flycosides against brain injury and neuroinflammation by inhibiting
the activation of NF-κB and STAT3 in transient focal stroke.
Pediatr Res. 80:1787–1799. 2016.
|
|
17
|
Zhang M, Yin HJ, Wang WP, Li J and Wang
XL: Over-expressed human TREK-1 inhibits CHO cell proliferation via
inhibiting PKA and p38 MAPK pathways and subsequently inducing G1
arrest. Acta Pharmacol Sin. 37:1190–1198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levitz J, Royal P, Comoglio Y, Wdziekonski
B, Schaub S, Clemens DM, Isacoff EY and Sandoz G: Long non-coding
RNA C2dat1 regulates CaMKIIδ expression to promote neuronal
survival through the NF-κB signaling pathway following cerebral
ischemia. Proc Natl Acad Sci USA. 113:pp. 266–271. 2016;
|
|
19
|
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X,
Chen F, Wang CS, Feng H and Lin JK: Curcumin attenuates acute
inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling
pathway in experimental traumatic brain injury. J
Neuroinflammation. 62:97–110. 2016.
|
|
20
|
Vivier D, Bennis K, Lesage F and Ducki S:
Daphnetin protects against cerebral ischemia/reperfusion injury in
mice via inhibition of TLR4/NF-κB signaling pathway. Biomed Res.
59:328–335. 2016.
|